Abstract
In the present study, the effects of sublethal concentrations of buprofezin on life-table traits of S. furcifera were evaluated for two consecutive generations (F0 and F1). Our results exhibited that the fecundity, life span (longevity) and hatchability of the F0 and F1 generations were significantly decreased at LC30 compared to the control. However, copulation was not significantly affected for the F0 or F1 generations at sublethal concentrations. The female life span was affected negatively at both treatments in F0 and at LC30 in F1, compared to the control. Furthermore, significant effects of the sublethal concentrations were found on the developmental rate of all instars except the 3rd instar of F1. However, the pre-adult period, total pre-oviposition period (TPOP) and adult pre-oviposition period (APOP) significantly increased in F1 individuals at LC30 and LC10 compared to the control. Our findings revealed that demographic characters (survival rate, intrinsic rate of increase (ri), finite rate of increase (λ), net reproductive rate (R 0), and gross reproductive rate (GRR)) of the F1 generation (from F0 parents) significantly decreased compared to the untreated group; however, the generation time (T) increased at LC10. Therefore, the results suggested that buprofezin could adversely affect individuals in the successive generation.
Similar content being viewed by others
Introduction
Rice (Oryza sativa L.) is the 2nd main food source for more than half of the world’s population and affects the livelihood and income of one hundred million people1. The white- backed planthopper (WBPH), Sogatella furcifera (Horvath), is a destructive rice pest throughout Asia, and it causes serious yield losses by sucking cell sap and ovipositing in rice stems2. Recently, outbreaks of the WBPH have damaged rice crops at the immature growth stage by transmitting southern rice black-streaked dwarf virus (SRBSDV). This virus was first reported at a location in Yangxi, Guandong Province, China in 20013. S. furcifera as well as the viral disease, causes heavy yield losses of rice in China and elsewhere in Asia3,4.
Buprofezin, is chitin synthesis inhibitor developed by Nihon-Nohyaku, with very low risks to the environment and human beings, is a thiadiazine insecticide that is especially used against sucking pests, such as the planthoppers. Its worldwide uses are in China, Japan, India and Southeast Asia and its normal application is 75–100 g a.i./ha5,6. Its initial effect is to inhibit with chitin deposition during moulting and to cause nymphal death during cuticle shedding5. In addition, reduced fecundity and egg hatching have been observed after adult females were treated5,6,7. Although buprofezin lacks an acute insecticidal effect, it offers the advantage of longer residual activity against N. lugens nymphs than conventional insecticides5. Therefore, buprofezin was thought to be a unique insecticide for controlling the planthopper5,8,9.
Sublethal effects are defined as physiological and or behavioural effects on individuals that survived from exposure to a pesticide at sublethal concentration10. The insect pests are exposed to sublethal concentrations of insecticides10, is a common approach in agro-ecosystems due to the fact that the pesticides degraded after initial applications in field11. Such exposure of insecticides may also impair various key biological traits of the exposed insects through sublethal effects10. For example, neurophysiology processes and biochemistry, longevity, fecundity, developmental time, the sex ratio, and immune capacity12,13,14,15, as well as behavioural changes (like feeding, learning ability, searching, mental capacity and oviposition10,16. Determining the sublethal effects on arthropods is very essential for impact analyse of pesticides17,18,19,20,21,22.
Some scientists have suggested that the sublethal concentrations of pesticides might persuade insect outbreaks in field23,24. For instance, organophosphorus, pyrethroid, and organochlorine pesticides have been revealed to cause the resurgence of pests when insecticide contaminants degraded to near a low lethal level23,24. Some reports have found that sublethal concentrations of insecticides affect growth and increased the productivity and developmental duration in insect, for S. furcifera, the population growth was inhibited by sublethal concentration of triazophos, chlorantraniliprole and imidacloprid25,26. In various investigations, increased fecundity and survival time were also observed in M. persicae after treatment with sublethal concentrations of azadirachtin, imidacloprid27.
The use of two-sex life tables is one of the most important tools for investigating sublethal effects, particularly in life cycle studies, as it can highlight population effects that may be underestimated at the individual level28,29,30. The possible sublethal effects of buprofezin on S. furcifera have not yet been reported. In our study, for data interpretation two-sex life table was used to observe the sublethal effects of buprofezin, with a particular focus on the trans-generational effects on S. furcifera.
Results
Buprofezin toxicity against S. furcifera
The toxicity level of buprofezin to 3rd instar S. furcifera is presented in Table 1; the estimated the LC10, LC30, LC50, and LC100 values are 0.173 mg a.i. L−1 (95% CI from 0.0132 to 0.483 mg a.i. L−1), 0.847 mg a.i. L−1 (95% CI from 0.217 to 1.526 mg a.i. L−1), 2.541 mg a.i. L−1 (95% CI from 0.731 to 6.647 mg a.i. L−1), and 332.460 mg a.i. L−1 (95% CI from 81.71 to 12072.15 mg a.i. L−1), respectively. Finally, these concentrations LC10 and LC30 were used as the sublethal concentrations for further experiments. In order to evaluate the sublethal effects, 3rd instar S. furcifera nymph were exposed to these sublethal concentrations of buprofezin, the 120-h mortality of nymphs were 6.616 ± 0.925, 12.21 ± 0.845 and 30.11 ± 1.352% for control, LC10 and LC30 of buprofezin, respectively.
Sublethal effects of buprofezin on parental (F0) S. furcifera
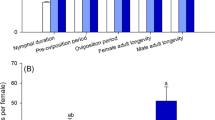
Third instars S. furcifera of the F0 generation were treated with two sublethal concentrations (LC10 and LC30) of buprofezin. Fecundity and longevity of female were significantly decreased by buprofezin (Fig. 1A,C), while hatchability (Fig. 1B) and copulation (data not shown) were not affected.
Trans-generational effects of buprofezin on individuals (F1) of S. furcifera
The developmental time (Table 2), fecundity, hatchability, emergence and longevity of S. furcifera F1 offspring produced by F0 parents treated with LC10 and LC30 buprofezin are shown in Fig. 1A–D. We found significant differences in fecundity, hatchability, longevity of female and emergence, for LC30 compared to LC10 and the control, whereas no significant effects on copulation were reported in the F1 offspring. Additionally, the developmental period of all instars (1st to 5th instar) of F1 individuals was significantly affected positively or negatively except for the 3rd instar at LC30 and LC10 compared to the control (Table 2). The overall nymph developmental period significantly affected in the treatments compared to the control. However, the pre-adult period, APOP (adult pre-oviposition period) and TPOP (total pre-oviposition period) in the offspring of the F0 parents was significantly increased compared to the control. The total male and female longevity in F1 offspring significantly decreased at LC30.
The trans-generational effects of the sublethal concentrations (LC10 and LC30) of buprofezin on population dynamics (Table 3) were calculated with bootstrap procedure based on a life cycle. The finite rate of increase (λ), intrinsic rate of increase (r i ), net reproductive rate (R 0) and gross reproduction rate (GRR) of F1 individuals significantly decreased in the LC30 treatment, while the net reproductive rate and gross reproduction rate were not affected by the LC10. In contrast to LC30, LC10 caused a significant increase in the mean generation time (T) of the exposed offspring.
The age-stage survival rate (s xj ) indicated the probability that a newly laid egg will survive to age x and stage j (Fig. 2). It represents variation in the developmental rate occurring among individuals, and the coinciding projected curves clearly showed the overlapping between the different stages for buprofezin (LC30) and control. The plotted peaks for each developmental stage under LC10 and control showed almost the same pattern, with the exceptions that the curves for male adults ended earlier than those for females, while the peaks of the plotted curves at LC30 were not high as for the control. The peaks also showed that male and female exhibited the same survival rate for LC10 and the control, while they exhibited a shortened survival period at LC30. The curves for the female and male adults showed that sexes emerged after 16 days in the buprofezin treatment but after 15 days in the control. Likewise, the age-specific survival rate (l x ) for the control and treatments is plotted in Fig. 3. It indicates that a similar l x pattern was observed for LC10 and the control, while the age-specific survival rate (l x ) declined more in the LC30-treated populations’ offspring at day 8 compared to the control. These results showed that the probability of new-born nymphs surviving to the adult stage was 0.80 at LC30 and 0.83 at LC10, whereas it was 0.87 in the control.
The age-stage reproductive values (v xj ) of the buprofezin treatments showed (Fig. 4) that the v xj of the LC30 buprofezin treatment was lower compared to LC10 and the control individuals in the 5th instar stage. At this stage (5th instar), the peak is sharper in cases of LC10 and the control than for LC30. However, in case of emerging females, the plotted curve for LC30 rose slightly higher and declined more rapidly compared to the control and LC10 but increased in an additional curve at later ages in LC10 treated individuals; the maximum v xj value 78 d−1 on 23rd day at LC10, whereas it was 60 d−1 on the 22nd day in the LC30 group and 60 d−1 on the 19th day in the control.
Discussion
This is the first study to evaluate the effects of sublethal exposure to buprofezin on the life cycle of S. furcifera. Buprofezin is an insect growth regulator and is highly effective against several sucking pests31,32.
Insecticides are usually distributed unequally as well as subjected to degradation after application in the field so the probability of targeted and non-targeted pests to low concentration of insecticides happens very often11,33,34, However, studies on the effects of sublethal concentrations of insecticides on target pests are of great importance to increase their rational use35,36,37,38. Therefore, buprofezin may also cause a wide range of sublethal effects to pests such as in S. furcifera. A thorough investigation of these possible effects would help to improve IPM in rice crops.
Lethal and sublethal effects of buprofezin have been studied in some arthropods, e.g Encarsia inaron (Hymenoptera: Aphelinidae)31, Eretmocerus mundus Mercet (Hymenoptera: Aphelinidae)32, Bemisia tabaci (Hemiptera: Aleyrodidae)32. Sublethal effects such as decreased fecundity, hatchability, longevity, and copulation could result in stimulatory effects on pest population growth10. In the present study, we investigated the sublethal effects of buprofezin for two consecutive generations and found a significant decrease in fecundity, hatchability, emergence and longevity of S. furcifera females in the F1 generation at LC30 but not copulation, whereas in the F0 generation, a significant difference was found in fecundity and female longevity at LC30. Our results are in line to Zhou et al.25 reported that sublethal concentrations of imidacloprid showed significant effects on the fecundity of S. furcifera.
Moreover, trans-generational effects on the F1 individuals of S. furcifera were also found. We found that the exposure to the LC10 and LC30 of buprofezin in the parent (F0) population significantly affected the F1 offspring growth rate, especially by increasing the duration of the pre-adult stage, TPOP and APOP and decreased the longevity, total longevity of males and females and fecundity at LC30 and vice versa at LC10. These effects are related to reductions in the intrinsic rate of increase (ri), finite rate of increase (λ), net reproductive rate (R 0), gross reproduction rate (GRR) and survival rate. Such effects on offspring growth have also been reported in the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae) and the small brown planthopper, Laodelphax striatellus (Homoptera: Delphacidae) through treatment with sublethal concentrations of sulfoxaflor and thiamethoxam39,40. Other insecticides, such as imidacloprid, chlorantraniliprole and triazophos have also lead to significant effects on the life cycle of the white-backed planthopper23,26,41. Although imidacloprid significantly affects the GRR in various cases, the generation time (T) was not affected in B. tabaci (Hemiptera: Aleyrodidae)40. Lashkari et al.42 observed that sublethal concentrations have an effect on the mean generation time in B. brassicae (Hemiptera: Aphididae) when treated with imidacloprid. Shorabi et al.32 also pointed out that biological character of B. tabaci have no significant effect at low concentration of imidacloprid and buprofezin. These reports revealed that insecticide concentrations effects life history and physiological state of the species21,35,36,37,43,44.
The analysis of the plotted curves of the age-specific survival rate (lx) showed more decline at LC30 compared to LC10 and the control, indicating that the LC30 of buprofezin is more effective. The age-stage reproductive value (v xj ) indicated that buprofezin at sublethal concentrations may significantly affect the duration of immature stage but have no significant effect on adult nymphs. This result could be interrelated to an invisible effect due to inhibition when treated with a sublethal concentration of buprofezin. Due to different physical and chemical processes, the pre-adult period, APOP, total pre-oviposition period (TPOP) and mean generation time (T) are longer when treated with buprofezin. Similar results have also been presented in various other reports32,34,37,38. Qu et al. found that sublethal concentrations of imidacloprid (0.20 mg a.i. L−1) can decrease the population growth of treated Aphis glycine (Hemiptera: Aphididae) compared to the control through reductions in the reproductive and survival rates36,45. In this study, we considered that hormesis is not the only important factor in terms of effects of buprofezin on S. furcifera. But all the biological processes may operate simultaneously after the exposure of arthropods to pesticides due to which they may develop, ultimately, hormesis and/or resistance responses against such chemicals33,34,46. Therefore, further research using numerous lethal and sublethal concentrations is needed to provide a detail evaluation report of nymphal responses to buprofezin in S. furcifera. In summary, this study found that sublethal concentrations of buprofezin affect the longevity and egg laying of parent (F0) individuals of S. furcifera and also cause some variation in the biological traits of the F1 generation of S. furcifera. However, non-stimulatory effects on copulation and hatchability were found in the F0 generation.
Overall, these observations of the present study under laboratory conditions emphasized the significance of assessing sublethal effects of the buprofezin on WBPH and to also determine how these effects may be interpreted to population dynamics in the field. Our research suggested the need to investigate further possible effects of buprofezin on WBPH in the aim to improve optimized IPM packages including this insecticide.
Materials and Methods
Insects and Insecticide
The WBPH (S. furcifera) population was initially sampled from rice fields in Xiaogan District Hubei Province, in 2014 and has been maintained on rice seedlings for 3 years at a temperature of 27 ± 1 °C, relative humidity (RH) of 70–80% and light/dark cycle of 16:8 h in a growth chamber in a laboratory at Huazhong Agricultural University without exposure to any insecticide. All experiments were performed in the above-mentioned growth chamber. Buprofezin (97.4%, technical grade) was purchased from Jiangsu Anpon Electrochemical Co., Ltd. China.
Bioassay
A bioassay test was carried out using the rice seedling dipping method with slight modifications46,47. Briefly, a stock solution of buprofezin (97.4%) was prepared in acetone and then serially diluted with water containing 0.1% Triton X-100 for five dilutions (16, 8, 4, 2, and 1 mg a.i. L−1). Rice plants were collected at the seedling stage and washed thoroughly with water, and then air dried at room temperature to eliminate excess water. Fifteen rice seedlings were grouped together and dipped into the serially diluted buprofezin solution treatments for 30 seconds25. After the treated rice stems were air dried at room temperature, the rice roots were wrapped with moistened cotton. Then, these wrapped stems were placed in 500 ml plastic cup. Forty-five 3rd instar nymphs were introduced into the plastic cups using a vacuum device. Distilled water containing 0.1% Triton-X was used as a control. Both control and treatments were replicated three times for each serial dilution. The treated and control insects were kept in a plant growth chamber maintained at a temperature of 27 ± 1 °C, RH of 70 ± 1% and a light/dark cycle (L:D) of 16:8 h. Mortality was recorded after 120 h. Individual nymphs were considered dead if they did not show movement after being slightly pushed with a soft brush.
Evaluation of sublethal effects of buprofezin on life history traits of F0 S. furcifera
The sublethal effects of buprofezin on the life cycle parameters of S. furcifera were followed by Liu and Han method with slight modifications47. Approximately 800–1000 adult WBPH were transferred to a clean cage with fresh and healthy rice to lay their eggs upon. After 24 h, the rice seedlings were removed and placed in another cage; these rice seedlings were retained for a number of days for nymphs to hatch out of the eggs and until these nymphs had developed into the 3rd instar. These 3rd instar nymphs were used as F0 generation individuals. Approximately 200–300 3rd instars were transferred to and reared separately in a glass tube containing rice seedlings dipped in a sublethal concentration (LC10 and LC30) of buprofezin. The live pests were collected after 5 days. The control pests were fed rice seedlings not treated insecticide. Each surviving pest was then transferred to a separate clean tube with a healthy rice stem, and each glass tube was numbered. As the nymphs became adult males and females, they were paired (40–60 per treatment) at once in a glass tube containing a single fresh rice seedling and kept under controlled temperature (27 ± 1 °C), RH (70 ± 1%) and light/dark cycle (16:8 h). The rice seedlings in each tube were changed every day during experiment. The fecundity and longevity of the couple was recorded, and measurements continued until the death of the couple. The experiment was repeated three times.
Effects of buprofezin on life cycle traits of F1 generation individuals of S. furcifera
Approximately 200 1st instar nymphs were selected randomly from each F0 couple as the founders of an experimental population (F1) and kept in separate tubes under the control conditions as discussed previously. These offspring were fed rice stems, and the stage and condition of the pests were observed daily. This method was followed for both the buprofezin treated and control groups. When these nymphs become adults, they were paired as described above. The population characteristics, including developmental time, longevity, fecundity and hatchability were checked daily until the couple died. The newly born nymphs were counted and discarded. Then, the rice stems were thoroughly checked using a microscope, and the number of unhatched eggs was recorded.
During this study, the following observations were noted; the developmental rate of each instar, the emergence of adults, the duration of the adult stage, mating, fecundity and hatchability. This whole experiment was repeated 3 times.
Statistical analysis
The Probit-MSChart48 program was used for probit analysis of the concentration-response data. The raw data of the life table of each S. furcifera individual was analysed using the age-stage, two-sex life table procedure28,29. The basic life-table parameters, such as age-stage survival rate (s xj ), age-specific survival rate (l x ), reproductive value (v xj ), intrinsic rate of increase (r), finite rate of increase (λ), net reproductive rate (R 0) and mean generation time (T), were analysed using the computer program TWOSEX-MS Chart49. The variances and standard errors of the population growth parameters were calculated using the bootstrap technique included in TWOSEX-MS Chart with 100,000 random resampling. Developmental growth, adult longevity, total preoviposition period (TPOP), adult pre-oviposition period (APOP), fecundity and population parameters (r, λ, R 0, and T) were compared using the paired bootstrap test based on the confidence interval of the differences. Therefore, the finite rate of increase (λ) and intrinsic rate of increase (r) are the most crucial parameters for determining the potential of population growth, and these have been used to represent the fitness of populations50,51,52. Survival rate and reproductive value curves were plotted using SigmaPlot 12.0 (Systat Software Inc., San Jose, CA).
References
International Rice Research Institute. Bringing hope, improving lives: Strategic Plan 2007–2015, http://www.worldcat.org/title/bringing-hope-improving-lives-strategic-plan-2007-2015/oclc/793814673 (2006).
Su, J. et al. Status of insecticide resistance of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Florida Entomologist 96, 948–956 (2013).
Zhou, G. H., Xu, D. L., Xu, D. G. & Zhang, M. X. Southern rice black-streaked dwarf virus: a white-backed planthopper-transmitted Fijivirus threatening rice production. Asia Front. Microbiol. 4, 270–279 (2013).
Wang, Q. et al. The complete genome sequence of two isolates of southern rice black‐streaked dwarf virus, a new member of the genus fijivirus. J. Phytopathol. 158, 733–737 (2010).
Wang et al. Buprofezin susceptibility survey, resistance selection and preliminary determination of the resistance mechanism in Nilaparvata lugens (Homoptera: Delphacidae). Pest Manag. Sci. 64, 1050–1056 (2008).
Prabhaker, N. & Toscano, N. C. Toxicity of the insect growth regulators, buprofezin and pyriproxyfen, to the glassy winged sharpshooter, Homalodisca coagulata say (Homoptera: Cicadellidae). Crop Prot. 26, 495–502 (2007).
Nagata, T. Timing of buprofezin application for control of the brown planthopper, Nilaparvata lugens (Stal) (Homoptera; Delphacidae). Appl. Entomol. Zool. 14, 357–368 (1986).
Chang, X. et al. The Toxicity and Detoxifying Mechanism of Cycloxaprid and Buprofezin in Controlling Sogatella furcifera (Homoptera: Delphacidae). J. Insect Sci. 15, 2–5 (2015).
Ishaaya, I., Mendelson, Z. & Melamed-Madjar, V. Effect of buprofezin on embryogenesis and progeny formation of sweet potato whitefly (Homoptera: Aleyrodidae). J Econ Entomol. 81, 781–784 (1988).
Desneux, N., Decourtye, A. & Delpuech, J. M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52, 81–106 (2007).
Desneux, N. et al. Diaeretiella rapae limits Myzus persicae populations after applications of deltamethrin in oilseed rape. J. Econ. Entomol. 98, 9–17 (2005).
Desneux, N., Ramirez-Romero, R. & Kaiser, L. Multi step bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ. Toxicol. Chem. 25, 2675–2682 (2006).
Desneux, N., Rafalimanana, H. & Kaiser, L. Dose-response relationship in lethal and behavioural effects of different insecticides on the parasitic wasp Aphidius ervi. Chemosphere. 54, 619–627 (2004).
Ceuppens, B. et al. Effects of dietary lambda-cyhalothrin exposure on bumblebee survival, reproduction, and foraging behavior in laboratory and greenhouse. J. Pest Sci. 88, 777–783 (2015).
He, Y. et al. Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly. Bemisia tabaci. Int. J. Biol. Sci. 9, 246–255 (2013).
Samuelson, E. E., Chen-Wishart, Z. P., Gill, R. J. & Leadbeater, E. Effect of acute pesticide exposure on bee spatial working memory using an analogue of the radial-arm maze. Sci. Rep. 6, 38957, https://doi.org/10.1038/srep38957 (2016).
Chen, Z. et al. Lethal and social-mediated effects of ten insecticides on the subterranean termite Reticulitermes speratus. J. Pest Sci. 88, 741–751 (2015).
Yao, F. L. et al. Lethal and sublethal effects of thiamethoxam on the whitefly predator Serangium japonicum (Coleoptera: Coccinellidae) through different exposure routes. Chemosphere 128, 49–55 (2015).
Guo, L. et al. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot. 48, 29–34 (2013).
Shi, X. et al. Toxicities and sublethal effects of seven neonicotinoid insecticides on survival, growth and reproduction of imidacloprid-resistant cotton aphid, Aphis gossypii. Pest Manag. Sci. 67, 1528–1533 (2011).
Stark, J. D. & Banks, J. E. Population-level effects of pesticides and other toxicants on arthopods. Annu. Rev. Entomol. 48, 505–519 (2003).
Huang, Z., Wang, Y. & Zhang, Y. Lethal and sublethal effects of cantharidin on development and reproduction of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 108, 1054–1064 (2015).
Bartlett, B. R. Outbreaks of two spotted spider mites and cotton aphids following pesticide treatment. I. Pest stimulation vs. natural enemy destruction. J. Econ. Entomol. 61, 297–303 (1968).
Cordeiro, E. M. G., de Moura, I. L. T., Fadini, M. A. M. & Guedes, R. N. C. Beyond selectivity: Are behavioral avoidance and hormesis likely causes of pyrethroid-induced outbreaks of the southern red mite Oligonychus ilicis? Chemosphere. 93, 1111–1116 (2013).
Zhou, C. et al. Sublethal effects of imidacloprid on the development, reproduction, and susceptibility of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). J. Asia-Pacific. Entomol. 20, 996–1000 (2017).
Liu, L. L., Dai, R. H., Yang, H. & Jin, D. C. Sublethal effects of triazophos on the life table parameters of Sogatella furcifera (Hemiptera: Delphacidae). Florida Entomologist 99, 292–296 (2016).
Cutler, C. G., Ramanaidu, K., Astatkie, T. & Isman, M. B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag. Sci. 65, 205–209 (2009).
Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 17, 26–34 (1988).
Chi, H. & Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 24, 225–240 (1985).
Akca, I., Ayvaz, T., Smith, C. L. & Chi, H. Demography and population projection of Aphis fabae (Hemiptera: Aphididae): with additional comments on life table research criteria. J. Econ. Entomol. 108, 1466–1478 (2015).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. S. Lethal and sublethal effects of buprofezin and imidacloprid on the whitefly parasitoid Encarsia inaron (Hymenoptera: Aphelinidae). Crop Prot. 32, 83–89 (2012).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. Lethal and sublethal effects of buprofezin and imidacloprid on Bemisia tabaci (Hemiptera: Aleyrodidae). Crop Prot. 30, 1190–1195 (2011).
Cutler, G. C. Insect, Insecticide and Hormesis: Evidence and Considerations for study. Dose-Response. 11, 154–177 (2013).
Guedes, R., Smagghe, G., Stark, J. & Desneux, N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annu. Rev. Entomol. 61, 43–62 (2016).
Xiao, D., Yang, T., Desneux, N., Han, P. & Gao, X. Assessment of sublethal and transgenerational effects of pirimicarb on the wheat aphids Rhopalosiphum padi and Sitobion avenae. PloS One 10, e0128936 (2015).
Liang, P., Tian, Y. A., Biondi, A., Desneux, N. & Gao, X. W. Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 21, 1889–1898 (2012).
Chen, X. et al. Sublethal and transgenerational effects of sulfoxaflor on the biological traits of the cotton aphid, Aphis gossypii Glover (Hemiptera: Aphididae). Ecotoxicology 25, 1841–1848 (2016).
Sohrabi, F., Shishehbor, P., Saber, M. & Mosaddegh, M. S. Lethal and sublethal effects of imidacloprid and buprofezin on the sweetpotato whitefly parasitoid Eretmocerus mundus (Hymenoptera: Aphelinidae). Crop Prot. 45, 98–103 (2013).
Xu, L., Zhao, C.-Q., Zhang, Y.-N., Liu, Y. & Gu, Z.-Y. Lethal and sublethal effects of sulfoxaflor on the small brown planthopper Laodelphax striatellus. J. Asia-Pac. Entomol. 19, 683–689 (2016).
Esmaeily, S., Samih, M. A., Zarabi, M. & Jafarbeigi, F. Sublethal effects of some synthetic and botanical insecticides on Bemisia tabaci (Hemiptera: Aleyrodidae). J. Plant Prot. Res. 54, 171–178 (2014).
Hong, Y., Zhao, W. & Dao-Chao, J. Sublethal effects of chlorantraniliprole on the experimental populations of the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Acta Entomologica Sinica 55, 1161–1167 (2012).
Lashkari, M. R., Sahragard, A. & Ghadamyari, M. Sublethal effects of imidacloprid and pymetrozine on population growth parameters of cabbage aphid, Brevicoryne brassicae on rapeseed, Brassica napus L. Insect Sci. 14, 207–212 (2007).
Haddi, K. et al. Sexual Success after Stress? Imidacloprid-Induced Hormesis in Males of the Neotropical Stink Bug Euschistus heros. PloS One 11, e0156616 (2016).
Qu, Y. et al. Sublethal and hormesis effects of imidacloprid on the soybean aphid Aphis glycines. Ecotoxicology 24, 479–487 (2015).
Kendig, E. L., Le, H. H. & Belcher, S. M. Defining hormesis: evaluation of a complex concentration response phenomenon. Int. J. Toxicol. 29, 235–246 (2010).
Wang, Y. et al. Susceptibility to neonicotinoids and risk of resistance development in the brown planthopper, Nilaparvata lugens (Stål)(Homoptera: Delphacidae). Pest Manag. Sci. 64, 1278–1284 (2008).
Liu, Z. W. & Han, Z. J. Fitness costs of laboratory-selected imidacloprid resistance in the brown planthopper, Nilaparvata lugens (Stål). Pest Manag. Sci. 62, 279–282 (2006).
Chi, H. P-MS C: a Computer Program for Probit Analysis, National Chung Hsing University, Taichung, Taiwan, http://140.120.197.173/Ecology/prod02.htm (2015).
Chi, H. TIMING-MSChart: a computer program for the population projection based on age-stage, two-sex life table. National Chung Hsing University, Taichung, Taiwan, http://140.120.197.173/Ecology/prod02.htm (2016).
Reddy, G. V. P. & Chi, H. Demographic comparison of sweetpotato weevil reared on a major host, Ipomoea batatas, and an alternative host, I. triloba. Sci. Rep. 5, 11871 (2015).
Akkopru, E. P., Atlıhan, R., Okut, H. & Chi, H. Demographic assessment of plant cultivar resistance to insect pests: a case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 11, 378–387 (2015).
Zheng, X., Tao, L. Y., Chi, H., Wan, H. F. & Chu, D. Adaptability of small brown planthopper to four rice cultivars using life table and population projection method. Sci. Rep 7, 42399 (2016).
Acknowledgements
This work was supported by a grant from the Special Fund for Agro-Scientific Research in the Public Interest of China (201503107), and the National Key Research and Development Program of China (2016YFD0200500).
Author information
Authors and Affiliations
Contributions
E.A., X.L., X.Z., A.S., W.H. and J.H.L. designed the experiment. E.A. performed experiment. E.A., K.K.M., Y.P., collected the pests. E.A. and X.L. analysed the data. E.A., M.S. and W.H. wrote the manuscript. E.A., L.X., X.Z., H.W. and J.H.L. read, corrected and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, E., Liao, X., Yang, P. et al. Sublethal effects of buprofezin on development and reproduction in the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Sci Rep 7, 16913 (2017). https://doi.org/10.1038/s41598-017-17190-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17190-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.