Abstract
Children of severe hand, foot, and mouth disease (HFMD) often present with same clinical features as those of mild HFMD during the early stage, yet later deteriorate rapidly with a fulminant disease course. Our goal was to: (1) develop a machine learning system to automatically identify cases with high risk of severe HFMD at the time of admission; (2) compare the effectiveness of the new system with the existing risk scoring system. Data on 2,532 HFMD children admitted between March 2012 and July 2015, were collected retrospectively from a medical center in China. By applying a holdout strategy and a 10-fold cross validation method, we developed four models with the random forest algorithm using different variable sets. The prediction system HFMD-RF based on the model of 16 variables from both the structured and unstructured data, achieved 0.824 sensitivity, 0.931 specificity, 0.916 accuracy, and 0.916 area under the curve in the independent test set. Most remarkably, HFMD-RF offers significant gains with respect to the commonly used pediatric critical illness score in clinical practice. As all the selected risk factors can be easily obtained, HFMD-RF might prove to be useful for reductions in mortality and complications of severe HFMD.
Similar content being viewed by others
Introduction
Hand, foot, and mouth disease (HFMD) is a common childhood illness caused by a group of enteroviruses such as enterovirus 71 (EV71) and coxsackievirus A16 (CA16)1,2. In recent years, outbreaks of HFMD have increased, and more and more severe cases and fatalities have appeared throughout most of the Asia-Pacific Region countries3. For example, data reported by the Western Pacific Regional Office of the World Health Organization showed that in China, a total of 2,468,174 cases of HFMD including 220 deaths were reported in 20164, representing a substantial public health threat.
HFMD is characterized by fever, general malaise, sore throat and vesicular eruptions on the hands, feet, tongue and oral mucosa5. Severe cases can also involve serious neurological, respiratory or circulatory complications, such as meningitis, encephalitis, cardiorespiratory failure, acute flaccid paralysis or even death6,7,8. Although the prognoses in most cases are good, children with severe HFMD usually have no typical clinical manifestations in the early stage and rapidly progress to severe or fatal disease in a short term. Consequently, the identification of high-risk patients who are going to develop a severe form of HFMD is a key goal in the management of this disease.
A variety of predictive factors have been utilized by clinicians and researchers to identify cases whose conditions are likely to deteriorate. A meta-analysis of 19 observational studies has identified variables that conferred risk for rapid progression to severe disease, including fever duration, peak temperature, age, vomiting, neutrophil count, hyperglycemia and EV71 infection9. Other groups have sought to correlate clinically useful biomarkers such as cytokines with disease severity10,11,12. Several single nucleotide polymorphisms might correlate with both susceptibility and progression of severe disease13,14. Moreover, MRI-related variables have also been involved in the identification of severe HFMD children with state-of-the-art machine learning algorithms15.
The most commonly used scoring system for disease severity in pediatric intensive care units is the pediatric critical illness score (PCIS), which was drawn up by the emergency group of Chinese pediatric society, Chinese medical association in 1995. One of the greatest challenges with PCIS application in HFMD lies in that it is not designed specifically for HFMD and thus cannot adequately evaluate the severity of HFMD16. To our knowledge, no simple, real-time and automatic prediction tools for severe HFMD identification have emerged as clinically practicable since 1995. Therefore, the interest of this study consists in staging HFMD early in hospitalization to provide effective and timely medical intervention.
The widespread implementation of Electronic Medical Record (EMR) brings the promise of abundant data resources for research purposes such as better prediction of clinical deterioration17. A number of machine learning algorithms and models have shown their advantages in improving real-time identification of sepsis shock, heart failure and other diseases18,19,20,21,22. In this paper we show whether machine learning methods and clinical data obtained from a relatively large population of HFMD patients can be efficiently applied to address the severe HFMD identification problems. We identified relevant predictive variables in severe HFMD and compared models of different complexity to provide to physicians a simple, robust and automatic decision-making support system. We also provided a comparison between the system and PCIS currently used in clinic.
Results
Basic Characteristics
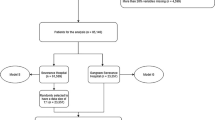
Table 1 shows the baseline characteristics of all subjects. A total of 2,532 HFMD hospitalizations were included in this study (mean age, 24.11 [standard derivation 17.14] months; 1,715 males [67.73%]). Of these hospitalizations, 365 (14.42%) progressed to the severe stage with complications including encephalitis (n = 237), meningitis (n = 36), acute flaccid paralysis (n = 19), cardiorespiratory failure (n = 258), or death (n = 5). For the population of severe cases, the median time from illness onset to diagnosis was 2 days (interquartile range 1–3), and 1 day (interquartile range 0–2) for diagnosis to severity. Distributions of the onset-to-diagnosis and diagnosis-to-severity time interval showed that 79.18% (289/365) of cases sought treatment in hospital within 3 days of illness onset and 74.52% (272/365) of cases developed severe complications after 1 day of admission (Supplementary Figure S1).
All the hospitalizations were randomly sorted 7:3 into a training set with 1,899 patients and a test set with 633 patients. There is no significant difference between the training and test sets in terms of baseline variables (Table 1).
Feature Selection
After removal of variables with missing rate >20%, a total of 153 variables consisting of laboratory results, vital signs, symptoms and demographics were extracted from the structured and unstructured data of EMR (Fig. 1). Then, we selected the predictors related to severe HFMD according to literature review, expert clinician opinion and univariate analysis. Eleven variables were chosen from the structured laboratory results: blood glucose, platelet, percentage of lymphocytes, lactate dehydrogenase, alkaline phosphatase, creatine kinase, creatine kinase-MB, creatinine, uric acid, blood chlorine, alanine aminotransferase, and five from the unstructured free text: age, respiratory rate, peak temperature, fever duration, vomiting.
Predictive efficiencies of the selected variables were supported by the Chi-square test or the two sample t-test to clarify the differences between the severe and mild HFMD groups. As expected, the null hypothesis was rejected for all the variables (Table 2), which showed that their statistical distributions should be considered to be different and the differences to be significant. These results confirmed the importance of variables from both the structured and unstructured data in identifying severe HFMD.
Prediction Results Using Machine Learning Algorithms
Figure 2 showed the receiver operating characteristic (ROC) curves for the four RF models basing on different variable sets. With all the 153 variables, the first model (Model 1) identified severe HFMD with a reasonable area under the ROC curve (AUC) of 0.862. Using the structured variables alone, the second model (Model 2) had comparable performance to that of Model 1, with an AUC of 0.855. Yet using the unstructured variables alone, the performance of the third model (Model 3) declined seriously to an AUC of 0.710. Interestingly, if both kinds of variables were used, the fourth model (Model 4) had even greater predictive ability than Model 1, yielding an AUC of 0.916. Overall, the performance of only one kind of variables lagged behind their combined performance in a RF model, demonstrating the importance of defining complex predictors and of combining their efficiencies in nonlinear models. This conclusion was supported by the similar changing patterns of AUC when applying other machine learning algorithms such as support vector machine, XGBoost, logistic regression and multi-layer perceptrons, although these algorithms performed a little worse than or equally to RF (Supplementary Figure S2).
We computed the importance score for each variable to identify the important features used by the RF classifier. The larger the score is, the more important the variable is. As shown in Fig. 3, the most important predictive variable was lactate dehydrogenase (LDH), followed by creatine kinase-MB (CK-MB), blood glucose and creatine kinase (CK). The symptom vomiting was the fifth important factor. Only a very small importance was found in respiratory rate, alanine aminotransferase, percentage of lymphocytes and platelet.
Overall Comparison of Predictive systems
Owing to its superb performance, we built a prediction system based on Model 4 of RF for predicting the progression of HFMD and named it HFMD-RF. The HFMD-RF system was then compared with the clinically used scoring system, PCIS. ROC curves for these two systems are plotted in Fig. 4. Their performance was presented in Table 3. In comparison to PCIS in the training and test sets, our system’s AUC, sensitivity, specificity and accuracy were significantly improved. The p-values comparing the two systems are less than 0.001 except for the sensitivity in the test set (p = 0.017). Our system’s sensitivity and specificity numerically improved by 21.3% and 25.2% in the test set. The accuracy and AUC of our model were both 0.916, which increased by 24.7% and 18.8% in comparison with PCIS.
Discussion
Due to the rapid progression of symptoms and the ambiguous boundary between disease stages, patients with severe HFMD are at high risk of insufficient quality of care and poor post discharge outcomes8. Rapid identification of patients with severe HFMD is thus urgently required to prevent deterioration and to reduce acute mortality. In this study, we developed and validated a prediction system for progression to severe HFMD through automated analysis of EMR data. Our major findings are as follows: (1) The prediction system can provide accurate identification of severe HFMD with an AUC of 0.916, basing on 16 clinical variables collected at the time of admission. (2) Results have shown that LDH, CK-MB, blood glucose, CK and vomiting are the top five indicators associated with the risk of severe HFMD. (3) Our system achieved significantly higher performance than application of the existing PCIS in clinic.
Special expertise and resource may be required to implement a sophisticated machine learning system in an EMR. As a result, the tradeoff between cost of implementation and benefit of performance improvement with sophisticated approaches should be considered. Through a process using literature review, expert clinician opinion, univariate analysis and machine learning, we built and compared four RF models based on different variable sets. Model 1 of 153 variables obtained a moderate performance in terms of AUC, a measurement of global classification. Model 2 was superior to Model 1 because it got a comparable AUC using only 11 structured variables and was relatively easier to implement. Model 3 and 4 are likely more difficult to implement because of their dependence on processing of unstructured data. However, this cost may be worth the improved performance in Model 4, which obtained the best AUC by integrating five unstructured and eleven structured variables. Combining the analysis of free-text notes and structured laboratory results appears to have the best predictive performance.
A similar machine learning model has been built recently for risk prediction of severe HFMD and achieved an AUC of up to 0.985 with several clinical and MRI-related features15, representing a great advance. However, MRI examination requires special equipment and is time- and cost-consuming, making this model difficult in general medical practice or for every patient. Jun Qiu et al. developed another model with good discrimination (AUC > 0.9) using only four laboratory parameters23. Whereas this model has a different purpose and can only discriminate children with high mortality risk from severe HFMD cases. Therefore, the present identification system HFMD-RF was developed to provide accurate risk stratification for mild HFMD patients in a timely and effective manner with easily-obtained variables.
Comparing HFMD-RF with the existing scoring system PCIS, HFMD-RF demonstrated superior performance in both the training and test sets in terms of sensitivity, specificity, accuracy and AUC. These results reveal that HFMD-RF may be more clinically practicable for the identification of severe HFMD patients than PCIS. One reason for this finding may lie in that we used 16 variables selected specifically for HFMD while the 10 variables in PCIS (see Methods) are aimed at all the serious diseases in pediatric intensive care units. Besides, unlike PCIS which combines sub-score in each variable linearly to create an overall score24,25, we used RF so that distinct variables can be integrated in nonlinear models for optimal prediction26.
Our research indicates that myocardial enzymes LDH, CK-MB, CK are very important risk factors for severe HFMD, as have been found in previous studies27,28,29. LDH is a cytoplasmic enzyme and its release into plasma reflects the degree of tissue damage. CK-MB and CK, which are mostly found in myocardial cells, are well-known and sensitive indicators of myocardial injury. Compared with the mild HFMD group, the severe HFMD group had significantly higher LDH, CK-MB, CK levels, indicating the degree of myocardial injury differs in children with HFMD. This suggests that LDH, CK-MB and CK may be valuable diagnostic markers in predicting the disease severity in HFMD children before they develop pulmonary oedema, pulmonary haemorrhage or heart failure.
The selected attributes in HFMD-RF can also be used to provide simple biomedical discriminatory rules for severe HFMD identification. We provide means or medians for the mild and severe HFMD groups, which can be of help in physicians’ decision-making process. Most of the variables used in our system have been reported previously, such as age, peak temperature, fever duration, vomiting, blood glucose and so on1,9,30,31. Besides, our results also indicated the prediction significance of other variables including alkaline phosphatase, creatinine and uric acid that are mainly related with the status of the liver and kidney and are not currently used as predictive factors in this disease. These newly found predictors might be useful to understand the potential cause of severe HFMD from a medical point of view.
It is crucially important to note that different combination lists of variables might exist with similar predictive performance due to the uncertainty space of the solutions in decision-making problems32. Besides superior performance, the variable list used in our model has a great advantage in that each variable was present in the EMR for clinical care. No additional reporting structure or extra clinical assessments were required.
Given these benefits, we plan to deploy the HFMD-RF system at our center to facilitate interventions that targeted hospitalized HFMD children. To implement this, EMR data will be exported to a secure server on which the system will run; the identification result of the system will be put back into the EMR for care delivery. In this way, our model can be replicated easily in other hospitals without considering EMR vendor.
Some important limitations of the HFMD-RF system should be noted. Firstly, as the system was developed in a retrospective manner that could introduce bias, a prospective validation is needed to demonstrate its predictive capability. Furthermore, we employed only one single center’s data for system development and evaluation, additional validation with local data should be carried out before its generalization to other hospitals. Due to these limitations, it would be useful to expand the model validation in prospective and multi-center clinical samples. In addition, another limitation of our study is a lack of a golden standard for severe HFMD. We, as well as other studies15,23, used the Chinese guidelines for HFMD diagnosis and treatment as the reference standard.
In conclusion, on a retrospective data set we successfully developed a RF model utilizing the EMR content to identify severe HFMD children in their mild stages. The novel model achieved higher sensitivity, specificity, accuracy and AUC than the existing scoring system PCIS.
Methods
Study population
We performed a retrospective study of hospitalizations at Guangzhou Women’s and Children’s Medical Center using data obtained from the inpatient EMR. Clinical diagnosis of HFMD was guided by the Chinese guidelines for HFMD diagnosis and treatment issued by the Ministry of Health of China (revised in 2010, http://www.moh.gov.cn/mohyzs/s3586/201004/46884.shtml). Children were clinically diagnosed as having HFMD, if they displayed maculopapular or vesicular rash on the hands, feet, oral mucosa, and/or buttock. In this study, we included 2,532 hospitalizations admitted between March 2012 and July 2015 for children <16 years suffering from newly diagnosed HFMD. Of these 2,532 children, 2,167 children were categorized as mild HFMD without any serious complications before discharge from hospital, and the other 365 children deteriorated to severe HFMD with serious complications, including encephalitis, meningitis, acute flaccid paralysis, cardiorespiratory failure or death after admission. The diagnosis of severe HFMD was reconfirmed by two senior physicians through reviewing physician progress notes.
This study was approved by the Ethics Committee and Institutional Review Board of Guangzhou Women’s and Children’s Medical Center, Guangzhou, China, and conducted in accordance with the ethical guidelines of the Declaration of Helsinki of the World Medical Association. The requirement to obtain informed consent was waived because of the retrospective nature of the study. All data were deidentified before they were provided to the investigators.
Feature extraction
For model development, we used variables from the examination done upon admission because our aim was to identify patients who might need more attention and resources at the start of their hospitalizations. All of the variables included in the investigation were collected by reviewing the patient medical records that were preserved in EMR. In the data set, structured data elements used for severe HFMD prediction were laboratory results, which include hematologic, biochemical, immunologic, humoral and microbiological findings. Potential unstructured data elements were admission notes, which include demographic characteristics (age and sex) and clinical parameters (signs and symptoms). To extract variables from unstructured clinical documentation in Chinese, we referred to Dong Xu’s work33 and used a data-driven framework which incorporated machine learning and natural language processing. Specifically, the framework combined the core lexica of medical terms (Systematized Nomenclature of Medicine-Clinical Terms in Chinese, Chinese Pharmacopoeia and Wanfang Med Online, see ref.33), an iterative bootstrapping algorithm to obtain more accurate terms and a random forest algorithm to compute correlations between terms and descriptions. Through this procedure, the input text was converted into an output record containing a clinical variable (a symptom, a vital sign, or a demographic feature, such as papule, myoclonic jerk, convulsion, vomiting, and so on), the time of the variable and an optional description. In total, 145 variables were extracted from the structured data and 45 from the unstructured text as candidate predictors.
Feature selection
First, we removed potentially difficult-to-obtain variables with missing rates more than 20%, leaving 153 features (126 from structured data and 27 from unstructured data) for analysis. Next, through literature review and consensus meeting between the authors including two senior physicians, nine variables (five from structured data and four from unstructured data) were selected according to importance reported by literatures. Although feature selection through using established clinical knowledge is a common method, a significant bias might be introduced in the selection process. Therefore, additional univariate analysis was used to select relevant variables for the prediction model. The top 10 variables selected by the data-driven univariate analysis based on Chi-square calculations were added to the feature set. After removal of duplicates, a total of 16 variables were selected, of which 11 were from structured data and 5 from unstructured data (Fig. 1).
Data preprocessing
Before applying machine learning algorithms, values that were missing or out of predefined physiological ranges were interpolated with a Nearest-Neighbor algorithm34, which impute an incomplete variable by giving the corresponding value of the closest sample within the set of fully-informed samples. This way of interpolation is able to avoid introducing additional outliers that are nonexistent in the original dataset. The standard minimum–maximum normalization to [0, 1] was then performed for the data to reduce the effect of large feature range variation. After preprocessing, the samples in the majority groups were randomly undersampled with the EasyEnsemble method35 to achieve data balance.
Model development
The random forest (RF) algorithm was adopted basing on its wide use in clinical decision systems and the excellent performance in classification tasks. A RF is a classifier that uses an ensemble of classification trees36,37,38,39. Each tree is unpruned (grown fully) and is built by using both bagging (bootstrap aggregation) and random variable selection, yielding low-bias and low-correlation trees. In contrast to the original publication36, in our models we used the Scikit-learn implementation40 to obtain the prediction by averaging the probability scores across the trees, rather than letting each tree vote for a single class. The number of input variables randomly chosen at each split was set as the square root of the number of features, and the number of trees in the forest was set as 500. Importance of each variable was evaluated based on the loss in predicting performance by its omission from the model.
Besides RF, a representative set of classification algorithms was also selected for the severe HFMD prediction task from the dataset. These algorithms were the support vector machine41, XGBoost42, logistic regression and multi-layer perceptrons43. The parameters were set to default values for the algorithms.
We developed 4 models with each algorithm for identification of patients who will progress to severe HFMD based on different numbers of variables: (1) 153 variables in the data set before feature selection; (2) 11 variables selected from structured data; (3) 5 variables from the unstructured text; (4) 16 variables from both the structured and unstructured data. All models were developed in Python using the package Scikit-learn40.
The holdout and cross validation methods were employed to reduce overfitting in the model and to derive a reliable estimate of the performance of the model. Mild and severe HFMD children were randomly split into two experimental datasets, a training set with 70% of cases (including 1,625 mild and 274 severe patients) and a test set with 30% of cases (including 542 mild and 91 severe patients). The training set was utilized for the feature extraction and feature selection processes described above and was used to generate the prediction models with a 10-fold cross validation step. The test set was employed to estimate the models’ performance. To measure the models’ predictive performance on practical, real world data, we calculated the sensitivity, specificity, accuracy and AUC in the training and test set44.
Experimental comparison
To compare the performance of the proposed model, we also evaluated PCIS, a widely used scoring system in China. Ten physiological indexes are enrolled: heart rate, spontaneous breath rate, systolic blood pressure, oxygen partial pressure under breathing room air, serum sodium, potassium, creatinine or urea nitrogen, hemoglobin, PH value of arterial blood gas, gastrointestinal system condition (stress ulcer hemorrhage and intestinal paralysis, only stress ulcer hemorrhage or other). PCIS is usually tabulated by hand by nurses and a score of 4, 6, or 10 is generated from each category and aggregated to a 40–100 total score. Patients were divided, according to PCIS scores, into noncritical group (>80), critical group (71–80) and extremely critical group (≤70). PCIS was calculated for most of the admitted HFMD children at our medical center. For a fair comparison, we extracted the PCIS values recorded right upon admission.
Statistical analysis
Continuous variables of each group are presented as mean ± standard deviation, and the categorical variables are expressed as absolute values. Chi-square test was used for analyzing categorical data and ratio values, while a two-sample t-test was used for analyzing the continuous data. The data that did not have a normal distribution are expressed as median (interquartile range) and were transformed logarithmically to assume a near-normal distribution for t-test. The accepted level of statistical significance for all analyses was p < 0.05. Statistical analyses were performed using SPSS version 17.0 (SPSS Inc, Chicago, IL).
References
Ooi, M. H., Wong, S. C., Lewthwaite, P., Cardosa, M. J. & Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol 9, 1097–1105, https://doi.org/10.1016/S1474-4422(10)70209-X (2010).
WHO. A guide to clinical management and public health response for hand, foot and mouth disease (HFMD) (2011).
Xing, W. et al. Hand, foot, and mouth disease in China, 2008-12: an epidemiological study. Lancet Infect Dis 14, 308–318, https://doi.org/10.1016/S1473-3099(13)70342-6 (2014).
WHO. Hand, Foot, and Mouth Disease Situation Update Number 505, <http://www.wpro.who.int/emerging_diseases/hfmd_biweekly_20170117.pdf (2017).
Sutton-Hayes, S., Weisse, M. E., Wilson, N. W. & Ogershok, P. R. A recurrent presentation of hand, foot, and mouth disease. Clin Pediatr (Phila) 45, 373–376 (2006).
Shah, V. A., Chong, C. Y., Chan, K. P., Ng, W. & Ling, A. E. Clinical characteristics of an outbreak of hand, foot and mouth disease in Singapore. Ann Acad Med Singapore 32, 381–387 (2003).
Shekhar, K. et al. Deaths in children during an outbreak of hand, foot and mouth disease in Peninsular Malaysia–clinical and pathological characteristics. The Medical journal of Malaysia 60, 297–304 (2005).
Chang, L. Y. et al. Clinical features and risk factors of pulmonary oedema after enterovirus-71-related hand, foot, and mouth disease. Lancet 354, 1682–1686, https://doi.org/10.1016/S0140-6736(99)04434-7 (1999).
Fang, Y. et al. Risk factors of severe hand, foot and mouth disease: a meta-analysis. Scand J Infect Dis 46, 515–522, https://doi.org/10.3109/00365548.2014.907929 (2014).
Lin, T. et al. Different proinflammatory reactions in fatal and non-fatal enterovirus 71 infections: implications for early recognition and therapy. Acta Paediatr 91, 632–635 (2002).
Wang, S. M. et al. Cerebrospinal fluid cytokines in enterovirus 71 brain stem encephalitis and echovirus meningitis infections of varying severity. Clin Microbiol Infect 13, 677–682 (2007).
Zhang, Y. et al. Comparative study of the cytokine/chemokine response in children with differing disease severity in enterovirus 71-induced hand, foot, and mouth disease. PLoS One 8, e67430 (2013).
Cai, Y. et al. Association analysis of polymorphisms in OAS1 with susceptibility and severity of hand, foot and mouth disease. Int J Immunogenet 41, 384–392 (2014).
Han, Z.-l, Li, J.-a & Chen, Z.-b Genetic polymorphism of CCL2-2510 and susceptibility to enterovirus 71 encephalitis in a Chinese population. Arch Virol 159, 2503–2507 (2014).
Zhang, B. et al. Machine Learning Algorithms for Risk Prediction of Severe Hand-Foot-Mouth Disease in Children. Sci Rep 7, 5368, https://doi.org/10.1038/s41598-017-05505-8 (2017).
Lu, X. et al. Role of Pediatric Critical Illness Score in evaluating severity and prognosis of severe hand-foot-mouth disease. Zhongguo dang dai er ke za zhi = Chinese journal of contemporary pediatrics 17, 961–964 (2015).
Das, S. et al. Meaningful use of electronic health records in otolaryngology: recommendations from the American Academy of Otolaryngology—Head and Neck Surgery Medical Informatics Committee. Otolaryngology–Head and Neck Surgery 144, 135–141 (2011).
Henry, K. E., Hager, D. N., Pronovost, P. J. & Saria, S. A targeted real-time early warning score (TREWScore) for septic shock. Sci Transl Med 7, 299ra122, https://doi.org/10.1126/scitranslmed.aab3719 (2015).
Blecker, S. et al. Comparison of Approaches for Heart Failure Case Identification From Electronic Health Record Data. JAMA Cardiol 1, 1014–1020, https://doi.org/10.1001/jamacardio.2016.3236 (2016).
Mani, S. et al. Medical decision support using machine learning for early detection of late-onset neonatal sepsis. J Am Med Inform Assoc 21, 326–336 (2014).
Duda, M., Ma, R., Haber, N. & Wall, D. Use of machine learning for behavioral distinction of autism and ADHD. Translational psychiatry 6, e732 (2016).
Pan, L. et al. Machine learning applications for prediction of relapse in childhood acute lymphoblastic leukemia. Sci Rep 7, 7402 (2017).
Qiu, J. et al. Derivation and Validation of a Mortality Risk Score for Severe Hand, Foot and Mouth Disease in China. Sci Rep 7, 3371, https://doi.org/10.1038/s41598-017-02658-4 (2017).
Yu, W.-L. et al. The epidemiology of acute respiratory distress syndrome in pediatric intensive care units in China. Intensive Care Med 35, 136 (2009).
Dang, H.-X., Liu, C.-J., Li, J., Chen, S.-J. & Xu, F. Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease. Nutrients 9, 478 (2017).
Zhang, J. et al. Machine-learning algorithms define pathogen-specific local immune fingerprints in peritoneal dialysis patients with bacterial infections. Kidney Int (2017).
Li, X. et al. Elevated levels of circulating histones indicate disease activity in patients with hand, foot, and mouth disease (HFMD). Scand J Infect Dis 46, 818–824, https://doi.org/10.3109/00365548.2014.943285 (2014).
Dang, H. X., Liu, C. J., Li, J., Chen, S. J. & Xu, F. Clinical Significance and Prognostic Effect of Serum 25-hydroxyvitamin D Concentrations in Critical and Severe Hand, Foot and Mouth Disease. Nutrients 9, https://doi.org/10.3390/nu9050478 (2017).
Li, W. et al. Study on risk factors for severe hand, foot and mouth disease in China. PLoS One 9, e87603, https://doi.org/10.1371/journal.pone.0087603 (2014).
Hsia, S.-H. et al. Predictors of unfavorable outcomes in enterovirus 71-related cardiopulmonary failure in children. The Pediatric infectious disease journal 24, 331–334 (2005).
Kim, S. J. et al. Risk factors for neurologic complications of hand, foot and mouth disease in the Republic of Korea, 2009. J Korean Med Sci 28, 120–127 (2013).
Fernández-Martínez, J. L., Fernández-Muñiz, Z., Pallero, J. & Pedruelo-González, L. M. From Bayes to Tarantola: New insights to understand uncertainty in inverse problems. Journal of Applied Geophysics 98, 62–72 (2013).
Xu, D. et al. Data-Driven Information Extraction from Chinese Electronic Medical Records. PLoS One 10, e0136270, https://doi.org/10.1371/journal.pone.0136270 (2015).
Troyanskaya, O. et al. Missing value estimation methods for DNA microarrays. Bioinformatics 17, 520–525 (2001).
Liu, X.-Y., Wu, J. & Zhou, Z.-H. Exploratory undersampling for class-imbalance learning. IEEE Transactions on Systems, Man, and Cybernetics, Part B (Cybernetics) 39, 539–550 (2009).
Breiman, L. Random forests. Machine learning 45, 5–32 (2001).
Svetnik, V. et al. Random forest: a classification and regression tool for compound classification and QSAR modeling. J Chem Inf Comput Sci 43, 1947–1958, https://doi.org/10.1021/ci034160g (2003).
Díaz-Uriarte, R. & De Andres, S. A. Gene selection and classification of microarray data using random forest. BMC Bioinformatics 7, 3 (2006).
Touw, W. G. et al. Data mining in the Life Sciences with Random Forest: a walk in the park or lost in the jungle? Briefings in bioinformatics, bbs034 (2012).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. Journal of Machine Learning Research 12, 2825–2830 (2011).
Vapnik, V. N. & Vapnik, V. Statistical learning theory. Vol. 1 (Wiley New York, 1998).
Chen, T. & Guestrin, C. In Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. 785–794 (ACM).
Conan-Guez, B. & Rossi, F. In Classification, Clustering, and Data Mining Applications: Proceedings of the Meeting of the International Federation of Classification Societies (IFCS), Illinois Institute of Technology, Chicago, 15–18 July 2004. 157 (Springer Science & Business Media).
Metz, C. E. Basic principles of ROC analysis. Semin Nucl Med 8, 283–298 (1978).
Acknowledgements
This work was supported by Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center (grant number KCP-2016-002), Guangzhou Science and Technology Bureau (grant number 2014Y2-00197 and 201707010018) and Science and Technology Planning Project of Guangdong Province, China (grant number 2016B030305006).
Author information
Authors and Affiliations
Contributions
Project design and implementation were conceived by Gansen Zhao, Huimin Xia, Yi Xu, Huiying Liang and Guangjian Liu. Data collection was performed by Xiaojun Cao, Xinming Wang, Xutian Zhuang, Liyan Pan and Yun Xi. Data analysis was performed by Huiying Liang, Huixian Li and Fangqin Lin. The experiments and programming were performed by Taishan Zeng, Xutian Zhuang, Yun Xi and Guangjian Liu. Manuscript drafting and editing were performed by Guangjian Liu, Huiying Liang, Xinming Wang and Gansen Zhao. All authors reviewed the manuscript in its final form.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, G., Xu, Y., Wang, X. et al. Developing a Machine Learning System for Identification of Severe Hand, Foot, and Mouth Disease from Electronic Medical Record Data. Sci Rep 7, 16341 (2017). https://doi.org/10.1038/s41598-017-16521-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16521-z
This article is cited by
-
Regional-level risk factors for severe hand-foot-and-mouth disease: an ecological study from mainland China
Environmental Health and Preventive Medicine (2021)
-
Risk prediction for delayed clearance of high-dose methotrexate in pediatric hematological malignancies by machine learning
International Journal of Hematology (2021)
-
Machine learning for the detection of early immunological markers as predictors of multi-organ dysfunction
Scientific Data (2019)
-
A method for hand-foot-mouth disease prediction using GeoDetector and LSTM model in Guangxi, China
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.