Abstract
Extreme climatic events, such as heatwaves, are predicted to be more prevalent in future due to global climate change. The devastating impacts of heatwaves on the survival of marine organisms may be further intensified by ocean acidification. Here, we tested the hypothesis that prolonged exposure to heatwave temperatures (24 °C, +3 °C summer seawater temperature) would diminish energy budget, body condition and ultimately survival of a subtidal gastropod (Thalotia conica) by pushing close to its critical thermal maximum (CTmax). We also tested whether ocean acidification (pCO2: 1000 ppm) affects energy budget, CTmax and hence survival of this gastropod. Following the 8-week experimental period, mortality was markedly higher at 24 °C irrespective of pCO2 level, probably attributed to energy deficit (negative scope for growth) and concomitant depletion of energy reserves (reduced organ weight to flesh weight ratio). CTmax of T. conica appeared at 27 °C and was unaffected by ocean acidification. Our findings imply that prolonged exposure to heatwaves can compromise the survival of marine organisms below CTmax via disruption in energy homeostasis, which possibly explains their mass mortality in the past heatwave events. Therefore, heatwaves would have more profound effects than ocean acidification on future marine ecosystems.
Similar content being viewed by others
Introduction
Since the onset of Industrial Revolution, atmospheric carbon dioxide (pCO2) concentration has been increasing rapidly and caused observable changes in marine ecosystems. For example, sea surface temperature increased by ~0.76 °C in the last century and is predicted to increase by 1.8–3.5 °C by the end of this century, depending on the CO2 emission scenario1. The increase in pCO2 is also leading to ocean acidification (OA), which is expected to have profound repercussions on a variety of marine organisms2,3. Indeed, meta-analyses suggest that OA and warming will likely modify community structures and functions of future marine ecosystems4,5.
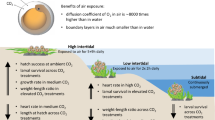
Given the slow rate of change in seawater chemistry, however, growing evidence shows that marine organisms may be able to acclimate to the predicted climatic conditions6,7,8. As such, it has been suggested that extreme climatic events, rather than climatic trends, will lead to more significant changes in future marine ecosystems due to their instant and catastrophic effects9. Heatwaves, one of the most devastating extreme climatic events, are of special concern because they can abruptly increase the sea surface temperature along coastlines by ~3 °C on average and persist for more than two months, causing substantial changes in marine ecosystems10,11,12. In view of global warming, it is conjectured that heatwaves will be more frequent and persistent in future9,13. Indeed, 71% of coastlines worldwide have already been warmed to some extent, while 38% have experienced anomalously high seawater temperature due to extreme hot events14.
Heatwaves can greatly influence the survival and distribution of marine organisms, and hence functions of marine ecosystems11,12. Cool-affinity species with low mobility are particularly vulnerable due to their limited ability to escape from thermal stress, while heatwaves favour the proliferation of warm-affinity species12. This implies that thermal stress is the key factor causing the mass mortality in the past heatwave events. Therefore, studying thermal tolerance of marine organisms can provide an initial insight into how heatwaves affect their fitness and survival15. Nevertheless, thermal tolerance could be modulated by OA through disruption in aerobic performance or energy homeostasis16,17, possibly increasing the susceptibility of marine organisms to heatwaves. Indeed, marine organisms are impacted by environmental stressors commonly via reduction in aerobic scope, defined as the excess metabolic energy available after basal metabolic cost is met18. As such, studying bioenergetics can shed light on the combined effects of multiple stressors on the performance, fitness and survival of marine organisms18. If heatwaves or OA causes energy deficit (i.e. negative energy budget) in the long term, energy reserves would be consumed or even depleted, eventually leading to mortality.
Here, we examined the effects of prolonged exposure to elevated pCO2 and temperature on the energy budget, body condition and survival of a subtidal grazing gastropod Thalotia conica, which is widely distributed in temperate Australia. Given its high abundance, this seagrass-associated gastropod plays a crucial role in energy flow in the seagrass community by regulating the abundance of epiphytes on seagrass leaves19, which can enhance the survival of seagrasses20. We first assessed the performance of aerobic metabolism of T. conica along a temperature gradient and determined its critical thermal maximum (CTmax), at which motor coordination is lost21, as a proxy for thermal tolerance. Then, we hypothesized that (1) prolonged exposure (8 weeks) to heatwave temperatures would lead to increased mortality of T. conica by diminishing its energy budget and body condition, when the temperature is close to CTmax; and (2) OA would reduce energy budget and thermal tolerance, resulting in greater mortality. Provided prolonged exposure to heatwaves diminishes the survival of this subtidal gastropod through energy depletion even below its thermal tolerance, this mechanism not only helps explain the mass mortality of marine organisms in the past heatwave events, but also suggests that persistent heatwaves are the main driver of the functions of both contemporary and future marine ecosystems.
Results
Performance of aerobic metabolism and CTmax
The respiration rate of T. conica gradually increased from 15 °C to 25 °C and then dropped from 25 °C to 35 °C (Fig. 1). The effect of pCO2 on respiration rate was not discernible, with few exceptions at some temperatures (29 °C and 31 °C). Regarding locomotory behaviour, individuals were inactive at 15 °C, but became gradually more active as temperature increased with the greatest activity at 25 °C. At 27 °C, foot strength was weakened and motor coordination was lost. Beyond 29 °C, the operculum was retracted, indicating acute thermal stress. Based on the locomotory behaviour, the CTmax of T. conica appeared at 27 °C.
Energy budget
The ingestion rate, absorption rate and scope for growth of T. conica at 24 °C were lower than those at 21 °C irrespective of pCO2 level (Fig. 2a,c and f; Table S1). Assimilation efficiency and excretion rate were not affected by both pCO2 and temperature (Fig. 2b and e; Table S1). Respiration rate at 24 °C was lower than that at 21 °C when pCO2 was at 1000 ppm (Fig. 2d; Table S1). Based on the patterns of energy budget parameters, absorption rate was the key factor determining scope for growth. Energy deficit was found at 24 °C, indicated by the negative scope for growth (Fig. 2f).
(a) Ingestion rate, (b) assimilation efficiency, (c) absorption rate, (d) respiration rate, (e) excretion rate and (f) scope for growth of Thalotia conica under different treatment conditions at the end of the experimental period (mean ± S.E.; n = 3). Different letters indicate significant difference between temperature groups (p < 0.05).
Body condition and survival
Following the 8-week experimental period, the total weight of T. conica increased at 21 °C, but decreased at 24 °C regardless of pCO2 level (Fig. 3a; Table S1). Organ weight to flesh weight ratio did not change significantly at 21 °C, but decreased at 24 °C at both pCO2 levels (Fig. 3b; Table S1), indicating the use of energy reserves. Mortality at 21 °C (~20%) was much lower than that at 24 °C (~70% at 400 ppm; ~80% at 1000 ppm) after the experimental period (Fig. 4; Table S1).
The change in (a) total weight and (b) organ weight to flesh weight ratio of Thalotia conica after the experimental period under different treatment conditions (mean + S.E.; n = 22 individuals for 21 °C, 400 ppm; n = 21 individuals for 21 °C, 1000 ppm; n = 8 individuals for 24 °C, 400 ppm; n = 5 individuals for 24 °C, 1000 ppm). Different letters indicate significant difference between temperature groups (p < 0.05).
Discussion
Studying bioenergetics can help decipher the fitness and survival of marine organisms in response to multiple environmental stressors18. Contrary to theory22, we revealed that OA did not significantly affect the thermal tolerance, energy budget, body condition and survival of a subtidal gastropod, even though the individuals had limited time to acclimate. The slightly reduced energy budget may be elicited by the extra energy required for the maintenance of acid-base balance and digestion efficiency under OA conditions and thus less energy can be allocated for foraging activity22, resulting in reduced energy gain by feeding. In contrast, we found that prolonged exposure to heatwave temperature markedly reduces energy budget, body condition and eventually survival. Therefore, heatwaves can probably pose greater adverse consequences than OA on the fitness and survival of marine organisms.
The detrimental effect of heatwaves on organisms can be mediated by reducing their aerobic scope (or excess metabolic energy)22. In general, energy demand increases with temperature and thus aerobic respiration needs to be upregulated to produce more metabolic energy and meet the elevated energy demand so that energy homeostasis can be maintained. When metabolic energy produced by aerobic respiration is insufficient to meet the elevated energy demand, aerobic scope will decrease18,23, which can be indicated when aerobic respiration starts to decline with increasing temperature24,25. As such, the foot strength of T. conica starts to weaken and its equilibrium is lost at 27 °C (i.e. CTmax), where the respiration rate can no longer increase with temperature probably due to reduced capacity of ventilatory and circulatory systems to deliver oxygen to foot tissues23,24. Consequently, the excess metabolic energy would not be adequate to support normal physiological functions and locomotory activity, and hence survival becomes time-limited, depending on the tolerance of individuals to starvation and thermal stress24. To avoid the impact of acute thermal stress, therefore, T. conica maximized locomotory activity at 25 °C, potentially as an escape behaviour. Since the respiration rate at 24 °C (i.e. heatwave temperature) was very close to the maximum, T. conica would be under sublethal thermal stress, which can compromise fitness and survival in the long term via disruption in energy homeostasis23,26.
As maintaining energy surplus is the key for long-term survival, bioenergetics-based models can be used to examine how environmental stressors affect the fitness and survival of marine organisms by changing their energy budget18. In this study, scope for growth was estimated to indicate energy budget, which was subject largely to absorption rate. We found that the heatwave temperature led to reduced absorption rate, which was in turn caused by reduced ingestion rate. The worsened feeding performance is likely attributed to the impact of sublethal thermal stress on aerobic metabolism. Based on the respiration rate along the temperature ramp, T. conica should have higher respiration rate at 24 °C than at 21 °C. After the experimental period, however, T. conica could not maintain higher respiration rate at 24 °C, which is likely caused by the reduced oxygen delivery capacity or impaired mitochondrial functions under long-term sublethal thermal stress23,27,28. As a result, energy production is suppressed, impairing various physiological functions and activities, including feeding29,30. Whether the reduced energy gain by feeding compromises growth and body condition depends on the net energy balance (i.e. scope for growth). We found that scope for growth is negative after the prolonged exposure to heatwave temperatures, indicating energy deficit which probably accounts for the loss of total weight. As shell weight is generally constant, the reduction in total weight plus negative energy budget suggest consumption of energy reserves, which is further substantiated by the reduced body condition (i.e. reduced organ weight to flesh weight ratio), indicating that energy supply was inadequate to support energy demand. Indeed, using energy reserves for basal maintenance becomes inevitable when energy deficit persists for a longer term. For instance, Ivanina et al.31 demonstrated that prolonged exposure to elevated temperature can cause depletion of energy reserves in bivalves Crassostrea virginica and Mercenaria mercenaria. Yet, using energy reserves can only provide a temporary relief against environmental stressors and is considered as the last resort for survival. When energy reserves are depleted, physiological processes will be hindered, or even arrested, explaining the rapid increase in the mortality of T. conica after six weeks of exposure to the heatwave temperature.
To date, heatwaves have received less attention due to their historical rarity in nature, but they can pose destructive impacts on marine ecosystems, especially survival of marine organisms. For example, recent heatwave events have abruptly increased the seawater temperature along coastlines by ~3 °C on average above normal level for more than eight weeks and caused mass mortality of marine organisms with little sign of recovery, possibly culminating in irreversible damage to ecosystem functions (Northwest Mediterranean coast in 1999 and 200310,11,32; Western Australian coast in 201112,33). Although underlying mechanisms (e.g. development of pathogens and modified species interactions) have been postulated to account for the mass mortality9,12,34, we suggest that persistent thermal stress is the overarching factor because it can directly impair aerobic metabolism, worsen feeding performance and eventually cause energy depletion18,23. Although subtidal organisms, especially in temperate regions, were shown to be resilient to elevated temperature because most of them are living below their thermal tolerance and have high acclimation capacity35,36, we showed that the adverse effects of thermal stress can be manifested after prolonged exposure to temperature below thermal tolerance. As many subtidal organisms are not highly mobile or even sessile (e.g. corals, polychaetes, gastropods, bivalves, sea urchins, etc.), they are subject to the instant thermal stress caused by heatwaves and have limited time to respond, ultimately leading to mortality.
In the past decade, a plethora of studies have investigated the potential impacts of OA on future marine ecosystems. Despite the merits of these studies, it is still difficult and challenging to accurately predict the impacts of OA in view of the inconsistent results and complexity of marine ecosystems4,8,37,38. Given the slow rate of change in seawater chemistry, the acclimation capacity of marine organisms will further make the consequences of future marine ecosystems unpredictable. In contrast, extreme climatic events, such as heatwaves, can remarkably modify the functions of marine ecosystems in a relatively short period of time11,12. In view of the dominance and role of T. conica, our findings imply that the ecological functions of seagrass community would be substantially altered by heatwaves, whereas OA poses negligible effects in the near future39,40.
Owing to global climate change, the frequency and persistence of heatwaves are predicted to increase in future, possibly jeopardizing the survival of marine organisms and modifying the functions of marine ecosystems. We revealed that prolonged exposure to heatwaves significantly diminishes the survival of a subtidal gastropod through reduction in energy budget and depletion of energy reserves, which are manifested at temperature below its thermal tolerance. This bioenergetics-based mechanism could help explain the mass mortality of marine organisms in past heatwave events and suggest that many subtidal organisms in temperate regions are vulnerable to persistent thermal stress, which would be the major driver affecting the integrity of both contemporary and future marine ecosystems.
Methods
Collection of gastropods and rearing conditions
Adult Thalotia conica were collected in summer from the subtidal region (0.5 to 4 m depth) at Wirrina Cove, South Australia (35°29′S, 138°15′E), where the seawater pH and temperature were similar to our laboratory control conditions at ~8.10 and 21 °C, respectively. Individuals were temporarily kept in a plastic holding aquarium (1 m × 40 cm × 30 cm) filled with natural seawater and allowed to acclimate under ambient conditions (pH: 8.10 ± 0.10, temperature: 21 ± 1 °C, salinity: 35 ± 1 psu and dissolved oxygen concentration: 6.9 ± 0.2 mg O2 L−1) with natural day/night cycles for two weeks prior to experimentation. Food was provided as epiphytes on rocks from the collection site. Seawater (ca. 50%) was replaced weekly.
Performance of aerobic metabolism and CTmax of gastropods
The performance of aerobic metabolism of T. conica was assessed by measuring respiration rate from 15 °C to 35 °C with an increasing temperature ramp of 2 °C h−1 25, whereas the locomotory behaviour was observed to estimate CTmax, indicated by the loss of motor coordination as the end point21,36. Prior to experimentation, individuals were transferred into plastic holding aquaria for a 2-day acclimation period at lower temperature. To avoid the shock effect due to a sudden decrease in temperature, the aquaria were submerged in a water bath and the seawater temperature in the aquaria was reduced from 21 °C to 15 °C at a decreasing rate of 3 °C day−1 using heater/chiller units (TC60, TECO, Italy). After the 2-day acclimation period, two individuals (shell height: ~11 mm) were transferred into a 73 mL airtight chamber filled with experimental seawater (i.e. pCO2 and temperature manipulated) at 15 °C and allowed to rest for one hour to minimize the effect of handling stress (n = 3 replicate chambers per pCO2 level, see section below for manipulation of pCO2 level). After the 1-hour resting period, the chamber seawater was fully replaced with oxygen-saturated experimental seawater with initial dissolved oxygen concentration measured using an automatic temperature compensation oxygen optode (LDO101, HACH, USA), which was calibrated at room temperature (~23 °C) following the manufacturer’s instructions. The chamber was then closed and put into a water bath at the target temperature to maintain the temperature of chamber seawater. After one hour, the chamber was opened to measure the final dissolved oxygen concentration, which was above 5.2 mg O2 L−1 for all samples. Then, the chamber seawater was fully replaced with oxygen-saturated experimental seawater at the next temperature level (i.e. +2 °C) immediately. The procedure for measuring respiration rate was repeated and locomotory behaviour was observed using the same individuals until measurement for the last temperature level (i.e. 35 °C) was completed. Blank samples without individuals were used to correct the background change in dissolved oxygen concentration. Respiration rate was expressed as mg O2 g−1 (flesh weight) h−1, where flesh weight was obtained after dissection.
Experimental setup for exposure to elevated pCO2 and temperature
Individuals of T. conica (shell height: 11.0 ± 1.30 mm, mean ± S.D.) were transferred into plastic aquaria (30 cm × 20 cm × 18 cm; n = 9 individuals per aquarium), which contained 5 L natural seawater, Ulva sp. and rocks from the collection site. They were then exposed to one of the crossed combinations of pCO2 (400 ppm vs. 1000 ppm) and temperature (21 °C vs. 24 °C) for eight weeks (n = 3 replicate aquaria per treatment). The “ambient” temperature (21 °C) is the average seawater temperature at the collection site in summer (average annual range: ~14 °C–22 °C), while the elevated temperature (+3 °C) simulates the average increase in seawater temperature based on the past heatwave events11,12. The desired seawater temperatures were achieved by submerging the aquaria in water baths maintained by heater/chiller units (TC60, TECO, Italy). The elevated pCO2 level, which simulates the predicted RCP8.5 scenario for the year 21001, was maintained by aerating the seawater with CO2-enriched atmospheric air using a gas mixer (Pegas 4000 MF, Columbus Instruments, USA). Food was provided as epiphytes on rocks from the collection site and replenished before totally consumed. Seawater (ca. 50%) was renewed weekly to prevent accumulation of metabolic waste. The seawater carbonate chemistry parameters throughout the experimental period are shown in Table S2.
Energy budget of gastropods
To estimate the effect of prolonged exposure to elevated pCO2 and temperature on energy budget, scope for growth was measured at the end of the experimental period (i.e. Week 8), where respiration rate, absorption rate and excretion rate were determined41. To measure respiration rate, two individuals from each aquarium were transferred into a 73 mL airtight chamber filled with seawater adjusted to their respective treatment conditions, and allowed to rest for one hour (n = 3 replicate chambers per treatment). The experimental procedures for measuring respiration rate are described in the section above. To quantify absorption rate (i.e. energy gain from the ingested food in a given period of time), two individuals which had been starved for one day were allowed to consume the epiphytes on rocks in their aquarium for one hour under their respective treatment conditions (n = 3 replicate aquaria per treatment). The initial and final total weights of each individual were measured by an electronic balance to the nearest 0.0001 g after blotting and removing the seawater inside the shell by gently tapping the operculum to obtain the fresh weight of epiphytes consumed8. The water content of epiphytes was determined by oven-drying so that ingestion rate was expressed as dry weight of epiphytes consumed per individual per hour. Then, the two individuals from the same feeding trial were put into a clean container filled with 200 mL natural seawater maintained under their respective treatment conditions. After four hours, the faeces in the container were collected, rinsed with deionized water and dried on a pre-weighed aluminium foil to obtain the dry weight of faeces. The ash-free dry weights of epiphytes and faeces were determined by weight loss on ignition at 550 °C in a muffle furnace for six hours so that assimilation efficiency was calculated according to equation (1) [ref.42]:
where AE is the assimilation efficiency; F′ is the ash-free dry weight to dry weight ratio of food; E′ is the ash-free dry weight to dry weight ratio of faeces.
Absorption rate is calculated by multiplying ingestion rate by assimilation efficiency, where the ingestion rate is converted into energy equivalent by the calorific value of epiphytes (8227 J g−1), determined by bomb calorimeter (C2000 Basic, IKA, Germany). To quantify excretion rate, the ammonium concentration of seawater in the container was analysed using a flow injection analyser (QuikChem 8500, Lachat Instruments, USA). Blank samples (i.e. natural seawater) were measured for correction of excretion rate. Scope for growth was calculated according to equation (2) [ref.41]:
where SfG is scope for growth (J ind−1 hr−1); AR is absorption rate; RR is respiration rate; ER is excretion rate. Conversion factors of 14.14 J mg O2 −1 and 0.025 J µg NH4 −1 were used to convert respiration rate and excretion rate, respectively, into energy equivalent43.
Body condition and survival of gastropods
Total weight of each individual was measured before and after the exposure experiment using an electronic balance to indicate the change in biomass. Mortality was checked daily and dead individuals were removed from the aquarium. Following the aforementioned measurements, all individuals were dissected to obtain the flesh weight which was further separated into organ weight and foot weight. Before the exposure experiment, 20 additional individuals which were collected at the same time as the experimental animals were dissected and weighed to obtain the initial organ weight to flesh weight ratio (i.e. body condition) so that the change in this ratio could be estimated8. All the weights were measured on a fresh weight basis.
Statistical analysis
Two-way permutational analysis of variance (PEMANOVA) was applied to examine the effects of pCO2 and temperature on ingestion rate, assimilation efficiency, absorption rate, respiration rate, excretion rate, scope for growth, total weight, organ weight to flesh weight ratio and mortality using software PRIMER 6 with PERMANOVA + add-on.
References
IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Stocker, T.F. et al. (eds.): Cambridge University Press (2013).
Fabry, V. J., Seibel, B. A., Feely, R. A. & Orr, J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008).
Doney, S. C., Fabry, V. J., Feely, R. A. & Kleypas, J. A. Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192 (2009).
Kroeker, K. J. et al. Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896 (2013).
Nagelkerken, I. & Connell, S. D. Global alteration of ocean ecosystem, functioning due to increasing human CO2 emissions. Proc. Natl. Acad. Sci. 112, 13272–13277 (2015).
Suckling, C. C. et al. Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J. Anim. Ecol. 84, 773–784 (2015).
Noisette, F., Bordeyne, F., Davoult, D. & Martin, S. Assessing the physiological responses of the gastropod Crepidula fornicata to predicted ocean acidification and warming. Limnol. Oceanogr. 61, 430–444 (2016).
Leung, J. Y. S., Russell, B. D. & Connell, S. D. Mineralogical plasticity acts as a compensatory mechanism to the impacts of ocean acidification. Environ. Sci. Technol. 51, 2652–2659 (2017).
Jentsch, A., Kreyling, J. & Beierkuhnlein, C. A new generation of climate-change experiments: Events, not trends. Front. Ecol. Environ. 5, 365–374 (2007).
Cerrano, C. et al. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (north-western Mediterranean), summer 1999. Ecol. Lett. 3, 284–293 (2000).
Garrabou, J. et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Change Biol. 15, 1090–1103 (2009).
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Change 3, 78–82 (2013).
Perkins, S. E., Alexander, L. V. & Nairn, J. Increasing frequency, intensity and duration of observed global heat waves and warm spells. Geophys. Res. Lett. 39, L20714 (2012).
Lima, F. P. & Wethey, D. S. Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat. Commun. 3, 704 (2012).
Verberk, W. C. E. P. et al. Can respiratory physiology predict thermal niches? Ann. N. Y. Acad. Sci. 1365, 73–88 (2016).
Pörtner, H. O. & Farrell, A. P. Physiology andclimate change. Science 322, 690–692 (2008).
Lannig, G., Eilers, S., Pörtner, H. O., Sokolova, I. M. & Bock, C. Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas – changes in metabolic pathways and thermal response. Mar. Drugs 8, 2318–2339 (2010).
Sokolova, I. M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 53, 597–608 (2013).
Jernakoff, P. & Nielsen, J. The relative importance of amphipod and gastropod grazers in Posidonia sinuosa meadows. Aquat. Bot. 56, 183–202 (1997).
McSkimming, C., Tanner, J. E., Russell, B. D. & Connell, S. D. Can herbivores compensate for nutrient pollution in seagrass meadows? J. Exp. Mar. Biol. Ecol. 471, 112–118 (2015).
Lutterschmidt, W. I. & Hutchison, V. H. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574 (1997).
Pörtner, H. O. Integrating climate-related stressor effects on marine organisms: Unifying principles linking molecule to ecosystem-level changes. Mar. Ecol. Prog. Ser. 470, 273–290 (2012).
Sokolova, I. M., Frederich, M., Bagwe, R., Lannig, G. & Sukhotin, A. A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 79, 1–15 (2012).
Pörtner, H. O. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp. Biochem. Physiol. A 132, 739–761 (2002).
Giomi, F. et al. The importance of thermal history: costs and benefits of heat exposure in a tropical, rocky shore oyster. J. Exp. Biol. 219, 686–694 (2016).
Gianguzza, P. et al. Temperature modulates the response of the thermophilous sea urchin Arbacia lixula early life stages to CO2-driven acidification. Mar. Environ. Res. 93, 70–77 (2014).
Peck, L. S., Pörtner, H. O. & Hardewig, I. Metabolic demand, oxygen supply, and critical temperatures in the Antarctic bivalve Laternula elliptica. Physiol. Biochem. Zool. 75, 123–133 (2002).
Ern, R. et al. Some like it hot: Thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Sci. Rep. 5, 10743 (2015).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Mertens, N. L., Russell, B. D. & Connell, S. D. Escaping herbivory: ocean warming as a refuge for primary producers where consumer metabolism and consumption cannot pursue. Oecologia 179, 1223–1229 (2015).
Ivanina, A. V. et al. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp. Biochem. Physiol. A 166, 101–111 (2013).
Perez, T. et al. Mass mortality of marine invertebrates: an unprecedented event in the Northwestern Mediterranean. C. R. Acad. Sci. 323, 853–865 (2000).
Pearce, A. F. & Feng, M. The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. J. Mar. Syst. 111–112, 139–156 (2013).
Bally, M. & Garrabou, J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: a new case of emerging disease linked to climate change. Glob. Change Biol. 13, 2078–2088 (2007).
Tomanek, L. Variation in the heat shock response and its implication for predicting the effect of global climate change on species’ biogeographical distribution ranges and metabolic costs. J. Exp. Biol. 213, 971–979 (2010).
Vinagre, C. et al. Vulnerability to climate warming and acclimation capacity of tropical and temperate coastal organisms. Ecol. Indic. 62, 317–327 (2016).
Kroeker, K. J., Kordas, R. L., Crim, R. N. & Singh, G. G. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434 (2010).
Connell, S. D. et al. How ocean acidification can benefit calcifiers. Curr. Biol. 27, R95–R96 (2017).
Russell, B. D. et al. Future seagrass beds: increased productivity leading to carbon storage? Mar. Poll. Bull. 73, 463–469 (2013).
Martínez-Crego, B., Olivé, I. & Santos, R. CO2 and nutrient-driven changes across multiple levels of organization in Zostera noltii ecosystems. Biogeosciences 11, 7237–7249 (2014).
Widdows, J. & Johnson, D. Physiological energetics of Mytilus edulis: Scope for Growth. Mar. Ecol. Prog. Ser. 46, 113–121 (1988).
Conover, R. J. Assimilation of organic matter by zooplankton. Limnol. Oceanogr. 11, 338–345 (1966).
Elliott, J. M. & Davison, W. Energy equivalents of oxygen consumption in animal energetics. Oecologia 19, 195–201 (1975).
Acknowledgements
Support was provided by the IPRS Scholarship from the University of Adelaide to JYSL, an Australian Research Council Discovery Program grant to BDR and SDC (DP150104263) and an ARC Future Fellowship to SDC (FT0991953).
Author information
Authors and Affiliations
Contributions
J.Y.S.L. designed and conducted the experiment, analysed the data and wrote the manuscript. S.D.C. helped revise the manuscript. B.D.R. designed the experiment and helped revise the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Leung, J.Y.S., Connell, S.D. & Russell, B.D. Heatwaves diminish the survival of a subtidal gastropod through reduction in energy budget and depletion of energy reserves. Sci Rep 7, 17688 (2017). https://doi.org/10.1038/s41598-017-16341-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16341-1
This article is cited by
-
Mussels show capacity for persistence under, and recovery from, marine heatwaves
Marine Biology (2023)
-
Do global environmental drivers’ ocean acidification and warming exacerbate the effects of oil pollution on the physiological energetics of Scylla serrata?
Environmental Science and Pollution Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.