Abstract
We tested the hypotheses that kidney cancer incidence was increasing globally whilst its mortality was reducing; and its incidence was positively correlated with country-specific socioeconomic development. The incidence and mortality figures of each country were projected to 2030. Data on age-standardized incidence/mortality rates were retrieved from the GLOBOCAN in 2012. Temporal patterns were examined for 39 countries from the Cancer Incidence in Five Continents volumes I-X and other national registries. We evaluated the correlation between the incidence/mortality rates and Human Development Index (HDI)/Gross Domestic Product (GDP]). The average annual percent change of its incidence and mortality in the most recent 10 years was obtained from joinpoint regression. The highest incidence rates were observed in Eastern Europe and North America, while its mortality rates were the highest in European countries. Incidence was positively correlated with HDI and GDP per capita. Many countries experienced incidence rise over the most recent 10 years, and a substantial reduction in mortality rates was observed for a significant number of countries, yet increases in mortality rates were observed in Eastern Europe. By 2030, Brazil and Ecuador may have the greatest rise in incidence both in men and women, which requires urgent need for planning healthcare resources.
Similar content being viewed by others
Introduction
Kidney cancer was the seventh most common malignancy and accounted for 3.3% of all newly diagnosed cancer in 20121. Renal cell carcinoma (RCC) constitutes approximately 90–95% of all kidney neoplasms2, and 25–30% of all patients had metastatic disease upon its diagnosis3. The estimated economic burden of metastatic RCC was $1.6 billion (2006 USD) in selected countries3. It is a rapidly evolving area of solid tumor oncology4. Recent studies showed that several European regions reported particularly high incidence rates5.
Recognized risk factors for RCC include cigarette smoking, obesity and hypertension6. Accumulating evidence suggests an etiologic role for physical inactivity, alcohol consumption, high parity among women, and occupational exposure to trichloroethylene. Many of these risk factors are amenable to lifestyle modifications and there exists a strong prospect for intervention. Previous studies examining its global trends were based on figures in the 1990s to early 2000s, did not make direct comparisons among countries, or focused on selected regions7,8,9. Based on existing literature10,11, it is worthwhile to analyze if the patterns and temporal trends of kidney cancer could quantify geographical variations, and identify modifiable factors that might have contributed to trend changes7. Projected estimates of its incidence and mortality are particularly useful to inform healthcare planning and priority setting.
There are two important knowledge gaps in kidney cancer research. Firstly, previous literature shows that the highest incidence occurs in more developed countries, and the recent decades witness increasing affluence and technological advancement especially in more developed nations. Temporal trends and projected changes in its future incidence and mortality are largely unaddressed. In addition, the role of socioeconomic status in RCC was still inconclusive when their associations were examined globally12. A recent study showed that among 9,623 patients with metastatic RCC at diagnosis, most were uninsured; residing in underprivileged regions, and were poorly educated13,14; but sporadic evidence suggested the contrary15. These findings highlight the need for a worldwide, across-country analysis. Furthermore, there have been no studies that have attempted to project the future trends of kidney cancer based on current epidemiological data.
Previous studies in the past decade showed that the number of new cases of kidney cancer increased and its mortality declined in populations of different ethnicities7,8,9,10,11. Hence, this study tested the a priori hypothesis that the temporal trends of its incidence increased and its mortality decreased. Also, we sought to test the hypothesis that its global incidence was positively correlated with country-specific socioeconomic development, and projected the incidence/mortality figures to 2020 and 2030. To standardize the methodology across published literature, we adopted the same analysis plan as reported in previous similar studies on prostate, colorectal, liver and pancreatic cancer16,17,18,19.
Methods
Data Source
We retrieved the incidence and mortality figures for kidney cancer in 2012 from the GLOBOCAN database for 167 countries1, excluding cancer of the renal pelvis and ureter. For all countries, data were matched with their Human Development Index (HDI) and Gross Domestic Product (GPD) per capita in the same year based on the United Nations Human Development Report20, which highlights the progress on human development over the past quarter century by reporting the different statistical indexes. HDI is a composite index of life expectancy, education period, and income per capita indicators20. For incidence figures, we extracted data from the Cancer Incidence in Five Continents series Volumes I-X21, which provided high-quality incidence statistics of cancer documented by local registries worldwide.
To acquire incidence data for more recent years, we also utilized publicly available information from the European Union Registration (EUREG)22, National Cancer Institute23, Nordic Cancer Registries24, Australian Cancer Incidence and Mortality Books25 and the Ministry of Health of New Zealand26. We used GLOBOCAN to report the latest incidence and mortality figures (2012) that are available for all countries, as well as the correlation between the socioeconomic indices and age-standardized rates of incidence/mortality. For evaluation of temporal trends of incidence and mortality in each country, we used the various databases highlighted in Table 1 to evaluate the Average Annual Percent Change (AAPC) by joinpoint regression analysis22,23,24,25,26. The incidence data were retrieved according to the International Classification of Diseases (ICD-10, C64; ICD-9-CM 189.0).
For mortality data, we made reference to the various national databases22,23,24,25,26, where the primary data source originated from death certificates. These data were categorized based on the ICD 9th or ICD 10th, according to the calendar year where the coding are available specific to each country. The WHO mortality database was not used as it does not consist of “kidney cancer” as a cause of death. Table 1 showed a more detailed description of the data sources and calendar years included for the present analysis. We adopted age-standardized rate (ASR) using the world standard population27. More developed regions include Europe, Northern America, Australia/New Zealand and Japan, whilst others are less developed regions1.
Statistical Analysis
We used joinpoint regression analysis to study the incidence/mortality trends28. A series of joined straight lines was fit to the ASR trend28. We performed logarithmic transformation of the ASRs and computed the standard errors adopting binomial approximation. A maximum number of three joinpoints were used as analysis options, and we evaluated the AAPC and the respective 95% confidence intervals (C.I.) for data available in the most recent 10 years. The AAPC was computerized as a geometrically weighted average of the generated APCs by the joinpoint trend analysis software. Their weights were equivalent to the length of each segment within the specified time interval29. We extracted all available global data for the incidence and mortality trends. We selected the most recent 10 years as the timeframe for examining temporal trend changes, as was commonly adopted in previous studies on global epidemiology of other cancers16,17,30. All AAPCs with their 95% C.I. lying above and below zero, respectively, were regarded as increasing and decreasing trends. AAPCs with 95% C.Is overlapping with zero was considered as stable trends16,17,30. AAPCs with 95% C.Is overlapping with zero was considered as stable trends. We plotted the ASRs of incidence and mortality against the HDI and GDP per capita, respectively, for each country. The HDI was defined as low (≤0.534), medium (0.534–0.710), high (0.710–0.796) and very high (>0.796)20. Logarithmic transformation of the ASR of incidence and mortality was applied in their correlations with HDI and GDP per capita as their associations were non-linear. Their correlation coefficients were evaluated. Also, we estimated the percent change in incidence and mortality by 2020 and 2030 when compared to the latest published figures based on the AAPC – a statistical technique employed by Bailey and colleagues in JAMA Surgery 30. The predicted incidence/mortality rates were assumed to change at a constant percentage of the rate of the previous year. All p values < 0.05 were considered statistically significant.
Results
Incidence and mortality in 2012
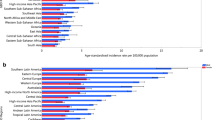
A total of 337,860 new cases of kidney cancer and 143,406 related deaths were reported in 2012. The highest incidence were found in Czech Republic (ASR 16.7 per 100,000), Lithuania (13.2), Slovakia (12.5) and Northern America (11.7), and the lowest were reported in Middle Africa (0.6), Western Africa (0.7) and Sub-Saharan Africa (0.9) (Fig. 1). The incidence was substantially higher in countries with very high HDI (9.1) than those with high (4.7), medium (2.5) and low HDI (1.0).
The mortality rates were higher in more developed than less developed regions (2.8 vs. 1.3). The highest mortality rates were reported in the Lithuania (4.9), Czech Republic (4.8), Latvia (4.7) and Estonia (4.6). The lowest estimated death rates were found in Micronesia/Polynesia (0.6), Middle Africa (0.6), Western Africa (0.6) and South-Central Asia (0.7). These geographical variations were similar when the worldwide incidence was stratified by gender (Table 2).
Correlation between incidence/mortality and socioeconomic development
Figure 2A,B and C showed the correlation between the incidence/mortality and HDI, evaluated by simple linear regression analysis. The ASR of incidence (r = 0.82, r2 = 0.67) and mortality (r = 0.68, r2 = 0.50) increased with higher levels of HDI. Similarly, the ASR of incidence (r = 0.55, r2 = 0.30) and mortality (r = 0.39, r2 = 0.16) was also correlated with GDP per capita (all p < 0.001; Fig. 3A,B and C). These significant correlations between the ASR figures and HDI/GDP were similar when analyzed separately for men and women.
(A) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Human Development Index (HDI) (Male). (B) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Human Development Index (HDI) (Female). (C) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Human Development Index (HDI) (Both sex).
(A) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Gross Domestic Product (GDP) (Male). (B) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Gross Domestic Product (GDP) (Female). (C) Correlation between age-standardised kidney cancer incidence (upper panel) and mortality (lower panel) and Gross Domestic Product (GDP) (Both sex).
Temporal trends
The incidence and mortality trend of each country was shown in Supplementary Figure 1, and the corresponding findings from the joinpoint regression analysis were presented in Supplementary Figures 2 and 3. Many countries experienced incidence rise, in particular Brazil, Ecuador, Thailand and Bulgaria. A substantial reduction in mortality rates was observed for many countries such as Sweden and Denmark, yet increases in mortality rates were observed in some Eastern European countries like Bulgaria.
Latin America and the Caribbean
Brazil (AAPC = 10.6, 95% C.I. = 3.4, 18.2, p = 0.009) and Ecuador (AAPC = 7.6, 95% C.I. = 3.7, 11.6, p = 0.002) showed substantial increase in incidence among men, and the rise in incidence among women was even more marked in these two countries (Brazil: AAPC = 16, 95% C.I. = 5.4, 27.6, p = 0.007; Ecuador: AAPC = 9.5, 95% C.I. = 2.6, 16.8, p = 0.01) (Fig. 4A).
Northern America
Its incidence showed a slight rise in male Americans (overall AAPC = 1.5, 95% C.I. = 0.3, 2.8, p = 0.01). Among women, Canada (AAPC = 1.9, 95% C.I. = 1, 2.9, p = 0.001) also showed a rise in incidence. There was a slight decline in mortality rates in Americans (Fig. 4B).
Asia
Thailand (AAPC = 5.3, 95% C.I. = 0.6, 10.3, p = 0.03), China (AAPC = 2.3, 95% C.I. 0.7, 4, p = 0.01), and India (AAPC = 1.9, 95% C.I. 0.1, 3.7, p = 0.04) were countries showing an increase in incidence among men, whilst Japan (AAPC = 2.5, 95% C.I. = 0.8, 4.2, p = 0.008) reported an incidence increase among women.
Oceania
The incidence in New Zealand was estimated to increase among men (AAPC = 2.1, 95% C.I. = 0.7, 3.6, p = 0.01), whilst Australia reported an increase among women (AAPC = 1.7, 95% C.I. = 0.7, 2.8, p = 0.006). The mortality decreased in Australia among both men and women (Fig. 4B).
Northern Europe
There was an incidence increase among men in the United Kingdom, Lithuania, Finland, Norway and Denmark (AAPC ranging from 1.8 to 2.8), as well as women in United Kingdom (AAPC = 2.2, 95% C.I. = 1.1, 3.3, p = 0.002). However, Sweden and Denmark showed a mortality decline in both men (−4.3, 95% C.I. −5.9, −2.7, p < 0.001 and −2.8, 95% C.I. −4.6, −1.1, p = 0.006) and women (−5.1, 95% C.I. −7.9, −2.3, p = 0.004 and −4.8, 95% C.I. −6.8, −2.7, p < 0.001).
Western Europe
The Netherlands (AAPC = 2.4, 95% C.I. = 1.1, 3.8, p < 0.001 [men]; AAPC = 2.2, 95% C.I. = 1.2, 3.1 p = 0.001 [women]), France (AAPC = 2.3, 95% C.I. = 0.8, 3.8, p = 0.007 [men]; AAPC = 2, 95% C.I. = 0.3, 3.8 p = 0.03 [women]) and Ireland (AAPC = 3.7, 95% C.I. = 1.4, 6.0, p = 0.005 [women]) reported an incidence increase. Austria (AAPC = −1.8, 95% C.I. = −2.7, −0.8, p = 0.003 [men]; AAPC = −2.2, 95% C.I. = −3.5, −0.9, p = 0.004 [women]) and Germany (AAPC = −3.5, 95% C.I. = −5.4, −1.5, p = 0.004 [women]) showed an incidence decline. Austria also showed a mortality reduction in men (AAPC = −2.1, 95% C.I. = −3.4, −0.7, p = 0.008).
Southern Europe
Spain had a slight increase in incidence among men (AAPC = 1.5, 95% C.I. = 0, 2.9, p = 0.048) and women (AAPC = 2.0, 95% C.I. = 0.1, 4, p = 0.04). Italy also reported a rise in incidence among men (AAPC = 2.3, 95% C.I. = 1.3, 3.3, p < 0.001). For mortality, Croatia was the only country showing a decline.
Eastern Europe
Bulgaria (AAPC = 4.7, 95% C.I. = 2.6, 6.8, p < 0.001 [men]; AAPC = 3.6, 95% C.I. = 0.6, 6.6, p = 0.02 [women]), Slovakia (AAPC = 3.6 95% C.I. = 2.1, 5.1, p < 0.001 [men]; AAPC = 3.4, 95% C.I. = 1.6, 5.2, p = 0.002 [women]), and Czech Republic (AAPC = 1.4, 95% C.I. = 0.02, 2.7, p = 0.047 [men]) reported a rise in incidence. Bulgaria also showed a significant increase in mortality (AAPC = 5.5, 95% C.I. = 2.8, 8.3, p = 0.002 [men]; AAPC = 5, 95% C.I. = 2.3, 7.7, p = 0.002 [women]).
AAPC-based projections of incidence and mortality to 2020 and 2030
By 2030, countries with the greatest rise in incidence included Brazil (911%), Ecuador (437%), Thailand (230%), Bulgaria (186%), and Malta (131%) in men. The most drastic rise in incidence by 2030 was found in Brazil (2,938%) and Ecuador (709%) in women. Bulgaria had the biggest rise in mortality in both men (227%) and women (192%) by 2030 (Supplementary Figures 4 and 5).
Discussion
This study presented the most updated global epidemiological profiles of kidney cancer, and we described the incidence and mortality patterns and trends based on high quality data. Both the incidence and mortality rates were positively correlated with human development levels and GDP per capita. The coefficients of correlation between incidence/mortality and HDI (0.62–0.73), and to a lesser extent GPD (0.36–0.55), were high. Incidence figures in the most recent 10 years reported that a total of 18 and 12 countries/regions, respectively, experienced increases in incidence rates in men and women. Brazil, Ecuador, Thailand and Bulgaria were some countries where incidence trends increased sharply. Many countries reported reduction in mortality trends, in particular Northern Europe (Sweden, Denmark) and Western Europe (Austria). Bulgaria was the only country that showed a substantially increasing mortality trend. We have also projected the incidence and mortality figures of kidney cancer for some selected countries.
Several reasons could explain the higher incidence of kidney cancer in more developed countries, and their positive correlation with HDI and GDP. Firstly, in developed nations with more rapid development and higher productivity, the prevalence of risk factors for RCC including smoking, obesity, physical inactivity and hypertension was higher7. Another explanation for the higher incidence could be attributed to the more liberal use of imaging techniques in more resource-privileged countries, such as abdominal ultrasound and computed tomography for non-specific symptoms presented by patients3. Indeed, most of the increases in incidence have been attributed to diagnosis of early, local stage tumors7. Yet another possible but more speculative contributing factor includes certain occupational and environmental exposures to carcinogenic agents, such as trichloroethylene, cadmium, arsenic, radon and nitrate3,7. As only 2–3% of all RCC were familial with distinct genetic phenotypes, hereditary factors seem to be rather remote in its influence on incidence rates. Our findings that some countries outside Europe and North America reported markedly increased incidence trends warrant further studies to elucidate the underlying etiological mechanisms.
The use of linear modelling for trend projection allows comparison between our findings and those reported from other literature. For instance, Bailey and colleagues have examined the incidence rates of colorectal cancer in the United States by using the Surveillance, Epidemiology and End Results (SEER) database, and the incidence was assumed to change at a constant proportion of the annual percent change (APC) of the previous year30. Other studies that projected future trends of cancer incidence and mortality also adopted linear modelling31,32. In addition, there has been a study that compared the validity of using 15 different models to predict cancer incidence, including linear and non-linear models and one on a smoothed version of the age-period-cohort model. It was found that none of the models significantly out-performed one another33. Nevertheless, despite the common use of linear regression for prediction of cancer statistics, there are some caveats that should be mentioned. Projected figures could be underestimated due to emergence of known and unknown risk factors, or on the other hand overestimated with more developed preventive efforts that could lead to a decrease in cancer incidence and mortality34. While risk factors related to lifestyle measures are not necessarily correlated with higher cancer incidence or mortality due to the relatively short latency period, an aging population and the growing proportion of elderly with longer life expectancy are associated with an escalated cancer burden in the future34.
With respect to reporting bias, cancer registration in relatively less-developed nations could suffer from higher chance of under-reporting. Incidence and mortality figures in more regional cancer registries might be less accurate due to limited communication infrastructure and less robust reporting systems to the healthcare management for new cancer cases and deaths. Other contributing factors to reporting bias include relative lack of clinical services in poor rural areas; low income levels of the general populations and thus lower willingness to utilize healthcare services; limited access to healthcare providers in underdeveloped regions; as well as attribution of cancer diagnoses and death to other diseases due to lower availability of confirmatory investigation tests required for cancer diagnosis. These factors could significantly underestimate the actual incidence and mortality figures when trends were estimated and future incidence/mortality rates were projected, in particular for less developed nations. These should alert cautious interpretation of this analysis that compared the incidence/mortality of kidney cancer among countries.
To address reporting bias as a major source of inaccuracy in analysis of incidence and mortality trends, resources are required to build national cancer registries that capture data in a systematic manner with comprehensive coverage of healthcare service providers. This should be in parallel with regular audit of the hospital case records on disease coding and clinical guidelines to enhance cancer incidence/mortality reporting to national databases. There have been studies conducted in the US that installed electronic health records and developed implementation strategy of new cancer-reporting module in rural clinics to increase cancer reporting rates and accuracy of reporting mortality causes35. Strategies to enhance service accessibility for patients with symptoms of cancer to facilitate timely diagnosis are also required. In addition, studies that adopt population-based designs, such as representative surveys that collect data directly from individuals, could supplement data for more precise estimation of cancer incidence and mortality.
The mortality patterns and their correlation with HDI/GDP were similar to that of the cancer’s incidence. Although more developed countries are equipped with technological advancement in disease treatment, it is well recognized that a significant proportion (up to 30%) of patients have metastatic disease during initial diagnosis, whilst another 20% of patients who receive nephrectomy will relapse and develop metastasis during follow-up3 - hence the close concordance between incidence and mortality. Nevertheless, the reduction in mortality trends in the recent decade could be explained by the earlier diagnosis leading to stage migration to earlier stage disease, which could be treated by curative intervention. Another driver for the mortality decline could be due to better systemic therapy for advanced diseases, such as interferon and interleukin-2 therapy36, and targeted therapy37.
Other limitations of this study should be discussed. Firstly, only one-third and one-fifth of the world’s countries, respectively, reported incidence and mortality data of high quality - data precision, coverage, and completeness in the national databases could be different from that of CI5. Furthermore, one could not establish cause-and-effect relationships in correlational analysis, and coincidental changes in lifestyle habits could parallel that of incidence/mortality rates. For instance, increased mortality rates due to kidney cancer is reminiscent of what is happening in Eastern Europe, particularly Bulgaria for coronary heart disease38, possibly related to limited preventive measures or poor dietary habits due to economic burden and increase in tobacco smoking and alcohol drinking that are heavily associated with physical inactivity, obesity and hypertension. Also, the analyses have not taken competition of risk into account39. Furthermore, we have used a cutoff value of significance at p < 0.05, similar to previous studies that examined the temporal patterns and trends of cancer incidence and mortality16,17,18,19,30. One should be cautious when our findings are interpreted as there are multiple testing in this study. Lastly, despite our most inclusive approach to analyze the most recent data, the figures used are from 2012 at the latest and the temporal trends will need continuous updates.
Conclusion
The incidence rates of kidney cancer increased in many countries analyzed in this study, and the mortality rates declined in a large number of nations, particularly in more developed regions. With population ageing and population growth, the absolute incidence of kidney cancer will show a continuing increasing trend out of proportion of the incidence increase here implies. Appropriate healthcare resources should be planned to cope with the increasing need for patient treatment, especially in more resource-deprived countries. Future studies are needed to explore the underlying mechanisms for these epidemiological trends with potential risk factors incorporated in further analysis.
References
Ferlay, J., Soerjomataram, I., Ervik, M. et al. GLOBOCAN 2012v1.0, Cancer Incidence and Mortality Worldwide. IARC Cancer Base No. 11. Lyon, France: International Agency for Research on Cancer (2013).
Ljungberg, B., Campbell, S. C. & Cho, H. Y. et al. The epidemiology of renal cell carcinoma. Eur Urol 60, 615–621 (2011).
Gupta, K., Miller, J. D. & Li, J. Z. et al. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev 34, 193–205 (2008).
Shuch, B., Amin, A. & Armstrong, A. J. et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol 67, 85–97 (2015).
Li, P., Znaor, A. & Holcatova, I. et al. Regional geographic variations in kidney cancer incidence rates in European countries. Eur Urol 67, 1134–1141 (2015).
Chow, W.-H., Dong, L. M. & Devesa, S. S. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7, 245–257 (2010).
Ma, J., Ward, E. M., Siegel, R. & Jemal, A. Temporal trends in mortality in the United States, 1969-2013. JAMA 314, 1731–1739 (2015).
Pérez-Farinós, N., López-Abente, G. & Pastor-Barriuso, R. Time trend and age-period-cohort effect on kidney cancer mortality in Europe, 1981–2000. BMC Public Health. 6, 119 (2006).
Kohlrt, B. A., Ward, E. & McCarthy, B. J. et al. Annual report to the Nation on the status of cancer, 1975–2007, featuring tumors of the brain and other nervous system. J Natl Cancer Inst 103, 714–736 (2011).
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncology; published online Dec 3. https://doi.org/10.1001/jamaoncol.2016.5688 (2016)
Mathew, A., Devesa, S. S., Fraumeni, J. F. & Chow, W.-H. Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev 11, 171–8 (2002).
Znaor, A., Lortet-Tieulent, J., Laversanne, M., Jemal, A. & Bray, F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67, 519–530 (2015).
Klaassen, Z., Augusta, G. A. & DiBianco, J. M. et al. Clear cell renal cell carcinoma: socioeconomic predictors of metastatic disease at diagnosis. J Urol 193, e48 (2015).
Mellemgaard, A., Engholm, G., McLaughlin, J. K. & Olsen, J. H. Risk factors for renal cell carcinoma in Denmark. I. Role of socioeconomic status, tobacco use, beverages, and family history. Cancer Causes Control. 5, 105–113 (1994).
Hellenthal, N. J. & Bermejo, C. E. The role of socioeconomic status in renal cell carcinoma. Urol Oncol. 30, 89–94 (2012).
Wong, M. C., Goggins, B. W., Wang, H. H. et al. Global incidence and mortality of prostate cancer: analysis of temporal patterns and trends in 36 countries. Eur Urol. https://doi.org/10.1016/j.eururo.2016.05.043 (2016).
Arnold, M., Sierra, M. S., Laversanne, M. et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. https://doi.org/10.1136/gutjnl-2015-310912 (2016).
Wong, M. C., Jiang, J. Y. & Liang, M. et al. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci Rep 9, 3165 (2017).
Wong, M. C., Jiang, J. Y. & Goggins, W. et al. International incidence and mortality trends of liver cancer: a global profile. Sci Rep 7, 45846 (2017).
Human Development Report 2013. The rise of the south: human progress in a diverse world. New York: United Nations Development Programme (UNDP), (2013).
Forman, D., Bray, F., Brewster, D. H. et al. Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon: IARC http://ci5.iarc.fr. (2013).
Steliarova-Foucher, E., O’Callaghan, M. & Ferlay, J. et al. The European Cancer Observatory: A new data resource. Eur J Cancer 51, 1131–1143 (2015).
National Cancer Institute. Surveillance, Epidemiology and End Results Program. http://seer.cancer.gov/faststats/selections.php?series=data.
Engholm, G., Ferlay, J., Christensen, N. et al. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 7.1 (09.07.2015). Association of the Nordic Cancer Registries. Danish Cancer Society. http://www.ancr.nu. (2015).
Australian Institute of Health and Welfare, Australian Government. Australian Cancer Incidence and Mortality (ACIM) books. http://www.aihw.gov.au/acim-books/.
New Zealand National Ministry of Health. Cancer: Historical summary 1948–2012. http://www.health.govt.nz/publication/cancer-historical-summary-1948-2012.
Segi, M., Fujisaku, S. & Kurihara, M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan 48, 219–225 (1957).
Kim, H.-J., Fay, M. P. & Feuer, E. J. et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 19, 335–351 (2000).
Clegg, L. X., Hankey, B. F. & Tiwari, R. et al. Estimating average annual percent change in trend analysis. Stat Med 28, 3670–3682 (2009).
Bailey, C. E., Hu, C. & You, Y. N. et al. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. JAMA Surgery 150, 17–22 (2015).
Malvezzi, M., Bertuccio, P. & Levi, F. et al. European cancer mortality predictions for the year 2013. Annals of Oncology 24, 792–800 (2013).
Jung, K. W., Won, Y. J. & Oh, C. M. et al. Prediction of cancer incidence and mortality in Korea, 2016. Cancer research and treatment: official journal of Korean Cancer Association. 48, 451 (2016).
Moller, B., Fekjaer, H. & Hakulinen, T. et al. Prediction of cancer incidence in the Nordic countries: empirical comparison of different approaches. Statist. Med. 22, 2751–2766 (2003).
Shamseddine, A., Saleh, A. & Charafeddine, M. et al. Cancer trends in Lebanon: a review of incidence rates for the period of 2003–2008 and projections until 2018. Population Health Metrics 12, 4 (2014).
Headd, A., Jackson-Thompson, J., Zachary, I. et al. Achieving increased cancer reporting from clinicians to public health: A local success with global implications. Presented in the American Public Health Association Available at: https://apha.confex.com/apha/141am/webprogram/Paper288290.html (2013).
Scott, C. S. & Chiu, W. A. Trichloroethylene cancer epidemiology: a consideration of select issues. Environ Health Perspect 114, 1471–1478 (2006).
Negrier, S., Escudier, B. & Lasset, C. et al. Recombinant human interleukin-2, recombinant human interferon alpha-2a, or both in metastatic renal-cell carcinoma. N Engl J Med 338, 1272–1278 (1998).
Puddu, P. E. & Menotti, A. Coronary heart disease differences in Eastern versus Western Europe: A demanding situation. Int J Cardiol 217, S60–S63 (2016).
Puddu, P. E., Piras, P. & Menotti, A. Lifetime competing risks between coronary heart disease mortality and other causes of death during 50 years of follow-up. Int J Cardiol 228, 359–363 (2017).
Acknowledgements
We thank the expert advice from the Division of Biostatistics of the School of Public Health and Primary Care on statistical analysis. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.C.S.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; conceived the study; wrote the first draft of the paper; and contributed to interpretation of the data; B.W.G. contributed to the study design and critically revised the manuscript; B.H.K.Y. critically revised the manuscript and contributed to statistical analysis; F.D.H.F. retrieved the data and composed the graphs; conducted the statistical analysis; and revised the paper; C.L. conducted the statistical analysis; contributed to the interpretation of the data and the writing and editing of the report; Y.F. critically revised the manuscript and contributed to statistical analysis; S.Y.S.W. interpreted the data and critically revised the manuscript; C.F.N. contributed to the interpretation of the data and the writing and editing of the report.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, M.C.S., Goggins, W.B., Yip, B.H.K. et al. Incidence and mortality of kidney cancer: temporal patterns and global trends in 39 countries. Sci Rep 7, 15698 (2017). https://doi.org/10.1038/s41598-017-15922-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15922-4
This article is cited by
-
Patient-specific, deliverable, and self-expandable surgical guide development and evaluation using 4D printing for laparoscopic partial nephrectomy
Scientific Reports (2024)
-
Multiphase CT radiomics nomogram for preoperatively predicting the WHO/ISUP nuclear grade of small (< 4 cm) clear cell renal cell carcinoma
BMC Cancer (2023)
-
Preliminary Study of Whole-Genome Bisulfite Sequencing and Transcriptome Sequencing in VHL Disease-Associated ccRCC
Molecular Diagnosis & Therapy (2023)
-
Sodium selenite inhibits proliferation and metastasis through ROS‐mediated NF‐κB signaling in renal cell carcinoma
BMC Cancer (2022)
-
Coffee intake and trace element blood concentrations in association with renal cell cancer among smokers
Cancer Causes & Control (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.