Abstract
Thrombopoietin (TPO) is the master cytokine regulator of megakaryopoiesis. In addition to regulation of megakaryocyte and platelet number, TPO is important for maintaining proper hematopoietic stem cell (HSC) function. It was previously shown that a number of lymphoid genes were upregulated in HSCs from Tpo −/− mice. We investigated if absent or enhanced TPO signaling would influence normal B-lymphopoiesis. Absent TPO signaling in Mpl −/− mice led to enrichment of a common lymphoid progenitor (CLP) signature in multipotential lineage-negative Sca-1+c-Kit+ (LSK) cells and an increase in CLP formation. Moreover, Mpl −/− mice exhibited increased numbers of PreB2 and immature B-cells in bone marrow and spleen, with an increased proportion of B-lymphoid cells in the G1 phase of the cell cycle. Conversely, elevated TPO signaling in Tpo Tg mice was associated with reduced B-lymphopoiesis. Although at steady state, peripheral blood lymphocyte counts were normal in both models, Mpl −/− Eµ-myc mice showed an enhanced preneoplastic phase with increased numbers of splenic PreB2 and immature B-cells, a reduced quiescent fraction, and augmented blood lymphocyte counts. Thus, although Mpl is not expressed on lymphoid cells, TPO signaling may indirectly influence B-lymphopoiesis and the preneoplastic state in Myc-driven B-cell lymphomagenesis by lineage priming in multipotential progenitor cells.
Similar content being viewed by others
Introduction
Platelets are produced from large precursor cells in the bone marrow called megakaryocytes. The process by which megakaryocytes produce platelets is unique and involves polyploidisation and shedding of platelets into the blood stream1. Thrombopoietin (TPO), produced mainly in the liver, stimulates both megakaryocyte colony formation and enhances megakaryocyte maturation in the bone marrow leading to increased platelet production. TPO binds to its receptor, TPOR/MPL, which is expressed on hematopoietic stem cells (HSCs), megakaryocytes and platelets2. Circulating TPO concentrations are controlled by MPL via receptor-mediated internalization and degradation3,4, and in part by sensing of senescent platelets by the Ashwell-Morell receptor in the liver5. Mpl −/− mice exhibit platelet counts ~10% of wild-type levels6, while Tpo Tg transgenic mice, engineered to overexpress TPO in the liver, have ~3.5 times higher platelet counts7 compared to wild-type mice. In addition to regulating megakaryopoiesis, TPO is known to affect HSC number and quiescence7. A low level of TPO leads to reductions in total HSCs, but with more of them in active cycle. This is evident in Mpl deficient mice, which not only exhibit thrombocytopenia, but also have increased cycling and a decline in the number of HSCs with age8,9,10. Conversely, a high TPO level leads to a greater proportion of quiescent HSCs7.
It has previously been reported that down-regulation of Mpl marks the transition from pluripotent to lymphoid-primed multipotent hematopoietic progenitor cells11 and that a number of lymphoid genes are upregulated in HSCs from Tpo −/− mice12. Here, we compared the effects of absent or enhanced TPO signaling on steady-state B-lymphopoiesis and in a model of over-active B-lymphopoiesis, the Eµ-myc transgenic mice, which display a characteristic preneoplastic phase with expansion of immature Pro- and Pre-B cell populations13,14. We found that, although Mpl is not expressed on lymphoid cells, absent TPO signaling in Mpl −/− mice led to lineage priming of lineage-negative Sca-1+c-Kit+ (LSK) cells with increased numbers of common lymphoid progenitors (CLPs), PreB2 and immature B-cells. Additionally, Mpl −/− Eµ-myc mice demonstrated an enhanced preneoplastic phase that resulted in earlier disease onset. By comparison, elevated TPO signaling modestly suppressed B-lymphopoiesis and resulted in delayed disease onset in Tpo Tg Eµ-myc transgenic mice. Thus, despite the absence of Mpl expression in B-lymphoid cells, changes induced in multipotential hematopoietic progenitor cells by altered TPO signaling, including altered gene expression and lineage priming, indirectly affect B-lymphopoiesis. The effects of altered Mpl signaling at steady-state were confined to changes in B-lymphoid precursor cell numbers, with no alteration in circulating B cell number. However, under conditions of excess proliferation modeled using Eµ-myc transgenic mice, the changes conferred by altered Mpl signaling in preneoplastic B-lymphopoiesis were sufficient to influence the onset of B-cell lymphoma.
Results
Lymphoid lineage skewing in Mpl−/− bone marrow and spleen
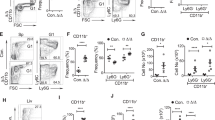
In accordance with previous findings reported in adult mice7,15, in the setting of increased TPO signaling, we found that 4–5 week old Tpo Tg mice had a 4-fold increase in numbers of LSK cells and corresponding LSK subpopulations long-term HSCs (LTHSCs), short-term HSCs (STHSCs) and lymphoid-primed multipotent progenitors (LMPPs) in the bone marrow when compared to wild-type mice (Fig. 1a). While augmented numbers of CLPs and B-cell primed progenitors (BLPs), could also be observed in Tpo Tg bone marrow, this effect was more modest (Fig. 1a, Supplementary Fig. S1). In contrast, as previously described7,15, Mpl −/− mice had a significantly reduced number of LSKs (Fig. 1a, Supplementary Fig. S1). Interestingly, despite a deficiency in the number of LSKs, numbers of CLPs and BLPs were significantly increased in Mpl −/− bone marrow (Fig. 1a, Supplementary Fig. S1). Prior evidence had suggested that lymphoid-specific gene transcription may be primed early in hematopoiesis16,17. Here, we observed more specifically, that relative to wild-type LSKs, a CLP gene expression signature18 was upregulated in Mpl −/− LSKs (p = 0.0001) (Fig. 1b, Supplementary Table S1) and conversely down regulated in Tpo Tg LSKs (p = 0.0001) (Fig. 1c, Supplementary Table S1). In confirmation of these results, lineage priming with early B-cell progenitor ProB and PreB gene expression signatures was also observed in Mpl −/− LSKs, and that these were conversely downregulated in Tpo Tg LSKs (Supplementary Table S2).
Lymphoid lineage priming in Mpl −/− LSKs. (a) Hematopoietic stem and progenitor cells per femur relative to WT in 4–5 weeks old mice. WT (n = 8), Mpl −/− (n = 6) and Tpo Tg (n = 7). Mean ± SEM. Statistical significance was generated by Student’s unpaired t-test. *p < 0.05. (b) Barcode plot of the CLP gene signature in Mpl −/− and (c) Tpo Tg LSKs. Gene set tests were performed using gene set obtained for the CLP population from the Hemopedia atlas18.
To determine any functional consequences of these altered gene expression profiles, the numbers of lymphoid cells were further enumerated in Mpl −/− and Tpo Tg mice. In 4–5 week old mice, Mpl deficiency led to increased PreB2 cell numbers in bone marrow along with elevated immature B-cell numbers in bone marrow and spleen (Fig. 2a,b). In contrast, Tpo Tg mice had reduced numbers of bone marrow ProB-PreB1, PreB2 cells and splenic mature B cells (Fig. 2a,b).
Lymphoid lineage priming in Mpl −/− bone marrow and spleen. (a) Bone marrow and (b) spleen cellularity of B-cell subsets in 4–5 week old mice. WT (n = 17), Mpl −/− (n = 16) and Tpo Tg (n = 15). (c-d) Ki-67/DAPI cell cycling assessment of B-cell subsets within bone marrow and spleen of 4–5 week old WT (n = 17), Mpl −/− (n = 17) and Tpo Tg (n = 13) mice. Statistical significance was generated by Student’s unpaired t-test. Mean ± SEM. *p < 0.05; **p < 0.005; ***p < 0.001
While Mpl is expressed on LSK cells, we found the receptor to be absent on CLPs, ProB-PreB1, PreB2, immature, and mature B cells (Supplementary Fig. S1), excluding an effect via direct TPO signaling in committed B-lineage cells. Limiting dilution assays revealed no significant difference in in vitro clonogenicity of purified Mpl −/− and Tpo Tg CLPs relative to wild-type cells (Supplementary Fig. S2). However, in the Mpl −/− spleen, immature and mature B-cells cycled significantly more than wild-type, as indicated by an increased proportion of cells in G1 and a reduced proportion in G0 (Fig. 2d). However, changes in cell cycle status were not evident in B-lymphoid cells in Tpo Tg mice (Fig. 2c,d), nor in Mpl −/− bone marrow (Fig. 2c), and peripheral blood lymphocyte counts in Mpl −/− and Tpo Tg mice were normal (Table 1)15. Thus, although altered TPO signaling may indirectly affect B-lymphopoiesis, other mechanisms were clearly contributing to circulating lymphocyte homeostasis at steady state.
TPO restricts Eµ-myc preneoplastic lymphoproliferation
We next investigated the changes in B-lymphopoiesis evident in Mpl −/− and Tpo Tg mice in the context of excess B-lymphoid proliferation in Eµ-myc transgenic mice14. We initially confirmed that preneoplastic Eµ-myc B-cell subsets did not express Mpl protein, when assessed by flow cytometry (Supplementary Fig. S3) as was the case for wild-type B-cell subsets. The preneoplastic phase in Eµ-myc mice is characterized by a polyclonal expansion of PreB cells accompanied by mild splenomegaly19. We observed that relative to 4–5 week-old Eµ-myc control mice, age matched Mpl −/− Eµ-myc mice displayed an increase in spleen weight of 1.4-fold (Fig. 3a) which was accompanied by a 1.9-fold increase in peripheral blood lymphocyte counts (Fig. 3b). In comparison to Eµ-myc controls, Mpl −/− Eµ-myc mice showed a modest reduction in red blood cell number, while, the number of platelets was reduced in the presence of the Eµ-myc transgene irrespective of whether mice were on a wild-type or Mpl −/− background (Fig. 3c,d). In order to investigate if the observed blood lymphocyte elevation corresponded with changes in B cell precursors in the bone marrow and spleen we examined B-cell subsets and cellularity in pre-neoplastic Eµ-myc, Mpl −/− Eµ-myc and Tpo Tg Eµ-myc mice (Fig. 3e,f). We found increased spleen cellularity and a doubling in the number of PreB2 cells as well as significant increases in the numbers of immature and mature B-cells in pre-neoplastic Mpl −/− Eµ-myc spleen, compared to Eµ-myc mice (Fig. 3f). Moreover, Mpl −/− Eµ-myc mice had increased numbers of immature B-cells in the bone marrow (Fig. 3e). Conversely, pre-neoplastic Tpo Tg Eµ-myc mice had reduced PreB2 cell numbers in bone marrow and spleen as well as significantly fewer immature and mature splenic B-cells compared to Eµ-myc control mice (Fig. 3e,f). To confirm these results, we next assessed the effect of exogenous TPO on the preneoplastic phase by administration of the TPO-mimetic Romiplostim20 in Eµ-myc and littermate control animals starting when they were 1 week old. When mice were assessed at 5-weeks of age, platelet counts were significantly increased in animals receiving Romiplostim compared to vehicle (Fig. 4a). As expected, 5-week old vehicle treated Eµ-myc mice had increased lymphocyte counts, spleen cellularity and bone marrow and spleen PreB2 cells compared to wild-type control mice (Fig. 4b,c,d). Interestingly, Romiplostim treated Eµ-myc mice had reduced lymphocyte counts, lower spleen cellularity and fewer PreB2 and immature B-cells in both bone marrow and spleen when compared to vehicle treated Eµ-myc mice (Fig. 4b,c,d).
Increased lymphoproliferation in preneoplastic Mpl deficient mice. (a) Spleen weight (b) Lymphocyte (c) Red blood cell (RBC) and (d) platelet counts in 4–5 week old mice. n = 11–18 mice per genotype. Whiskers: Min to Max. (e) Bone marrow and (f) spleen cellularity and Pro-PreB1, PreB2, immature and mature B-cells in 4–5 week old mice assessed by flow cytometry. Data are presented as mean ± SEM. n = 11–18 mice per genotype. Statistical significance was generated by Student’s unpaired t-test. *p < 0.05; **p < 0.005; ***p < 0.001
Effects of TPO-mimetic on preneoplastic phase. 1-week-old Eμ-myc mice and their wild-type littermates were injected subcutaneously every 3 days with 100 μg/kg Romiplostim or saline vehicle. At 5 weeks of age (a) platelet and (b) lymphocyte counts (c) spleen cellularity, PreB2, and immature B-cells, and (d) bone marrow cellularity, ProB PreB1, PreB2 and Immature B-cells. n = 8–14 mice per group. Data are presented as mean ± SEM. Statistical significance was generated by Student’s unpaired t-test. *p < 0.05; **p < 0.005; ***p < 0.001. BM = bone marrow.
Eµ-myc mice have previously been shown to succumb to aggressive lymphoma within 3–6 months after birth14. Mpl −/−, Tpo Tg and wild-type mice aged up to 1 year showed no signs of tumor development during the course of the study (Fig. 5a). However, the changes conferred by altered Mpl signaling in preneoplastic B-lymphopoiesis were sufficient to influence the onset of B cell lymphoma in Eµ-myc mice. Mpl −/− Eµ-myc mice became unwell with a reduced median survival of 76.5 days, compared to conventional Eµ-myc mice (87 days, p < 0.0001) (Fig. 5a). Conversely, Tpo Tg Eµ-myc mice exhibited delayed illness (p = 0.0019) with a median survival of 107 days (Fig. 5a). Moribund mice of all three genotypes had splenomegaly (Fig. 5b) and many presented with elevated blood lymphocyte counts (Table 1) as well as enlarged lymph nodes (Supplementary Fig. 4b). Spleen weights and cellularity were significantly increased in ill Mpl −/− Eµ-myc mice when compared to control Eµ-myc mice (Fig. 5b, Supplementary Fig. S4a). The bone marrow revealed diffuse tumor infiltrate of large cells with multiple nucleoli, as well as frequent extravasation into surrounding tissues (Supplementary Fig. S5). The tumor phenotypes, classified as PreB2, immature B-cell or a mixture of the two (mixed), were similarly distributed among the genotypes bearing the Eµ-myc transgene (Fig. 5c). Hematocrit and platelet counts often drop in the late stages of hematological malignancy including in Eµ-myc induced lymphoma (Table 1). Of note, Mpl −/− Eµ-myc mice were substantially more anemic than Eµ-myc and Tpo Tg Eµ-myc mice (Table 1). At necropsy, lymph node hemorrhage was often observed in Mpl −/− Eµ-myc mice, a phenotype not commonly present in the other genotypes (Supplementary Fig. S6).
TPO delays B-cell lymphoma development in Eµ-myc mice. (a) Survival of Eµ-myc (n = 83), Mpl −/− Eµ-myc (n = 200), Tpo Tg Eµ-myc (n = 58), WT (n = 14), Mpl −/− (n = 11) and Tpo Tg (n = 13) mice. P-values were generated with Logrank (Mantel-Cox) test. (b) Spleen weight in terminally ill mice and 1-year old control mice. Eµ-myc (n = 47), Mpl −/− Eµ-myc (n = 59), Tpo Tg Eµ-myc (n = 56), WT (n = 5), Mpl −/− (n = 7) and Tpo Tg (n = 6). Whiskers: Min to Max. Statistical significance was generated by Student’s unpaired t-test. *p < 0.05; **p < 0.005; ***p < 0.001. (c) Bone marrow disease phenotype in terminally ill mice assessed by flow cytometry. Eµ-myc (n = 31), Mpl −/− Eµ-myc (n = 36) and Tpo Tg Eµ-myc (n = 25) mice.
Enhanced cycling of B-lymphoid cells in pre-neoplastic Mpl−/− Eµ-myc mice
Our results support a model in which the pre-malignant phase of Eµ-myc lymphomagenesis is affected by the underlying cellular changes in mice with altered TPO signaling. Since increased B-lymphoid proliferation in pre-malignant Eµ-myc mice is characterized by changes in apoptosis and cell cycling19,21, we examined these processes in the compound mutant mice. Bone marrow cells from 4–5 week old Eµ-myc, Mpl −/− Eµ-myc, and Tpo Tg Eµ-myc mice were cultured for 24 hours under conditions of cytokine deprivation, and apoptosis was measured in B-cell subsets by Annexin-V binding. In accord with previous studies21, Eµ-myc B-cell subsets were highly sensitive to apoptosis compared to wild-type control cells (Supplementary Fig. S7). However, no differences in B-cell apoptosis were observed in Mpl −/− Eµ-myc or Tpo Tg Eµ-myc cells (Supplementary Fig. S7).
TPO has an established role in the control of HSC number and quiescence6,7, and we confirmed reduced quiescence (G0) in Mpl −/− LSKs and conversely increased quiescence in Tpo Tg LSKs (Supplementary Fig. S8c). Eµ-myc LSKs expressed Mpl to the same extent as wild-type cells (Supplementary Fig. S8a) and similar changes in LSK cell number and cell cycling were observed in Mpl −/− and Tpo Tg mice in the presence and absence of Eµ-myc (Supplementary Fig. S8b,c). We next investigated if there were differences in cycling of ProB-PreB1, PreB2, immature and mature B-cells in bone marrow and spleen of preneoplastic Mpl −/− Eµ-myc, Tpo Tg Eµ-myc, and Eµ-myc mice. Previous studies have shown that Eµ-myc mice have more than double the number of PreB cells and mature B cells in active cycle compared with controls19. In keeping with these findings we found an increased proportion of PreB2, immature and mature B-cells in active cycle in Eµ-myc mice compared to healthy controls (Figs 2 and 6). Consistent with our findings in Mpl −/− mice, this effect was augmented in Mpl −/− Eµ-myc PreB2-, immature and mature B-lymphocytes, while a reverse effect was observed in immature and mature B-cells in Tpo Tg Eµ-myc mice, particularly in the spleen (Fig. 6a,b). Thus, the accumulation of B-lymphoid cells in preneoplastic Mpl −/− Eµ-myc mice is likely to be the result of further deregulation of the already increased rate of proliferation of Eµ-myc B-lymphoid cells, with a converse attenuating effect of excess TPO signaling in Tpo Tg Eµ-myc mice.
Increased B-cell cycling in preneoplastic Mpl −/− Eµ-myc mice. Cell cycle status of (a) bone marrow and (b) spleen B-cell subsets in 4–5 week old Eµ-myc (n = 13), Mpl −/− Eµ-myc (n = 22) and Tpo Tg Eµ-myc (n = 13) mice assessed by Ki-67/DAPI staining profiles. Statistical significance was compared to Eµ-myc. Statistical significance was generated by Student’s unpaired t-test. Mean ± SEM. *p < 0.05; **p < 0.005; ***p < 0.001.
Discussion
Our findings demonstrated that perturbed TPO signaling had a clear effect on the lymphoid compartment at steady state using genetically modified Mpl −/− and Tpo Tg models. The earliest stages of the hematopoietic hierarchy where this effect was apparent was observed in Mpl expressing LSK cells, where an upregulation of CLP, ProB and PreB gene expression signatures in Mpl −/− LSK cells confirmed prior data from Sanjuan-Pla et al. that showed an upregulation of lymphoid genes in HSCs from Tpo −/− mice, while Tpo −/− bone marrow cells gave lymphoid-biased reconstitution when transplanted12. Together, these data suggest absence of TPO signaling on LSK cells results in lineage priming with enhanced early lymphoid gene expression. In support of the biological significance of the upregulated CLP, ProB and PreB signatures in Mpl −/− LSKs, we found an increased number of CLPs in Mpl −/− bone marrow. Moreover, we demonstrate that 4–5 week old Mpl −/− mice exhibit increased PreB2 and immature B-cell counts in bone marrow and spleen, with an increased proportion of immature and mature splenic B-cells in active cycle. Conversely, elevated TPO signaling in Tpo Tg mice negatively affected bone marrow and spleen B-lymphopoiesis. These effects on committed B-lymphoid precursor cell numbers must be indirect, since previous studies, also confirmed here, have shown that while LSK cells express the Mpl receptor, CLP and B-cell subsets do not11,15,22,23,24. The in vitro clonogenicity of CLPs from Mpl −/− and Tpo Tg mice was unaltered, but changes in the cell cycling profiles of B-lymphoid precursor cells correlated with altered cell numbers in these mice. Interestingly, the master erythroid cytokine regulator, EPO, has also been shown, in addition to stimulating erythropoiesis, to negatively affect B-lymphopoiesis at the ProB-cell and PreB-cell stage of maturation, by an indirect mechanism within the bone marrow environment25.
The altered B-lymphopoiesis observed in Mpl −/− and Tpo Tg bone marrow and spleen was, however, not sufficient to influence lymphocyte counts in the peripheral blood at steady state. Nevertheless, we hypothesized that changes to B-lymphopoiesis evident in mice with altered TPO signaling might have more dramatic consequences in a perturbed system. Thus, we investigated the preneoplastic phase in Eµ-myc transgenic mice, which is characterized by increased B-lymphoid cell cycling and lymphocytosis. We observed accumulation of PreB2, immature and mature B-cells in preneoplastic Mpl −/− Eµ-myc mice relative to that in Eµ-myc controls, an effect that was reversed in Tpo Tg Eµ-myc mice. Furthermore, the effect on preneoplastic Eµ-myc mice was also countered by 4 weeks of Romiplostim administration, when mice were 1 to 5 weeks of age. While the increased apoptosis characteristic of Eµ-myc B-lymphoid cells21 was unaltered in Mpl −/− Eµ-myc and Tpo Tg Eµ-myc mice, the increased B-lymphoid cell cycling caused by Eµ-myc expression was augmented in Mpl −/− Eµ-myc mice and attenuated in Tpo Tg Eµ-myc animals. The effects on the preneoplastic phase in Eµ-myc mice were sufficient to influence disease outcome: illness from B-lymphoma developed with a reduced latency in Mpl −/− Eµ-myc mice and was delayed in Tpo Tg Eµ-myc mice. This result is in agreement with a recent study which reported that Mpl deficiency increased precursor B-ALL development in the Lnk −/− Tp53 −/− mouse model26.
Our results support a model in which the direct influence of the Eµ-myc transgene and the indirect effect of loss of TPO signaling, at least in part due to lymphoid lineage priming in multipotential hematopoietic cells, appear to have an additive effect on cell cycling and accumulation of B-lymphoid populations resulting in an enhanced preneoplastic state and earlier disease onset in Mpl −/− Eµ-myc mice. The suppressive indirect impact of excess TPO signaling in Tpo Tg mice was also evident in the presence of the Eµ-myc transgene and could account for the delayed disease initiation in Tpo Tg Eµ-myc mice. Lymph node intratumor hemorrhage was occasionally observed in terminally ill Mpl −/− Eµ-myc mice, which is consistent with previous recognition of hemorrhaging of solid tumors in severely thrombocytopenic mice27,28. Thus, while excess bleeding may have contributed to the terminal stage of disease in Mpl −/− Eµ-myc mice, the significant preneoplastic expansion of splenic B lymphoid cell numbers (PreB2, immature and mature B-cells) and blood lymphocyte counts support a significant contribution by altered B-lymphopoiesis.
While no megakaryocyte-associated fibrosis29,30 was observed in 4–5 week old Tpo Tg mice (data not shown), our data cannot exclude an effect on B-lymphopoiesis from altered environmental niches or competition for space arising from abnormal megakaryocyte number in the bone marrow and spleen as Mpl −/− mice have 10% of normal megakaryocyte and platelet numbers, while Tpo Tg mice have ~3.5-fold increased platelet counts with 3-fold elevated bone marrow and spleen megakaryocytes7,15,31 (Supplementary Fig. S5b).
In addition, platelets have been shown to protect tumor vasculature28 and to promote hematogenous metastasis in mouse models32,33. Although limited information is available on roles of platelets in lymphoma and leukemia, some recent studies have examined interactions between lymphoma and leukemia cell lines and activated platelets or platelet-released molecules34,35,36. Although, the present study investigated the preneoplastic phase in Eµ-myc mice, future studies are needed to explore potential effects of altered megakaryocyte or platelet numbers that may alter the biological behavior of established lymphoma.
Material and Methods
Mice
Eµ-myc 14, Mpl −/− 6 and Tpo Tg 7 mice have been previously described. All mutations were backcrossed onto the C57BL/6 background for at least 10 generations prior to this study. Experiments include balanced groups of male and female mice if not otherwise stated. Eµ-myc mice included in the survival study were taken at an ethical endpoint when displaying lymphadenopathy and/or splenomegaly. All animal experiments complied with the regulatory standards of, and were approved by the Walter and Eliza Hall Institute (WEHI) Animal Ethics Committee.
Peripheral blood counts
Automated cell counts were performed on blood collected from the retro-orbital plexus or by cardiac puncture into Microtainer tubes containing EDTA (Sarstedt, Ingle Farm, SA Australia), using an Advia 2120 hematological analyser (Siemens, Munich Germany).
Progenitor and B-cell subset analysis
4–5 week old, 1–year old or terminally ill mice were analyzed. Organ processing: Bone marrow was flushed from femurs and tibias in 10 mL of balanced salt solution (BSS; 150 mM NaCl, 3.7 mM KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 7.4 mM HEPES.NaOH, 1.2 mM KH2PO4, 0.8 mM K2HPO4, pH 7.2) supplemented with 2% fetal calf serum (FCS) and gently pipetted to create a single-cell suspension. Cell suspensions were centrifuged at 1500 g, 5 min at 4 °C. Supernatant was then aspirated and the pellet was resuspended in 3 ml of BSS 2% FCS. Cells were filtered using a 100 μm cell strainer. Spleen cell suspensions were prepared by physical dissociation in 3 ml of BSS 2% FCS. Subsequently, 7 ml of Red Cell Removal Buffer (156 mM NH4Cl, 0.1 mM EDTA, and 12 mM NaHCO3) was added for 1 min at room temperature. After centrifugation the supernatants were aspirated and the pellet was resuspended in 5 ml of BSS 2% FCS. Cell suspensions were filtered using a 100 μm cell strainer. B-cell populations were defined as ProB-PreB1 (B220+ cKit+), PreB2 (B220+ cKit− IgD– IgM−), Immature (B220+ cKit− IgD− IgM+), Mature (B220+ cKit− IgD+ IgM+). LSK (Lin− Sca1+cKit+), LTHSC (Lin− Sca-1+ cKit+ CD34low/−), STHSC (Lin− Sca-1+ cKit+ CD34+ FLT3−), LMPP (Lin− Sca-1+ CD34+ FLT3+), CLP (Lin− Sca-1int cKitint IL7R+ FLT3+), ALP (Lin− Sca-1int cKitint IL7R+ FLT3+ Ly6D−), BLP (Lin− Sca-1int cKitint IL7R+ FLT3+ Ly6D+). Data was acquired using a LSRFortessa flow cytometer (BD, Franklin Lakes, NY, USA), and data analysis was performed using FlowJo software 10.3.0 (Treestar Inc, Ashland, USA).
LSK gene expression analysis
Gene set tests were performed on microarray data generated from sorted LSK cells from adult wild-type C57BL/6, Mpl −/− and Tpo Tg mice. Total RNA was hybridized to Illumina MouseWG-6v2 bead chip arrays15. The microarray data are available at Array express (www.ebi.ac.uk/arrayexpress) under accession no. E-MTAB-2389. Results were normalized using the neqc(x) function in limma and gene expression was fitted to the linear model using empiric Bayes and array weights. Gene set tests were performed using gene sets obtained for the CLP, ProB and PreB populations from the Hemopedia atlas (haemosphere.org)18 using the top 77 upregulated genes for each population compared to the highest gene expression across the entire dataset. Using the Roast function in Limma, a self-contained rotational gene set testing method37, gene set enrichment was tested in Mpl −/− and Tpo Tg LSKs when compared to wild-type and leading-edge genes contributing to the enriched CLP expression signature in Mpl −/− LSK cells, as well as downregulated CLP expression signature in Tpo Tg LSK cells, were extracted (Supplementary Table S1).
Analysis of B-lymphoid cell cycling
Single-cell suspensions from bone marrow and spleen of 4–5 week old mice were harvested as described above. Nucleated cell count was performed using trypan blue exclusion and 20 × 106 cells were stained with antibodies to B220 (clone RA3-6B2; BD Pharmingen Franklin Lakes NY USA), CD19 (clone 1D3; ebiosciences San Diego CA USA), c-Kit (clone ACK4; WEHI mAb Facility, Parkville VIC Australia), IgD (clone 11–26 c.2a; Biolegend San Diego CA USA), and IgM (clone 5.1; WEHI mAb Facility) for 30 min on ice. Cells were then washed with BSS 2% FCS and stained with the appropriate secondary streptavidin-conjugated fluorochromes for another 30 min on ice. Subsequently, cells were fixed and permeabilized with Cytofix/Cytoperm (BD) according to the manufacturer’s instruction for 30 min on ice. Cells were then incubated with either FITC-conjugated anti-Ki-67 antibody or the appropriate FITC-conjugated isotype control (BD) overnight at 4 °C. Lastly, 4′,6-diamidino-2-phenylindole (DAPI) (10 μg/ml) (Sigma-Aldrich, St. Louis, MO, USA) was added for 30 min at room temperature for analysis of DNA content. Cells were subsequently washed and resuspended with BSS 2% FCS and filtered using 40 μm cell strainer prior to analysis. Data was acquired using a LSR II flow cytometer (BD), and data analysis was performed using FlowJo software. Here, B-cell subsets were defined as: ProB-PreB1 (B220+ cKit+), PreB2 (B220+ CD19+ IgD– IgM−), Immature (B220+ CD19+ IgD− IgM+) and Mature (B220+ CD19+ IgD+ IgM+).
Romiplostim
At 6–8 days old, Eμ-myc mice and their wild-type littermates were injected subcutaneously with 100 μg/kg Romiplostim38 (Nplate®, Amgen, Thousand Oaks, CA, USA) or saline vehicle. Injections were repeated every 3 days until mice were 5-weeks old.
Statistics
Statistical significance between two treatment groups was analyzed using an unpaired Student’s t test with two-tailed p-values. One-way ANOVA with multiple comparison test was applied where appropriate (GraphPad Prism Version 7). *p < 0.05; **p < 0.005; ***p < 0.001 or as otherwise stated. Data are presented as mean ± SD or SEM, indicated in figure legends.
Data availability statement
Figure 1 and Tables S1 and S2 have an associated data source. The microarray data are available at Array express (www.ebi.ac.uk/arrayexpress) under accession no. E-MTAB-2389.
References
Italiano, J. E. Jr., Patel-Hett, S. & Hartwig, J. H. Mechanics of proplatelet elaboration. Journal of thrombosis and haemostasis: JTH 5(Suppl 1), 18–23, https://doi.org/10.1111/j.1538-7836.2007.02487.x (2007).
Alexander, W. S. Thrombopoietin and the c-Mpl receptor: insights from gene targeting. Int J Biochem Cell Biol 31, 1027–1035 (1999).
Kuter, D. J. & Rosenberg, R. D. The reciprocal relationship of thrombopoietin (c-Mpl ligand) to changes in the platelet mass during busulfan-induced thrombocytopenia in the rabbit. Blood 85, 2720–2730 (1995).
Stoffel, R., Wiestner, A. & Skoda, R. C. Thrombopoietin in thrombocytopenic mice: evidence against regulation at the mRNA level and for a direct regulatory role of platelets. Blood 87, 567–573 (1996).
Grozovsky, R. et al. The Ashwell-Morell receptor regulates hepatic thrombopoietin production via JAK2-STAT3 signaling. Nature medicine 21, 47–54, https://doi.org/10.1038/nm.3770 (2015).
Alexander, W. S., Roberts, A. W., Nicola, N. A., Li, R. & Metcalf, D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood 87, 2162–2170 (1996).
de Graaf, C. A. et al. Regulation of hematopoietic stem cells by their mature progeny. Proceedings of the National Academy of Sciences of the United States of America 107, 21689–21694, https://doi.org/10.1073/pnas.1016166108 (2010).
Kimura, S., Roberts, A. W., Metcalf, D. & Alexander, W. S. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proceedings of the National Academy of Sciences of the United States of America 95, 1195–1200 (1998).
Qian, H. et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684, https://doi.org/10.1016/j.stem.2007.10.008 (2007).
Yoshihara, H. et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697, https://doi.org/10.1016/j.stem.2007.10.020 (2007).
Luc, S. et al. Down-regulation of Mpl marks the transition to lymphoid-primed multipotent progenitors with gradual loss of granulocyte-monocyte potential. Blood 111, 3424–3434, https://doi.org/10.1182/blood-2007-08-108324 (2008).
Sanjuan-Pla, A. et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature 502, 232–236, https://doi.org/10.1038/nature12495 (2013).
Adams, J. M. & Cory, S. Transgenic models of tumor development. Science 254, 1161–1167 (1991).
Adams, J. M. et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature 318, 533–538 (1985).
Ng, A. P. et al. Mpl expression on megakaryocytes and platelets is dispensable for thrombopoiesis but essential to prevent myeloproliferation. Proceedings of the National Academy of Sciences of the United States of America 111, 5884–5889, https://doi.org/10.1073/pnas.1404354111 (2014).
Ng, S. Y., Yoshida, T., Zhang, J. & Georgopoulos, K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity 30, 493–507, https://doi.org/10.1016/j.immuni.2009.01.014 (2009).
Mansson, R. et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity 26, 407–419, https://doi.org/10.1016/j.immuni.2007.02.013 (2007).
de Graaf, C. A. et al. Haemopedia: An Expression Atlas of Murine Hematopoietic Cells. Stem cell reports 7, 571–582, https://doi.org/10.1016/j.stemcr.2016.07.007 (2016).
Langdon, W. Y., Harris, A. W., Cory, S. & Adams, J. M. The c-myc oncogene perturbs B lymphocyte development in E-mu-myc transgenic mice. Cell 47, 11–18 (1986).
Kuter, D. J. Romiplostim. Cancer Treat Res 157, 267–288, https://doi.org/10.1007/978-1-4419-7073-2_16 (2011).
Jacobs, J. J. et al. Bmi-1 collaborates with c-Myc in tumorigenesis by inhibiting c-Myc-induced apoptosis via INK4a/ARF. Genes & development 13, 2678–2690 (1999).
Drexler, H. G. & Quentmeier, H. Thrombopoietin: expression of its receptor MPL and proliferative effects on leukemic cells. Leukemia 10, 1405–1421 (1996).
Graf, G., Dehmel, U. & Drexler, H. G. Expression of thrombopoietin and thrombopoietin receptor MPL in human leukemia-lymphoma and solid tumor cell lines. Leukemia research 20, 831–838 (1996).
Erickson-Miller, C. L. et al. Thrombopoietin receptor levels in tumor cell lines and primary tumors. J Oncol 2010, 135354, https://doi.org/10.1155/2010/135354 (2010).
Singbrant, S. et al. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 117, 5631–5642, https://doi.org/10.1182/blood-2010-11-320564 (2011).
Cheng, Y. et al. LNK/SH2B3 regulates IL-7 receptor signaling in normal and malignant B-progenitors. The Journal of clinical investigation 126, 1267–1281, https://doi.org/10.1172/jci81468 (2016).
Ho-Tin-Noe, B., Goerge, T., Cifuni, S. M., Duerschmied, D. & Wagner, D. D. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer research 68, 6851–6858, https://doi.org/10.1158/0008-5472.can-08-0718 (2008).
Ho-Tin-Noe, B., Goerge, T. & Wagner, D. D. Platelets: guardians of tumor vasculature. Cancer research 69, 5623–5626, https://doi.org/10.1158/0008-5472.can-09-1370 (2009).
Abbonante, V. et al. Thrombopoietin/TGF-beta1 Loop Regulates Megakaryocyte Extracellular Matrix Component Synthesis. Stem cells (Dayton, Ohio) 34, 1123–1133, https://doi.org/10.1002/stem.2285 (2016).
Kakumitsu, H. et al. Transgenic mice overexpressing murine thrombopoietin develop myelofibrosis and osteosclerosis. Leukemia research 29, 761–769, https://doi.org/10.1016/j.leukres.2004.12.009 (2005).
Lebois, M. et al. Regulation of platelet lifespan in the presence and absence of thrombopoietin signaling. Journal of thrombosis and haemostasis: JTH 14, 1882–1887, https://doi.org/10.1111/jth.13397 (2016).
Gay, L. J. & Felding-Habermann, B. Platelets alter tumor cell attributes to propel metastasis: programming in transit. Cancer cell 20, 553–554, https://doi.org/10.1016/j.ccr.2011.11.001 (2011).
Stegner, D., Dutting, S. & Nieswandt, B. Mechanistic explanation for platelet contribution to cancer metastasis. Thrombosis research 133(Suppl 2), S149–157, https://doi.org/10.1016/s0049-3848(14)50025-4 (2014).
Au, A. E. et al. Activated platelets rescue apoptotic cells via paracrine activation of EGFR and DNA-dependent protein kinase. Cell death & disease 5, e1410, https://doi.org/10.1038/cddis.2014.373 (2014).
Velez, J. et al. Platelets promote mitochondrial uncoupling and resistance to apoptosis in leukemia cells: a novel paradigm for the bone marrow microenvironment. Cancer microenvironment: official journal of the International Cancer Microenvironment Society 7, 79–90, https://doi.org/10.1007/s12307-014-0149-3 (2014).
Ohana, O. M., Ozer, J., Prinsloo, I., Benharroch, D. & Gopas, J. Hodgkin lymphoma cell lines bind to platelets. Incubation with platelets induces CD15 and P-selectin dependent adhesion of the cell lines to Human Umbilical Vein Endothelial cells (HUVEC). Cancer biology & therapy 16, 1651–1659, https://doi.org/10.1080/15384047.2015.1095411 (2015).
Ng, A. P. et al. Early lineage priming by trisomy of Erg leads to myeloproliferation in a Down syndrome model. PLoS genetics 11, e1005211, https://doi.org/10.1371/journal.pgen.1005211 (2015).
Leon, C. et al. Romiplostim administration shows reduced megakaryocyte response-capacity and increased myelofibrosis in a mouse model of MYH9-RD. Blood 119, 3333–3341, https://doi.org/10.1182/blood-2011-08-373811 (2012).
Acknowledgements
The authors thank J. McManus, J. Lochland, S. Lee, L. Cengia, L. Di Rago, K. Stoev and S. Ross for outstanding assistance. This work was supported by grant funds received from the following: Leukaemia Foundation Australia Grant-in-Aid (E.C.J., K.D.M.), Sir Edward Dunlop Medical Research Foundation (E.C.J.), the Australian National Health and Medical Research Council Project Grant (E.C.J. 1079250, A.P.N. 1060179), Program Grant (1016647), Fellowships (W.S.A. 1058344; B.T.K. 1063008; K.D.M. 1090500) and Independent Research Institutes Infrastructure Support Scheme Grant (9000220) and a Victorian State Government Operational Infrastructure Support Grant. E.C.J. is the recipient of a fellowship from the Lorenzo and Pamela Galli Charitable Trust.
Author information
Authors and Affiliations
Contributions
M.L., S.A.S., P.C., J.C., P.G., C.D.H., D.M., A.R., F.Y. and K.D.M. performed research and analyzed data. A.E.A. and A.P.N. designed and performed research, analyzed data, prepared figures and revised the paper. B.T.K. provided reagents and advice. W.S.A. designed research and revised the paper. E.C.J. designed research and wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Au, A.E., Lebois, M., Sim, S.A. et al. Altered B-lymphopoiesis in mice with deregulated thrombopoietin signaling. Sci Rep 7, 14953 (2017). https://doi.org/10.1038/s41598-017-15023-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-15023-2
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.