Abstract
Proteus vulgaris L-amino acid deaminase (pvLAAD) belongs to a class of bacterial membrane-bound LAADs mainly express in genus Proteus, Providencia and Morganella. These LAADs employ a non-cleavable N-terminal twin-arginine translocation (Tat) peptide to transport across membrane and bind to bacterial surface. Recent studies revealed that a hydrophobic insertion sequence (INS) in these LAADs also interacts with bacterial membrane. However, the functional significance of INS-membrane interaction is not clear. In this study, we made site-directed mutagenesis on the surface-exposed hydrophobic residues of pvLAAD INS, and we found that these mutations impaired the INS-membrane interaction but did not affect pvLAAD activity in the solution. We further found that when cell membrane is present, the catalytic activity can be increased by 8~10 folds for wild-type but not INS-mutated pvLAAD, indicating that the INS-membrane interaction is necessary for increasing activity of pvLAAD. Molecular dynamic (MD) simulations suggested that INS is flexible in the solution, and its conformational dynamics could lead to substrate channel distortion. Circular dichroism (CD) spectroscopy experiments indicated that bacterial membrane was able to maintain the conformation of INS. Our study suggests the function of the membrane binding of INS is to stabilize pvLAAD structure and increase its catalytic activity.
Similar content being viewed by others
Introduction
L-amino acid oxidases/deaminases (LAAOs/LAADs, EC 1.4.3.2) are flavin-containing enzymes that catalyze the oxidative deamination of L-amino acids to corresponding α-keto acids with strict stereospecificity1. LAAOs/LAADs are widely expressed in snake venom, insects, mammals, fungi, fishes and some bacterial species2,3,4,5, and play various functional roles such as anti-microbes, anti-tumor, anti-leishmaniasis, anti-HIV, platelet aggregation inhibition and innate immune defenses of animals6,7. While most LAAOs/LAADs are secreted or cytosolic enzymes8, bacteria from genus Proteus, Providencia and Morganella express a class of membrane-bound LAADs9. Studies revealed that these membrane-bound LAADs could produce α-keto acids as siderophores to capture irons from the environment. Proteus is one of major pathogens causing infections in urinary tract (UTI) where the iron concentration is low10. Membrane-bound LAAD was suggested as an essential factor for Proteus survival since the knockout of LAAD was lethal for Proteus mirabilis 11,12. However, another study showed that the plasmid containing the LAAD gene was unable to restore the iron-limiting survival of a siderophore negative E. coli strain13, which put the physiological role of LAADs in debate. Despite this, these LAADs are gaining increasing interests for their potential applications on producing various α-keto acids in eco-friendly manners. Such as, phenylpyruvic acid14, α-Keto-γ-methylthiobutyric acid15, α-ketoglutaric acid16, α-keto isocaproate17 and pyruvate18 can be produced from their corresponding L-amino acids by either purified LAAD enzymes or the whole-cell biocatalysts overexpressing LAADs.
The bacterial membrane-bound LAADs encode a twin-arginine translocation (Tat) signal peptide at the N-termini, which exists in many extracellular flavin-containing proteins for their cross-membrane transportation19. Because of the sequence mutation at the cleavage site, the membrane-inserted signal peptide escapes from the peptidase cleavage after transportation and tethers LAADs onto the extracellular side of bacterial membrane15,20. When the transmembrane peptide was truncated, LAADs can still fold well in solution and present significant activity16. The activities can increase several folds when LAADs binding to bacterial membrane20. One possible reason is that the LAADs need other bacterial membrane proteins, such as cytochrome b, as the electron acceptor to re-oxidize the reduced cofactor FADH2 to FAD21. It is quite different from typical LAAOs/LAADs, which directly use O2 to re-oxidize the cofactor and generate H2O2 as the side product. Snake LAAOs were reported to inhibit bacterial growth by binding to bacterial surface and producing H2O2 locally22,23. The cofactor re-oxidization mechanism proposed for membrane-bound LAADs can also well explain why the LAADs do not inhibit the bacteria themselves. This proposed mechanism is supported by the following facts: 1) the H2O2 production has not been detected from Proteus myxofaciens LAAD (pmaLAAD) even peroxidase/catalase inhibitor NaN3 was added, and 2) the O2 consumption and α-keto acid production were almost equal for pmaLAAD even excess amount of catalase was added24. In contrast, because the catalase converts a H2O2 into one H2O and a half O2, the O2 consumption is half to the α-keto acid production in typical LAAOs/LAADs. Recently, purified pvLAAD was found to produce significant amount of H2O2 in vitro without bacterial membrane, suggesting LAADs could alternatively utilize O2, although less efficient, to re-oxidize FADH2 25.
The crystal structures of bacterial membrane-bound LAADs from Proteus myxofaciens (pmaLAAD) and Proteus vulgaris (pvLAAD) have been reported21,25. This opens a door to understand the catalytic mechanism of this class of enzymes and invent the improved biocatalysts26. A hydrophobic insertion sequence (INS) was discovered in both pvLAAD and pmaLAAD21,25. The deletion of INS in pvLAAD can have pvLAAD almost completely lose the catalytic activity. This implies that the INS plays an important role in catalytic process. The INS of pvLAAD (from Val321 to Met375) is located nearby the active site, and forms a hydrophobic module on protein surface. Structural analysis and liposome-binding assays suggested that the INS interacts with bacterial membrane25. However, it is not known whether membrane binding of INS plays a role in catalysis.
In the present study, we found that INS-mediated membrane-binding is responsible for membrane-caused catalytic activity enhancement and it also significantly changes the substrate preference. Our molecular dynamic (MD) simulations found that the INS is flexible before binding to membrane and tended to disturb the conformation of the substrate tunnel and catalytic center. Circular dichroism difference spectroscopy (CD) results indicate that the INS-membrane interaction stabilizes the INS structure. We propose that the structure stabilization is a mechanism to enhance the activity for bacterial membrane-bound LAADs.
Results
Binding with the lipid bilayer enhances the catalytic activity of pvLAAD
Membrane electron transfer chain-mediated cofactor re-oxidization is an important mechanism for bacterial membrane to enhance LAADs activities. To figure out whether it is the sole mechanism for the activity enhancement by membrane, we detected the catalytic activity of pvLAAD associated with the membrane whose electron transfer chain was blocked or not.
When membrane was added (2 mg/mL), 10 μM of purified full-length pvLAAD (FL-pvLAAD) produced 7655 ± 320 μM of PPA in 60 min, which was 9.3-fold higher than that of pvLAAD without membrane (820 ± 35 μM). Further increase of pvLAAD activity by adding more excess bacterial membrane was not significant (Supplementary Fig. S1). When 10 mM of sodium azide, an inhibitor of cytochrome oxidase of the electron transfer chain27, was added, the activity of pvLAAD with membrane was only slightly reduced (6382 ± 236 μM of PPA in 60 min), and still remained 7.8-fold higher than that of pvLAAD without membrane (Fig. 1a). This suggested that a mechanism other than the electron transfer chain-coupling is involved in membrane-caused activity enhancement of pvLAAD.
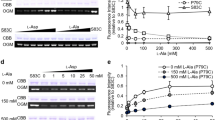
Membrane binding enhances pvLAAD activity. (a) Catalytic activities of pvLAAD with or without membrane presence were tested by measuring the transformation of L-phenylalaine to phenylpyruvic acids (PPA) in 1, 10 and 60 min. The activity of full-length pvLAAD with E. coli crude membrane was significantly enhanced compared to that without membrane, and the enhancement was only slightly reduced by adding the cytochrome oxidase inhibitor sodium azide. Pronase E-treated protein-free membrane, as well as the liposome, also holds the capability to enhance the enzymatic activity, although the fold change is smaller than crude membrane. The results here and later are all from three independent assays, and the error bars are SEM (standard error of the mean). The inset shows that almost all of the proteins on the E. coli crude membrane have been digested after 12 hr of treatment with 0.1 mg/mL pronase E at 37 °C. (b) The production of H2O2 was measured by HRP-coupled methods. Significant enhancement of H2O2 production in the reactions containing protein-free membrane and the liposome, indicating the O2 was used as the substrate by pvLAAD in the deamination of amino acids. H2O2 production has not been detected in the reactions with the crude membrane, which might be due to the strong catalase activity associated with crude membrane.
The crude extract of E. coli membrane contains a large amount of membrane integrating and association proteins, which may play roles in membrane-associated activity enhancement of pvLAAD. Pronase E was employed to treat the membrane. Protein electrophoresis showed that almost all of the membrane proteins have been digested after 12 h treatment with 0.1 mg/ml pronase E at 37 °C (the insert of Fig. 1a). This protein-free E. coli membrane still significantly increased the activity of pvLAAD (5.2 folds at 60 min). This result suggested that the membrane-caused activity enhancement did not completely rely on the functional membrane proteins. To further test this idea, liposomes without any functional proteins were constructed using E. coli lipids and incubated with pvLAADs. The PPA production was still increased to 2.9 folds (in 60 minutes). The smaller fold change by liposomes than E. coli membrane can be due to the incomplete reconstruction of liposomes from lipid molecules.
At the same time, the production of H2O2 was also quantified. Our results showed that the H2O2 production was significantly increased by adding protein-free E. coli membrane or liposomes, and the fold change was similar to that of the α-keto acid production (Fig. 1b). No H2O2 was observed when pvLAAD was incubated with crude membrane, which was due to the strong catalase activity associated with E.coli membrane28. In conclusion, our results suggested that both cell membrane and artificial lipid bilayer can significantly enhance the catalytic activity of pvLAAD, and O2 can be used to re-oxidize the cofactor FAD if the membrane electron transfer chain was blocked.
INS-membrane interaction is important for activity enhancement of pvLAAD
pvLAAD has two membrane-binding elements. The N-terminal transmembrane Tat peptide commonly exists in many membrane-bound or secreted proteins containing redox cofactors19,29. Its function is more likely for providing membrane binding affinity rather than catalytic activity regulation. Consistently, truncating the N-terminal Tat peptide (ΔN-pvLAAD, res.30–471) strongly reduces membrane binding of pvLAAD25,30, but does not affect the catalytic activity of pvLAAD in solution (Supplementary Fig. S2). The INS is an assistant membrane-binding element identified in membrane-bound LAADs25. It locates near the catalytic site and is involved in forming the substrate channel. pvLAAD without INS ((res.1–325)-GGSS-(res.375–471), ΔINS-pvLAAD) lost all of the catalytic activity, suggesting that the INS plays an critical role in the catalytic activity(Supplementary Fig. S2).
Previous structural analysis suggested that the INS binds to membrane through hydrophobic interactions25. The INS contains several surface-exposed hydrophobic residues, and seven of them (V321, F326, I345, L347, L351, I352 and F355, Fig. 2a) are conserved in LAADs among genera Proteus and Providencia bacteria (Supplementary Fig. S3)25. Mutating these residues were expected to block the hydrophobic interactions between the INS and membrane. Double mutations (L347A/L351A-ΔN-pvLAAD, I352A/F355A-ΔN-pvLAAD) and quintuple mutations (I345A, L347A, L351A, I352A and F355A, named as 5M-ΔN-pvLAAD) were generated (Supplementary Fig. S3), and their binding with membrane were tested. FL-pvLAAD was used as the positive control, and double-truncated ΔN-ΔINS-pvLAAD was used as a negative control. Because the N-terminal Tat peptide is the major membrane-binding site25, most ΔN-pvLAAD stayed in the supernatant and only a small amount of ΔN-pvLAAD co-pelleted with the E. coli membrane (Fig. 2b). Double mutations (L347A/L351A-ΔN-pvLAAD, I352A/F355A-ΔN-pvLAAD) did not further reduce the membrane binding of ΔN-pvLAAD. However, the mutations (5M-ΔN-pvLAAD) completely abolished the membrane binding (Fig. 2b). The catalytic activity of pvLAAD mutants in solution was also measured. All the pvLAAD mutants, except ΔN-ΔINS-pvLAAD, kept the similar catalytic activity compared to the wild-type FL-pvLAAD (Supplementary Fig. S4), suggesting that the quintuple mutations only blocked INS-membrane interaction but did not affect the catalytic activity of pvLAAD in solution.
The INS-membrane interactions enhance the enzymatic activities and modulate the substrate preference of pvLAAD. (a) The cartoon drawing of the INS (blue) of pvLAAD. Seven of the conserved surface-exposed hydrophobic residues are drawn as sticks (magenta). (b) Membrane binding of INS-mutated pvLAADs were detected by membrane co-pelleting assays. pvLAADs co-pelleting with membrane or staying in supernatant were analyzed with SDS-PAGE. FL-pvLAAD was used as the positive control, and the double-truncated ΔN-ΔINS-pvLAAD was used as the negative control. Although the N-terminal Tat peptide is the major membrane-binding site of pvLAAD, a small fraction of ΔN-pvLAAD still co-pelleted with the liposome through the INS-mediated membrane binding. Two double-mutated ΔN-pvLAAD proteins showed the similar membrane-binding capability to the wild-type ΔN-pvLAAD. But, co-pelleting of the quintuple-mutant ΔN-pvLAAD (5M-ΔN-pvLAAD) could not be observed, and all of the 5M-ΔN-pvLAAD protein stayed in the supernatant, indicating that the quintuple mutation completely abolished the membrane-binding capability of the INS. (c) Catalytic activities of FL-pvLAAD and 5M-FL-pvLAAD with or without membrane presence were tested at three different time points (1, 10 and 60 min). FL-pvLAAD and 5M-FL-pvLAAD gave the similar activity when in the solution, but activity enhancement by membrane binding could be observed only for wild-type FL-pvLAAD but not for 5M-FL-pvLAAD. (d,e) Catalytic activities of wild-type FL-pvLAAD and 5M-FL-pvLAAD against twenty proteinogenic L-amino acids were tested by measuring the α-keto acid production using chromogenic reaction with 2,4-dinitrophenylhydrazine (DNP). Membrane binding enhanced the activities of FL-pvLAAD for most of the twenty proteinogenic L-amino acids, but with different folds. The highest activities were observed for Asn, Asp, His, Leu, Met, Phe, Trp and Tyr when membrane was added, which are significantly different to the favorable substrates of pvLAAD without membrane. In contrast, no activity enhancement and substrate spectrum shift were observed for the 5M-FL-pvLAAD.
The N-terminal Tat peptide was added back to 5M-ΔN-pvLAAD to generate 5M-FL-pvLAAD, which could anchor to membrane through the N-terminal peptide. Without membrane, FL-pvLAAD and 5M-FL-pvLAAD exhibits the similar activity in solution. When E. coli protein-free membrane was added (2 mg/mL), the PPA production by FL-pvLAAD reached to 4325 ± 290 μM in 60 min, which was about 5.5-fold higher than that by FL-pvLAAD without membrane (780 ± 35 μM in 60 min). In contrast, no obvious activity increase were observed for 5M-FL-pvLAAD when E. coli membrane was added (803 ± 52 μM versus 962 ± 75 μM in 60 min), and the PPA production by 5M-FL-pvLAAD was similar to wild-type FL-pvLAAD in solution (Fig. 2c). The amount of H2O2 produced by pvLAADs were also detected after 60 min. When membrane was added, the significant increase of H2O2 production was only observed for wild-type FL-pvLAAD (about 4.7 folds) but not for 5M-FL-pvLAAD (Supplementary Fig. S5), which is similar to the situation of PPA production. These results indicate that the INS-mediated membrane-binding plays a major role in enhancing the catalytic activity of pvLAAD.
Membrane binding regulates pvLAAD substrate spectrum
To prove the membrane binding can affect the substrate preference of pvLAAD, all the 20 proteinogenic L-amino acids were tested with or without adding membrane for wild-type FL-pvLAAD and 5M-FL-pvLAAD. In solution, two proteins showed the same substrate preference that L-alanine, L-asparagine, L-leucine, L-methionine, L-phenylalanine, L-tryptophan, L-tyrosine and L-valine are the preferred substrates for both proteins (Fig. 2d and e). When bacterial membrane was added, the activities of FL-pvLAAD against L-asparagine, L-aspartate, L-histidine, L-leucine, L-methionine, L-phenylalanine, L-threonine, L-tryptophon and L-tyrosine were increased by 6.8–13.6 folds, and the activities against the L-arginine, L-isoleucine, L-lysine were increased by 1.6–3.3 folds. In contrast, no significant activity increase was observed against L-alanine, L-serine and L-valine (Fig. 2d). Other L-amino acids, such as L-cysteine, L-glutamate, L-glutamine, L-proline and glycine, are not preferred substrates of pvLAAD, although some activity enhancement by membrane binding could also been observed for these amino acids. Because of the different fold changes, the top substrates of FL-pvLAAD switched to L-asparagine, L-aspartate, L-histidine, L-leucine, L-methionine, L-phenylalanine, L-tryptophon and L-tyrosine when membrane was present, and L-alanine and L-valine were no longer the preferred substrates.
The substrate preference of 5M-FL-pvLAAD is similar to FL-pvLAAD in solution. They have the same activity for most of the twenty L-amino acids except L-isoleucine, which is more preferred by 5M-FL-pvLAAD (Fig. 2e). Although 5M-FL-pvLAAD can still bind to membrane through the N-terminal Tat peptide, however, Tat peptide-mediated membrane binding did not cause significant activity change to any of the twenty L-amino acids (Fig. 2e). Therefore, membrane did not affect the substrate preference for 5M-FL-pvLAAD. These results indicated that only the INS-mediated membrane binding could modulate the substrate preference of pvLAAD.
Structural flexibility of the INS
The crystal structures of pvLAAD and pmaLAAD have been determined21,25. Their overall structures are quite similar to each other. The root-mean-square derivation (RMSD) between two structures is 0.723 Å for 386 comparable Cα atoms. The INS in both structures consists of three α-helices and one β-strand, and their structural differences are mainly in the first α-helix and the following loop links to the β-strand, which are closed to the substrate channel. The B-factors of the residues in the first α-helix and the following loop are very high in both structures, implying a dynamic structure. MD simulations were conducted to study the dynamic conformations of the INS using the crystal structure of ΔN-pvLAAD (PDB code 5hxw) as the start conformation. The RMSD of the systems is reasonable and achieves equilibrium during the simulation, indicating that the force field and simulation protocol used here are adequate for the current systems (Fig. 3a). The structure of pvLAAD excluding the INS stayed stable during simulation. In contrast, the INS underwent dramatic conformational rearrangements, indicating the highly dynamic nature of the INS in the solution phase. The confirmations of the INS were clustered, and the representative structures for each cluster were depicted in Fig. 3b. In the INS, the first and second α-helices and the short β-strand were more dynamic compared to the third α-helix in all clusters. They showed large movement during simulations, and their secondary structure was destructed in some clusters. Because of their movement, the substrate channel was closed in cluster 1 (9.4% population), cluster 4 (29.2% population) and cluster 5 (28.1%), and was opened only in cluster 2 (26.9% population) and cluster 3 (6.4% population) (Fig. 3b).
Molecular dynamics simulations of pvLAAD. 160 ns of MD simulation was performed with AMBER12 program package using the crystal structure of ΔN-pvLAAD (PDB code 5hxw) as the start model. (a) The RMSD-based trajectory analysis showed that the overall structure of ΔN-pvLAAD (purple) was stable, but the INS was flexible. The conformations of the INS were clustered to five classes. (b) The representative structures of each cluster. The INS was colored blue and the rest part of pvLAAD was colored white. The INS showed large movement among different clusters. The substrate channel opened in cluster 2 (26.9% population) and cluster 3 (6.4% population), but tended to close in cluster 1, 4 and 5. (c) The catalytic pockets of the representative structures of each cluster. While key residues Y97, Q99, Q278, S313, G437 and W438 were stable during MD simulations, residue R315, which could interact with the carboxyl group of amino acid substrates, and residue I317, which could contact with the side chain of the substrate amino acids, deviate from their initial positions in catalytic site during MD simulations. The apo pvLAAD structure (PDB code 5hxw) was colored orange, and its complex with L-methionine was modeled24 and colored cyan.
At the catalytic center, most residues (such as Tyr97, Gln99, Gln278, Ser313 and Gly437) were stable during MD simulations (Fig. 3c). However, residues Arg315 and Ile317 are just a few residues before INS on the protein sequence, and the conformation rearrangement of the INS moved both residues a few angstroms away from their starting positions during the simulations (Fig. 3c). Arg315 is the key residue for stabilizing the carboxyl group of substrate amino acids shown by previous MD simulations of pvLAAD complexed with L-methionine and by enzyme-inhibitor cocrystal structure of pmaLAAD (Arg316 in pmaLAAD)21. The mutation of this residue could significantly reduce the catalytic activity of pvLAAD25. Ile317 is the key residue that interacts with the side chain of the substrate L-amino acids, and mutation of this residue changed the substrate spectrum of pvLAAD25. The structural instability of these key residues in catalytic center would reduce the substrate binding affinity and slow down the transformation rate of pvLAAD in the solution.
Membrane binding stabilizes the INS structure
The structural dynamics of the INS might impair the catalytic activity of pvLAAD in the solution, therefore, we wonder if the INS-membrane interaction can stabilize the INS structure. The circular dichroism (CD) spectra of FL-pvLAAD and 5M-FL-pvLAAD were recorded with or without liposome presence. The spectrum of liposome alone was also recorded and subtracted in calculation. The CD spectra of FM-FL-pvLAAD with and without liposome presence were similar and only a small shift was observed (Fig. 4a), indicating that the liposome binding of N-terminal Tat peptide only caused a small structural change. In contrast, a significant shift was observed between the two CD spectra of the wild-type FL-pvLAAD, suggesting that the INS-liposome interaction caused a significant secondary structure reorganization (Fig. 4a). The percentages of secondary structure components of FL-pvLAAD were calculated based on the CD spectra using K2D method31. The calculated secondary structure components of pvLAAD based on CD spectra were closed to those shown in the crystal structures (Supplementary Table S1). The results of FL-pvLAAD with liposome presence revealed that the random coil content decreased by 5% (from 45% to 40%), the β-sheet content increased by 2% (from 24% to 26%) and the α-helix content increased by 3% (from 31% to 34%). The increase of the secondary structure content indicated that the INS-membrane interaction stabilizes the structure of the INS.
Membrane binding stabilized the structure of pvLAAD. (a) Liposome-binding-caused secondary structure changes of the wild-type and mutated pvLAAD were detected by CD. The CD spectra of FL-pvLAAD and 5M-FL-pvLAAD were recorded with or without adding the liposome. The CD spectrum of the liposome was also recorded (dashed line), and was subtracted as background from the spectra of pvLAADs with the liposome. An obvious bathochromic shift was observed between the CD spectra in solution (Red line) and with liposome (Blue line) of FL-pvLAAD. However, such bathochromic shift was less significant for 5M-FL-pvLAAD, suggesting the secondary structure reorganization was mainly caused by the INS-liposome interaction. (b) Enzymatic kinetic parameters of FL-pvLAAD and 5M-FL-pvLAAD with or without membrane presence were measured. In comparison with pvLAAD in the solution, membrane enhanced the substrate binding affinity (K m ) for about 5.4 folds and substrate transformation rate (V max ) for about 2.6 folds for wild-type pvLAAD. The enzymatic kinetic parameters of 5M-FL-pvLAAD were quite similar to those of wild-type pvLAAD in the solution, and they were not changed by adding the membrane.
Then, we studied how the structural stabilization effect by INS-membrane interaction could improve the catalytic kinetics of FL-pvLAAD. The catalytic activities of FL-pvLAAD and 5M-FL-pvLAAD were measured with or without membrane (Fig. 4b). When a wild-type FL-pvLAAD was incubated with the membrane, the substrate affinity increased by about 5.3 folds (Km value was changed from 12.56 ± 3.19 mM to 2.34 ± 1.28 mM), and the maximal velocity of the reaction increased by about 2.6 folds (V max was changed from 0.25 ± 0.01 μmol min−1 mg−1 to 0.64 ± 0.02 μmol min−1 mg−1). In contrast, the membrane did not affect the activity of 5M-FL-pvLAAD. With or without membrane presence, the enzymatic kinetic parameters of mutated pvLAAD were similar to those of wild-type pvLAAD without membrane (Fig. 4b). These results proved that the membrane binding-mediated structural stabilization of the INS can enhance both the substrate binding affinity and transformation rate, while substrate binding was significantly enhanced.
Discussion
The LAADs from genera Proteus, Providencia and Morganella bacteria are different from the typical LAAOs/LAADs in two aspects: (1) these bacterial LAADs are membrane-bound enzymes and their activities are several folds higher when on membrane than in the solution; (2) these LAADs do not produce H2O2 during catalytic process. It was believed that the bacterial LAADs cannot efficiently transfer the electrons directly to O2 for cofactor re-oxidization. Instead, they transfer electrons to membrane electron transfer chain. This hypothesis could well explain why the membrane could enhance the activity of these bacterial LAADs, and also explain why these LAADs do not generate H2O2, a strong oxidant that may cause severe damage to bacterial membrane. However, this hypothesis could not explain the fact that the protein-free membrane could still significantly enhance the activity of LAADs in vitro. pvLAAD has two membrane binding sites. The N-terminal non-cleavable Tat signal peptide was the major membrane-binding site, but we found Tat peptide-mediated membrane binding could not increase the enzymatic activity. Because the central helix of Tat peptide is less hydrophobic, its binding to membrane is weaker than typical transmembrane helices32. We previously proposed that the INS of bacterial LAADs may serve as an assistant membrane-binding site to avoid the risk of dissociating from the cell membrane25.
In the current study, we found that the membrane-mediated structure stabilization is a new mechanism, which is independent to the mechanism of the electron transfer chain-coupled cofactor re-oxidization, for how the membrane binding enhance the catalytic activity of pvLAAD. Membrane binding-induced structure stabilization is involved in the enzymatic activation in many peripheral membrane-bound proteins, such as RNase E33. RNase E contains a conserved segment, which has the propensity to form an amphipathic α-helix to mediate membrane binding. This membrane binding stabilizes RNase E in its activate conformation, which leads to a stronger substrate binding affinity34. Another case is the E. coli pyruvate oxidase (EcPOX). When EcPOX stays in the cytosol, its C-terminal domain covers the active site and blocks the access of the substrate35. The membrane binding of the C-terminus stabilizes EcPOX at an open conformation, which makes its active site fully accessible to the substrate pyruvate and electron acceptor Q8 and increases the catalytic activity for about 30 folds35. pvLAAD and other membrane-bound LAADs are the new examples of membrane binding-induced enzyme activation. We propose that protein engineering which stabilize the INS-membrane interactions could help to maintain the active conformation of LAADs, therefore results in the biocatalyst with higher transformation activity.
The membrane-binding affinity of the INS is relatively weak. It is possible that the INS can switch between the membrane-association and membrane-disassociation states. This may make the enzyme switch between the fully active and partially active states. Based on the current data of bacterial membrane-bound LAADs, we suggest that the two membrane-binding elements have evolved two different roles. The N-terminal Tat peptide is responsible for the cross-membrane transportation of the LAAD and finally anchoring it to out-surface of bacterial membrane where the enzyme activity is required. The INS is in charge of the enzyme activation when the enzyme activity is needed (Supplementary Fig. S6). Both roles could be important for these LAADs to function properly. The further studies are needed to see if other membrane proteins could regulate the INS-membrane interaction and then regulate the enzymatic activity of the bacterial membrane-bound LAADs.
Methods
Protein expression and purification
The expression plasmids for FL-pvLAAD, ΔN-pvLAAD and ΔN-ΔINS-pvLAAD were constructed previously25. FL-pvLAAD and ΔN-pvLAAD plasmids containing I345A, L347A, L351A, I352A and F355A mutations were generated using QuikChange XL site-directed mutagenesis kit (Agilent Genomics) following the instruction manual and confirmed by DNA sequencing. The E. coli M15 (Qiagen) cells carrying pvLAAD plasmids were grown at 37 °C overnight in LB medium supplemented with ampicillin (100 μg/mL). The overnight culture was inoculated (1:100) into fresh LB and shaken at 37 °C until the culture reached an OD600 of about 0.5. Then protein expression was induced with 0.1 mM isopropyl-β-d-thio-galactoside (IPTG) for 16 h at 20 °C. The cells were harvested by centrifugation at 4000 rpm for 30 min at 4 °C and resuspended with binding buffer (500 mM NaCl, 50 mM Tris-HCl, pH 8.0, 20 mM imidazole, 5% glycerol). After sonication, cell lysates were centrifuged at 18000 rpm for 30 min, and the supernatants were loaded onto Ni-NTA columns (Qiagen) pre-equilibrated with the binding buffer. The Ni-NTA columns were washed with 20 column volumes of binding buffer to remove impurity, and then the target proteins were eluted with 30 mL of elution buffer (500 mM NaCl, 50 mM Tris-HCl pH 8.0, 200 mM imidazole, 5% glycerol). The pvLAAD proteins were further purified with size-exclusion chromatography (HiLoad 16/60 Superdex 200 pg, GE healthcare). The purified pvLAAD proteins were desalted and concentrated to about 30 mg/mL in storage buffer (50 mM NaCl, 5 mM Tris-HCl pH 8.0, 10% glycerol), and stored at −80 °C.
Liposome preparation
E. coli total lipid extracts were purchased from Avanti Polar Lipids, Inc. Firstly, 10 mg of lipids were dissolved in 1 mL of chloroform in a round bottom bottle. The solvent was evaporated under reduced pressure on a rotary evaporator. The sample was further dried under nitrogen gas for 2 hours, which resulted a thin lipid film at the bottom of the bottle. The lipid film was resuspended with 2.5 mL of membrane-binding buffer (50 mM NaCl, 5 mM Tris-HCl, pH 8.0) and shaken at 25 °C, 200 rpm for one hour. Then, the lipid suspension was sonicated (200 W, 6 mm microtip probe) for 15 minutes to produce a translucent liposome solution. The liposome solution was transferred to a new eppendorf tube and stored at 4 °C before use.
E. coli membrane preparation
The E. coli membrane was prepared using DH5α cells, which do not express any membrane-bound LAAO/LAAD. 500 mL of DH5α cells were grown overnight at 37 °C and harvested by centrifugation (4,000 rpm, 30 min, 4 °C). Cells were suspended in 20 mL of washing buffer (400 mM NaCl, 50 mM Tris-HCl, pH 8.0) on ice, and disrupted by sonicator (20 min, 200 W). Then cell lysate was centrifuged at 20,000 × g for 30 min to remove insoluble proteins and cell debris. The supernatant was collected and subjected to next centrifugation at 100,000 × g for 1 h at 4 °C. After dumped the supernatant, the membrane pellet was weighted and solved to a concentration of 4 mg/mL with buffer of 50 mM potassium phosphate pH 7.5. In order to remove the proteins integrated in or associated with membrane, the membrane solution was treated with pronase E (0.1 mg/mL) at 37 °C. Then the membrane was re-pelleted by centrifugation, and was washed with washing buffer twice to remove the residual pronase E. The membrane fraction was finally suspended in buffer, and the residual proteins were analyzed with 15% SDS/PAGE.
Membrane binding assay
Each pvLAAD protein (20 μM in final) was incubated with protein-free E. coli membrane (4 mg/mL in final) in 400 μL of membrane-binding buffer (50 mM NaCl, 5 mM Tris-HCl, pH 8.0) at 25 °C for 30 min. The membrane was pelleted by centrifugation using a Beckman 90 Ti rotor (30 min, 100,000 × g, 4 °C), and the supernatant containing the unbound proteins were collected into another tube. The membrane pellet was washed in membrane-binding buffer once and re-pelleted by centrifugation. The resulting pellets were resuspended in 200 μL buffer of 50 mM NaCl, 5 mM Tris-HCl, pH 8.0 and 5% SDS. Then, the pvLAAD proteins co-pelleted with membrane were analyzed using 15% SDS/PAGE. The supernatants during the membrane binding assays were also concentrated to 200 μL and analyzed by SDS/PAGE.
Catalytic activity measurement
L-phenylalanine, one of the best substrates for pvLAAD, was chosen to evaluate the catalytic activities of different pvLAAD truncations and mutants. The transformation of L-phenylalanine by pvLAADs was measured by detecting the production of phenylpyruvic acid (PPA), which has an absorption peak at 321 nm. In brief, L-phenylalanine was dissolved to the concentration 25 mM in 500 μL of reaction buffer (50 mM potassium phosphate, pH 7.5) with or without 2 mg/mL of E. coli membrane. The pvLAADs were added at the final concentration of 10 μM to start the reactions. Reactions were incubated at 25 °C, and at the three time points (1 min, 10 min and 60 min), aliquots of 100 μL of the reactions were transferred into a 96-wells plate containing 50 μL of 3 M NaOH. The absorption at 321 nm was measured using microplate reader (Flex Station 3, Molecular Devices). The reaction without adding pvLAAD enzyme was used as the blank control. For quantification, phenylpyruvic acid (Sigma-Aldrich) at different concentrations (100 μM, 500 μM, 1000 μM, 2500 μM, 5000 μM, 10000 μM) were used to calculate the standard curve.
Kinetic parameter determination
K m and V max values of the pvLAADs against L-phenylalanine were calculated by measuring the phenylpyruvate production rate at the increasing substrate concentrations (0.78125, 1.5625, 3.125, 6.25, 12.5, 25, 50, and 100 mM) in reaction buffer at 37 °C. The reaction initiated by addition of a final concentration of 5 μM of the pvLAADs. Reactions were incubated for 5 min and then stopped by adding 3 M NaOH (final concentration is 1 M). The absorption at 321 nm was measured for the reactions. The kinetic parameters Km and Vmax were calculate using the Lineweaver-Burk plotting36, which follows the Eq. (1):
where V is the reaction rate (the amount of PPA produced by 1 mg of the pvLAAD protein per min), Vmax is the maximum reaction rate, K m is the Michaelis constant (mM), and [S] is the concentration of L-phenylalanine (mM). Each reaction group was repeated three times, and the results were expressed as the mean ± standard deviation (n = 3).
Peroxide detection
Peroxide was determined by a method based on the commercial Amplex® Red Glutamic Acid/Glutamate Oxidase Assay Kit (Invitrogen). The reaction mixture contained 25 mM L-phenylalanine, 10 μM pvLAAD protein and with or without 2 mg/mL of E. coli membrane. Reactions were incubated at 37 °C for 60 min. Then, working solution (100 μM Amplex® Red reagent, 0.25 U/mL HRP) was added, and the reactions were incubated at 37 °C for 40 min. Then, fluorescence emission at 585 nm (excited at 571 nm) were recorded using a microplate reader (Flex Station 3, Molecular Devices). The reactions without pvLAAD protein were used as the blank control. The standard curve of H2O2 concentrations was generated using H2O2 samples at different concentrations (5000, 2000, 1000, 500, 250, 125, 62.5 μM).
Substrate selectivity measurement
10 μL of enzymes (200 μM) were mixed with 90 μL of one of the twenty native L-amino acids (10 mM) with or without E. coli membrane (2 mg/mL). Reactions were incubated at 25 °C for 60 min, and then 100 μL trichloroacetic acid (20%) were added to stop the reactions. After that, 40 μL 2,4-dinitrophenylhydrazine (DNP, 20 mM) was added to produce the corresponding 2,4-dinitrophenylhydrazone derivatives. After incubating at 37 °C for 15 min, 800 μL of NaOH (0.8 M) was added to stop the reactions. Additional 5 min incubation was applied at room temperature. Finally, the reactions were transferred into the plastic cuvettes and absorbance at 520 nm were measured. The concentrations of α-keto acid were calculated according to the standard curve generated by different concentrations (25, 100, 250, 500, 1000 and 2000 μM) of phenylpyruvate (Sigma-Aldrich).
Circular dichroism difference spectroscopy
CD spectra were recorded using a Chirascan with the 0.1 cm cuvettete (Hellma). Spectra for the liposome (2 mg/mL), pvLAADs (20 μM) and their mixture were collected in the wavelength range of 200–260 nm using the bandwidth of 1 nm and the resolution of 1 nm. Three parallel spectra for each sample were recorded and averaged. Spectrum for the buffer (50 mM NaCl, 5 mM Tris-HCl, pH 8.0) was collected as baseline and was subtracted during data process. Spectrum of the pvLAADs bound with the liposome was calculated by deducting the spectrum of the liposome sample from that of pvLAAD-liposome mixture. Data were expressed as mean residue ellipticity: [θ] (degree·cm2/dmol). The fractional percentage of the secondary structure was calculated by the Dichro-Web37.
Molecular dynamic simulations
Crystal structure of ΔN-pvLAAD (residues 30–471, PDB code 5hxw) was used as the initial model, and prepared using Molecule Operating Environment (MOE, Chemical Computing Group Inc.) package. The protonation states of charged residues were determined by H++ program38, and then the model was neutralized by adding Na+ or Cl− ions at protein surface using Amber39. The model was solvated into a box with a 10 Å buffer distance between the solvent box wall and the nearest solute atoms. The TIP3P model40 and Amber99SB force field41 were applied for water molecules and the proteins, respectively. The model was firstly minimized, and then gradually heated from 0 to 300 K over a period of 50 ps, followed by another 100 ps of isothermal-isobaric ensemble (NPT) MD simulations to relax the system density to about 1.0 g/cm3, with the target temperature of 300 K and the target pressure of 1.0 atm. Afterward, 160 ns of canonical ensemble (NVT) MD simulations with a target temperature of 300 K via employing the periodic boundary condition were performed to produce trajectories. RMSD-based clustering was performed with the program ptraj implemented in Amber12.
References
Guo, C., Liu, S., Yao, Y., Zhang, Q. & Sun, M. Z. Past decade study of snake venom L-amino acid oxidase. Toxicon 60, 302–311 (2012).
Samel, M. et al. L-Amino acid oxidase from Naja naja oxiana venom. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 149, 572–580 (2008).
Aitken, J. B. et al. Characterization of an L-amino acid oxidase in equine spermatozoa. Biol. Reprod. 92, 125 (2015).
Shen, Y., Fu, G. H., Liu, F. & Yue, G. H. Cloning and characterization of the gene for L-amino acid oxidase in hybrid tilapia. Mol. Biol. Rep. 42, 1593–1601 (2015).
Hahn, K. et al. Recombinant expression and characterization of a L-amino acid oxidase from the fungus Rhizoctonia solani. Appl. Microbiol. Biotechnol. 101, 2853–2864 (2016).
Hughes, A. L. Origin and diversification of the L-amino oxidase family in innate immune defenses of animals. Immunogenetics 62, 753–759 (2010).
Izidoro, L. F. et al. Snake venom L-amino acid oxidases: trends in pharmacology and biochemistry. Biomed. Res. Int. 2014, 196754 (2014).
Yu, Z. & Qiao, H. Advances in non-snake venom L-amino acid oxidase. Appl. Biochem. Biotechnol. 167, 1–13 (2012).
Takahashi, E., Ito, K. & Yoshimoto, T. Cloning of L-amino acid deaminase gene from Proteus vulgaris. Biosci. Biotechnol. Biochem. 63, 2244–2247 (1999).
Jacobsen, S. M., Stickler, D. J., Mobley, H. L. & Shirtliff, M. E. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21, 26–59 (2008).
Coker, C., Poore, C. A., Li, X. & Mobley, H. L. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes. Infect. 2, 1497–1505 (2000).
Massad, G., Zhao, H. & Mobley, H. L. Proteus mirabilis amino acid deaminase: cloning, nucleotide sequence, and characterization of aad. J. Bacteriol. 177, 5878–5883 (1995).
Burall, L. S. et al. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72, 2922–2938 (2004).
Hou, Y. et al. Production of phenylpyruvic acid from L-phenylalanine using an L-amino acid deaminase from Proteus mirabilis: comparison of enzymatic and whole-cell biotransformation approaches. Appl. Microbiol. Biotechnol. 99, 8391–8402 (2015).
Hossain, G. S. et al. Bioconversion of L-glutamic acid to alpha-ketoglutaric acid by an immobilized whole-cell biocatalyst expressing L-amino acid deaminase from Proteus mirabilis. J. Biotechnol. 169, 112–120 (2014).
Liu, L. et al. One-step production of alpha-ketoglutaric acid from glutamic acid with an engineered L-amino acid deaminase from Proteus mirabilis. J. Biotechnol. 164, 97–104 (2013).
Song, Y. et al. One-step biosynthesis of alpha-ketoisocaproate from L-leucine by an Escherichia coli whole-cell biocatalyst expressing an L-amino acid deaminase from Proteus vulgaris. Sci. Rep. 5, 12614 (2015).
Hossain, G. S. et al. Transporter engineering and enzyme evolution for pyruvate production from D/L-alanine with a whole-cell biocatalyst expressing L-amino acid deaminase from Proteus mirabilis. RSC Adv. 6, 82676–82684 (2016).
Berks, B. C. A common export pathway for proteins binding complex redox cofactors? Mol. Microbiol. 22, 393–404 (1996).
Baek, J. O. et al. Expression and characterization of a second L-amino acid deaminase isolated from Proteus mirabilis in Escherichia coli. J. Basic. Microbiol. 51, 129–135 (2011).
Motta, P., Molla, G., Pollegioni, L. & Nardini, M. Structure-function relationships in L-amino acid deaminase, a flavoprotein belonging to a novel class of biotechnologically relevant enzymes. J. Biol. Chem. 291, 10457–10475 (2016).
Toyama, M. H. et al. Isolation of a new L-amino acid oxidase from Crotalus durissus cascavella venom. Toxicon 47, 47–57 (2006).
Barbosa, P. S. et al. Renal and antibacterial effects induced by myotoxin I and II isolated from Bothrops jararacussu venom. Toxicon 46, 376–386 (2005).
Pantaleone, D. P., Geller, A. M. & Taylor, P. P. Purification and characterization of an L-amino acid deaminase used to prepare unnatural amino acids. J. Mol. Catal. B Enzym. 11, 795–803 (2001).
Ju, Y. et al. Crystal structure of a membrane-bound L-amino acid deaminase from Proteus vulgaris. J. Struct. Biol. 195, 306–315 (2016).
Li, R. et al. Rational molecular engineering of L-amino acid deaminase for production of α-ketoisovaleric acid from L-valine by Escherichia coli. RSC Adv. 7, 6615–6621 (2017).
Yoshikawa, S. & Orii, Y. The inhibition mechanism of the cytochrome oxidase reaction. II. Classification of inhibitors based on their modes of action. J. Biochem. 71, 859–872 (1972).
Mishra, S. & Imlay, J. Why do bacteria use so many enzymes to scavenge hydrogen peroxide? Arch. Biochem. Biophys. 525, 145–160 (2012).
Palmer, T. & Berks, B. C. The twin-arginine translocation (Tat) protein export pathway. Nat. Rev. Microbiol. 10, 483–496 (2012).
Zhang, C., Feng, J., Ding, S., Xu, Q. & Chen, N. Expression and characterization of recombinant L-amino acid deaminase of Proteus mirabilis isolated from acute pyelonephritis patients. J. Chem. Pharm. Res. 6, 2394 (2014).
Andrade, M. A., Chacon, P., Merelo, J. J. & Moran, F. Evaluation of secondary structure of proteins from UV circular dichroism spectra using an unsupervised learning neural network. Protein Eng. 6, 383–390 (1993).
Bachmann, J., Bauer, B., Zwicker, K., Ludwig, B. & Anderka, O. The Rieske protein from Paracoccus denitrificans is inserted into the cytoplasmic membrane by the twin-arginine translocase. FEBS J. 273, 4817–4830 (2006).
Khemici, V., Poljak, L., Luisi, B. F. & Carpousis, A. J. The RNase E of Escherichia coli is a membrane-binding protein. Mol. Microbiol. 70, 799–813 (2008).
Murashko, O. N., Kaberdin, V. R. & Lin-Chao, S. Membrane binding of Escherichia coli RNase E catalytic domain stabilizes protein structure and increases RNA substrate affinity. Proc. Natl. Acad. Sci. USA 109, 7019–7024 (2012).
Neumann, P., Weidner, A., Pech, A., Stubbs, M. T. & Tittmann, K. Structural basis for membrane binding and catalytic activation of the peripheral membrane enzyme pyruvate oxidase from Escherichia coli. Proc. Natl. Acad. Sci. USA 105, 17390–17395 (2008).
Lineweaver, H. & Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 56, 658–666 (1934).
Whitmore, L. & Wallace, B. A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic. Acids. Res. 32, W668–673 (2004).
Gordon, J. C. et al. H++: a server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic. Acids. Res. 33, W368–371 (2005).
Case, D. A. et al. The Amber biomolecular simulation programs. J. Comput. Chem. 26, 1668–1688 (2005).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Maier, J. A. et al. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory. Comput. 11, 3696–3713 (2015).
Acknowledgements
This work was supported by National Natural Science Foundation of China (81473138), Guangdong Frontier & Key Technology Innovation Program (2015B010109004) to J.X., National Natural Science Foundation of China (81603030) to Y.J., and by grants from Sun Yat-sen University to H.Z. (No. 36000–18811200).
Author information
Authors and Affiliations
Contributions
H.Z. and J.X. supervised this project, H.Z., J.X. and Y.J. designed the experiments, Y.J., Z.L., Z.Z and L.D. performed the experiments, H.Z., J.X. and Y.J. wrote the paper, Y.J., Z.L., Z.Z., L.D., Q.L., Q.G., C.Z., J.X. and H.Z. discussed the results and approved the final version.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ju, Y., Liu, Z., Zhang, Z. et al. Membrane binding of the insertion sequence of Proteus vulgaris L-amino acid deaminase stabilizes protein structure and increases catalytic activity. Sci Rep 7, 13719 (2017). https://doi.org/10.1038/s41598-017-14238-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14238-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.