Abstract
Mice deficient in the megakaryoblastic leukaemia 1 (Mkl1) gene experience less severe dextran sulphate sodium (DSS)-induced colitis, implying that Mkl1 plays a pathological role in inflammatory bowel disease (IBD). However, the contribution of Mkl1 to the development of colitis remains to be elucidated. The expression of Mkl1 is higher in the colonic lamina propria macrophages (LPMac) of DSS-treated mice than in those of control mice. Therefore, we established a transgenic mouse line that overexpresses human MKL1 (MKL1-Tg) specifically in cells of the monocyte/macrophage lineage, in order to investigate the potential role of macrophage MKL1 in the pathogenesis of colitis. MKL1-Tg mice displayed spontaneous colon shortening and rectal prolapse. Flow cytometric and quantitative RT-PCR analyses revealed that, in MKL1-Tg mice compared to littermate controls, the population of LPMac was decreased and had an altered inflammatory phenotype indicative of impaired anti-inflammatory properties, whereas bone marrow-derived macrophages from MKL1-Tg mice skewed towards M1 polarisation. In addition, MKL1-Tg mice had higher susceptibility to DSS-induced colitis than their littermate controls. These observations indicated that MKL1 crucially contributes to the development of colitis via the regulation of the function of macrophages, suggesting that it may be a potential therapeutic target for the prevention of IBD.
Similar content being viewed by others
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, is characterised by chronic, progressive inflammation of the gastrointestinal tract. IBD arises from immune dysfunction, in which aberrant immune responses evoke excessive inflammation1,2. It is widely accepted that both genetic and environmental factors are involved in the pathogenesis of IBD1,3; however, the precise aetiology is still not well understood.
Megakaryoblastic leukaemia 1 (MKL1), also called myocardin-related transcription factor A, is an actin-binding protein that shuttles between the cytoplasm and nucleus in response to Rho–Rock signalling4. In the nucleus, MKL1 mediates various cellular functions as a co-activator of serum response factor. For example, MKL1 plays a key role in the regulation of the proliferation and migration of vascular smooth muscle cells5. The functions of MKL1 in leukocytes have been intensively investigated. The loss of MKL1 in neutrophils results in the disruption of actin filaments and impaired phagocytosis, and a patient with homozygous nonsense mutations in MKL1 failed to efficiently clear bacteria6. In macrophages, MKL1 mediates the upregulation of pro-inflammatory cytokines in response to inflammatory stimuli7. Interestingly, murine experimental colitis is less severe in Mkl1-knockout mice7, suggesting the involvement of Mkl1 in IBD. Given that the immune system plays a central role in IBD, it is likely that MKL1 in leukocytes contributes to the development of colitis.
Macrophages constitute the most abundant population in the intestinal lamina propria. Through frequent interactions with the enteric microflora and crosstalk with the adaptive immune system, lamina propria macrophages (LPMac) govern the immune network, which is indispensable for gut defence and homeostasis8. Recent studies have indicated that LPMac derived from Ly6C+ blood monocytes are highly ‘educated’; despite being phagocytic, they are unresponsive to inflammatory stimuli and generally display anti-inflammatory properties8,9. Collapses of gut homeostasis are often associated with alternations in the inflammatory phenotypes of LPMac10,11, a hallmark of IBD.
In this study, we investigated the involvement of MKL1 in IBD. We found that the expression of Mkl1 was significantly elevated in LPMac, but not in whole colon tissue, in the setting of murine experimental colitis, compared to that in the steady state, suggesting an important role for MKL1 in macrophages in the development of IBD. We established a line of transgenic mice, which overexpress human MKL1 specifically in monocyte/macrophage lineage cells (MKL1-Tg). We found that LPMac from MKL1-Tg mice had a perturbed inflammatory phenotype, and that dextran sulphate sodium (DSS)-induced colitis in MKL1-Tg mice was fulminant compared to that in littermate controls. In addition, we found that MKL1 overexpression was associated with skewed macrophage polarisation, which may underlie the molecular pathogenesis of IBD in MKL1-Tg mice.
Results
Elevated expression of Mkl1 in colonic LPMac in experimental colitis
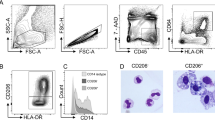
We analysed the phenotypes of colonic LPMac by flow cytometry, using antibodies against CD45, CD11b, CD11c, CD64, Ly6C, and MHC-II10,11. We observed that mice that received 3% DSS had a decreased proportion of LPMac accompanied by a significant increase in the proportion of lamina propria monocytes (LPMo) (Fig. 1A and Supplementary Fig. S1). We next investigated the expression of Mkl1 in colonic tissues. In the steady state, isolated LPMo and LPMac showed comparable expression of Mkl1 (Fig. 1B). However, LPMac, but not LPMo, had increased expression of Mkl1 in DSS-treated mice (Fig. 1B). In addition, the expression of Mkl1 was unchanged in the colons of control and DSS-treated mice (Supplementary Fig. S2). The upregulation of Mkl1 in LPMac was associated with decreased expression of Pparg (Supplementary Fig. S3). These observations suggested a potential role of macrophage Mkl1 in the pathogenesis of experimental colitis.
Perturbation of the LPMac phenotype was associated with upregulation of Mkl1 during experimental murine colitis. C57BL/6 J mice were treated with 3% DSS or control drinking water for 4 days. (A) Representative cytograms of Ly6C and MHC-II expression on CD45+CD11b+CD11clow to midCD64+ cells. We calculated the proportion (rate, %) of the cells in the red frames, designated LPMac. (B) The expression levels of Mkl1 in CD45+CD11b+CD11clow to mid CD64+Ly6C+ LPMo and CD45+CD11b+CD11clow to mid CD64+Ly6C—MHC-II+ LPMac were determined by quantitative RT-PCR. All experiments were repeated 5 times and data are shown as mean ± standard deviation (SD). *P < 0.05.
Establishment of MKL1-Tg mice
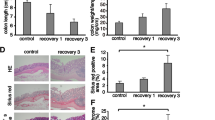
We established a transgenic mouse line that overexpresses human MKL1 specifically in monocytes/macrophages, driven by the human CD68 promoter element (Supplementary Fig. S4A and B). Compared with bone marrow monocytes, bone marrow-derived macrophages (BMDMs) from MKL1-Tg mice expressed higher levels of the transgene, MKL1 (Supplementary Fig. S4C). We found that MKL1-Tg mice had lower body weights than their non-Tg littermates, which reached statistical significance approximately 8 months after birth (Fig. 2A). At approximately 4 months of age, MKL1-Tg mice showed spontaneous shortening of the colon (Fig. 2B) and occasionally developed rectal prolapse (Fig. 2C). Histological analysis revealed scattered cryptitis in the colonic epithelial layer of MKL1-Tg mice (Fig. 2D). In addition, we observed infiltration of CD68+ cells into the colonic submucosal layer of MKL1-Tg mice (Fig. 2E).
Characterisation of MKL1-Tg mice. (A) Body weights of male MKL1-Tg mice and non-Tg littermates were measured monthly. (B) Representative photograph of whole colon from female MKL1-Tg mice and control littermates at 4 months after birth. Colon length was measured (n = 3 for each group). Data are shown as mean ± SD. *P < 0.05. (C) Representative photographs of the perianal region of male control and MKL1-Tg mice with rectal prolapse. (D) Representative histological image showing cryptitis (arrowheads) in H&E-stained colonic tissue of 8-month-old MKL1-Tg mice. (E) Representative immunohistochemical images showing CD68+ cells stained with rat anti-CD68 antibody and Alexa Fluor® 488-conjugated goat anti-rat IgG. Green cells represent CD68+ cells (arrowhead) infiltrating the colonic submucosal layer of MKL1-Tg mice.
LPMac from MKL1-Tg mice had aberrant inflammatory phenotypes
Flow cytometric analysis showed that MKL1-Tg mice had more LPMo and fewer LPMac than control littermates (Fig. 3A and Supplementary Fig. S5). To further investigate the perturbation of LPMac in MKL1-Tg mice, we isolated mRNA from LPMac and performed quantitative RT-PCR. We found that the expression of the tissue-resident macrophages markers Mrc1 and Cd163, and the receptors for the anti-inflammatory cytokines IL-10 and TGF-β, were lower in MKL1-Tg mice than in control littermates (Fig. 3B). In addition, the expression of Pparg was significantly decreased in LPMac from MKL1-Tg mice (Supplementary Fig. S6). Furthermore, cultured colonic lamina propria lymphocytes (LPLs) from MKL1-Tg mice produced more IFN-γ, IL-4, and IL-17 than those from non-Tg littermates (Fig. 3C).
MKL1 overexpression affected the inflammatory phenotype of LPMac. (A) Representative cytograms showing the Ly6C and MHC-II expression on CD45+CD11b+CD11clow to midCD64+ cells from 6-month-old MKL1-Tg and non-Tg littermates. We also calculated the proportions of LPMac. (B) The expression levels of Mrc1, Cd163, Il10ra, and Tgfbr2 in CD45+CD11b+CD11clow to midCD64+Ly6C—MHC-II+ LPMac were determined by quantitative RT-PCR. (C) The concentration of IFN-γ, IL-4, IL-17, and IL-10 in supernatants from LPLs were measured by ELISA. All experiments were repeated 3 times and data are shown as mean ± SD. *P < 0.05.
MKL1 orchestrated macrophage polarisation
Treatment with IFN-γ+ lipopolysaccharide (LPS) or IL-4 in vitro polarises macrophages into classically activated (M1) or alternatively activated (M2) types, respectively. It is known that the transcriptional activity of MKL1 is regulated by its nuclear localisation, which depends on actin dynamics4. We observed that, in BMDMs, expression of ectopic MKL1 was markedly increased in nuclei (Supplementary Fig. S7A); this was associated with increased formation of cytoplasmic filamentous actin (F-actin) (Supplementary Fig. S7B), and led to downregulation of Pparg (Supplementary Fig. 7C). Given that PPARγ regulates the polarisation of M2 macrophages12, we hypothesised that MKL1 overexpression would affect macrophage polarisation. Using flow cytometric analysis, we demonstrated that the expression of the M2 marker CD206 was suppressed both in vehicle-treated and IL-4-treated BMDMs from MKL1-Tg mice, compared to its expression in non-Tg controls (Fig. 4A and Supplementary Fig. S8). Quantitative RT-PCR analysis showed impaired expression of the M2 marker genes Mrc1, Arg1, Chi3l3, and Retnla, in M2-polarised BMDMs from MKL1-Tg mice (Fig. 4B). On the other hand, upon LPS stimulation, we detected significantly higher NF-κB p65 transcriptional activity in nuclear extracts of BMDMs from MKL1-Tg mice than in those from control mice (Supplementary Fig. 7D). Furthermore, expression of MKL1 promoted M1 polarisation, as indicated by increased expression of the pro-inflammatory genes Nos2, Il12b, Tnfa, and Il6 in MKL1-Tg mice (Fig. 4C).
MKL1 modulated macrophage polarisation. (A) BMDMs from MKL1-Tg mice and non-Tg littermates were treated with rIL-4 (20 ng/ml) for 24 hours. The expression of CD206 on CD11b+F4/80+ BMDMs (histograms) was evaluated by flow cytometry. We calculated and plotted the mean fluorescence intensity (MFI). BMDMs from MKL1-Tg mice and non-Tg littermates were either treated with rIL-4 (20 ng/ml), or rIFN-γ (100 ng/ml) + LPS (50 ng/ml) for 16 hours. The expression levels of (B) M2 markers Mrc1, Arg1, Chi3l3, and Retnla, and (C) M1 markers Nos2, Il12b, Tnfa, and Il6 were determined by quantitative RT-PCR. All experiments were repeated 3 times and data are shown as mean ± SD. *P < 0.05.
DSS-induced colitis is fulminant in MKL1-Tg mice
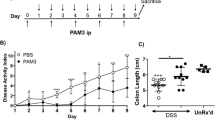
As MKL1-Tg mice did not show spontaneous inflammation in the colonic mucosa until 8 weeks of age (Supplementary Fig. S9), we examined their susceptibility to DSS-induced colitis at this age. Compared to control littermates, MKL1-Tg mice showed more prominent weight loss (Fig. 5A) and shortening of the colon (Fig. 5B) after administration of 3% DSS for 7 days. In addition, histopathological analyses indicated that lesions of immune infiltration and ulceration extended from the proximal to the distal region of the colon in MKL1-Tg mice, whereas control littermates showed similar, but relatively mild lesions, mainly in the distal colon (Fig. 5C). Detailed histological assessment revealed that MKL1-Tg mice experienced more damaging colitis, especially of the proximal colon, as evidenced by the massive infiltration and penetration of inflammatory cells into the colonic tissues, including the lamina propria, submucosa, and muscle layers, and the destruction of the epithelial structure, accompanied by ulceration (Fig. 5D). Notably, the LPMac compartment was further decreased in MKL1-Tg mice upon treatment with 3% DSS (Fig. 5E and Supplementary Fig. S10).
MKL1-Tg mice developed fulminant DSS-induced colitis. MKL1-Tg mice and non-Tg littermates (8 weeks old) received 3% DSS in their drinking water for 7 days. (A) Male body weights were measured on day 0, 1, 4, and 7, and are shown as the percentage of initial body weight. (B) Representative image of whole colons from female MKL1-Tg mice and control littermates. Colon length was measured (n = 3 for each group). (C) Representative histological images (low-power field) of Swiss-rolled whole colon stained with H&E. Regions specified with blue lines were affected by colitis. Positions of anuses are indicated by circles. Lesion area was measured and plotted (n = 5 for each group). (D) Representative histological images (high-power field) of the proximal region of the colon stained with H&E. We calculated the histological score (n = 5 for each group). (E) MKL1-Tg mice and non-Tg littermates received 3% DSS in the drinking water for 4 days. Representative cytograms showing Ly6C and MHC-II expression on the CD45+CD11b+CD11clow to mid CD64+ cells. We calculated the proportions of LPMac. Experiments were repeated 3 times. Data are shown as mean ± SD. *P < 0.05.
In order to evaluate the histopathological features of the recovery, rather than the acute, phase of DSS-induced colitis, we induced colitis by a modified protocol. We administered 3% DSS for 5 days, followed by regular water for another 3 days (Supplementary Fig. S11A). In the colitis recovery phase, MKL1-Tg mice still experienced more prominent colon shortening (Supplementary Fig. S11B). Immunohistochemical analysis revealed prominent B cell infiltration and/or follicle formation in the colonic mucosa of MKL1-Tg mice, compared to that in control littermates, during the recovery phase (Supplementary Fig. S11C).
Discussion
IBD is globally focused because of the difficulty in understanding its complex aetiology. Murine DSS-induced colitis is a classical model for studying the pathogenesis of IBD13, and Mkl1-knockout mice are known to develop DSS-induced colitis of mitigated severity7. We observed elevated expression of Mkl1 in LPMac, but not in LPMo or the colon tissue, in DSS-treated mice. Given that MKL1 is involved in the regulation of macrophage activation7, we hypothesised that MKL1 in LPMac could modulate intestinal inflammation and, hence, contribute to the development of IBD.
CD68, or macrosialin in the mouse, is one of the canonical molecular markers of macrophages. Expression of CD68 increases substantially during the differentiation of monocytes into macrophages14. In order to investigate the role of MKL1 in LPMac in vivo, we selected the CD68 promoter element, which was reported to effectively drive transgene expression in macrophages, but not in dendritic cells, lymphocytes, or granulocytes, in the colon15, to generate an MKL1-Tg line. The human MKL1 transgene was successfully overexpressed in macrophages from MKL1-Tg mice. The transgene was also expressed in monocytes. However, its expression was much higher in BMDMs, indicating that the CD68 promoter was largely activated during differentiation into macrophages. We observed that MKL1-Tg mice had a decreased proportion of colonic LPMac and a reciprocally increased LPMo population, compared to non-Tg controls. Perturbation of LPMac phenotypes has been reported to affect the function of effector T and/or regulatory T cells in murine colitis models11,16, and we detected increased production of IFN-γ, IL-4, and IL-17 by LPLs from MKL1-Tg mice compared to those from controls, indicating enhanced effector T cell responses.
Several molecules play key roles in maintaining the tolerogenic phenotype of LPMac. For example, IL-10 and TGF-β are major regulatory cytokines that shape gut homeostasis17,18,19,20, and expression of their receptors, Il10ra, Il10rb, and Tgfbr2, on macrophages is essential for anti-inflammatory signalling15,16,21. The alterations to LPMac phenotypes found in MKL1-Tg mice were likely due to a loss of their anti-inflammatory properties; LPMac from MKL1-Tg mice had lower expression of Il10ra and Tgfbr2 than those from controls.
Although the evidence suggested that MKL1-Tg mice would be colitogenic, MKL1-Tg mice did not show overt symptoms of colitis, other than rectal prolapse. Nevertheless, cryptitis and infiltration of CD68+ cells into the submucosal layer indicated a pro-inflammatory environment in the MKL1-Tg mice. These observations indicate that MKL1-Tg mice are predisposed to develop colitis. Indeed, when MKL1-Tg mice were subjected to a DSS-induced colitis model, they showed higher susceptibility than non-Tg controls. Of note, the proximal region of the colon, which is relatively resistant to DSS-induced damage, experienced worse histopathology in the MKL1-Tg mice.
B cell infiltration or follicle formation in the colonic mucosa is one of the particular histological features of the DSS-induced colitis model22; although it could also reflect a reaction against epithelial irritation or local infection in the colon caused by DSS treatment23. DSS-induced migration of B cells into the mucosa mainly occurs relatively late after treatment24. To avoid excessive ablation and disruption of the mucosal layer by continuous DSS treatment, we examined the histopathology of the colon during the recovery phase of DSS-treated mice23,24, and observed remarkable B cell infiltration and follicle formation in the colons of MKL1-Tg mice, suggesting that MKL1-Tg mice underwent fulminant colonic inflammation.
Consistent with a previous report, we found that MKL1 overexpression increased its nuclear localisation and promoted the formation of F-actin, both of which have been reported as prerequisite molecular events to suppress the expression of Pparg 25. The nuclear receptor PPARγ is mainly expressed in adipose tissue and the colon. PPARγ in macrophages plays a protective role against the development of inflammatory diseases, such as atherosclerosis, obesity, and colitis12,26,27. PPARγ is also a master regulator of macrophage M2 polarisation12. In this regard, we found that MKL1 overexpression blunted M2 macrophage polarisation. In addition, we found that, even without IL-4 treatment, BMDMs from MKL1-Tg mice had compromised CD206 expression, which is consistent with previous reports that M-CSF primes M2-prone macrophages28,29. One of the most important functions of MKL1 is to regulate the expression of pro-inflammatory cytokines, which it accomplishes by interacting with NF-κB p657. We found that, under pro-inflammatory conditions, MKL1 overexpression significantly enhanced the transcriptional activity of NF-κB p65 and boosted M1 macrophage polarisation. These observations indicate that MKL1 orchestrates macrophage M1/M2 polarisation through interactions with other transcription factors; our results support the hypothesis that M2 macrophages protect against DSS-induced and other forms of murine experimental colitis16,30.
In conclusion, our study revealed the impact of MKL1 in macrophages on the development of colitis. In particular, we found that MKL1 regulates the inflammatory properties of macrophages. Our observations suggest that MKL1 may be a potential therapeutic target for the treatment of IBD.
Methods
Animal experiments
C57BL/6 J mice were purchased from CLEA Japan. For the establishment of MKL1-Tg mice, a complementary DNA (cDNA) fragment encoding human MKL1 was tagged with a FLAG cDNA sequence at the 5′ end, then cloned downstream of a modified human CD68 promoter element (a kind gift from Peter. J. Murray, St. Jude Children’s Research Hospital). The designed expression cassette, containing the driving promoter, IVS1, the target gene, and the BGH polyA tail, was excised using SalI and DraIII, then injected into the male pronucleus of a C57BL/6 J egg, which was implanted into a pseudo-pregnant female recipient mouse. The transgenic offspring were genotyped by PCR. For the DSS-induced colitis model, mice (8–12 weeks) received 3% DSS (160110, M.W. = 36,000–50,000, MP Biomedicals), dissolved in drinking water, for specified periods, depending on the assay. All animal care and experimental procedures were in accordance with the guidelines for the Care and Use of Laboratory Animals published by the National Research Council (The National Academy Presses, 8th edition, 2011), and were subjected to prior approval by the local animal protection authority of the Tokyo Medical and Dental University (TMDU).
Histological and immunohistochemical analyses
We euthanised mice and excised theirs colons, which were cut open longitudinally and rinsed in 1 × PBS to remove faeces, then fixed in a Swiss roll shape in 10% Formalin Neutral Buffer Solution (Wako). Paraffin-embedded sections were stained with haematoxylin and eosin (H&E) for histological assessment. The disease severity was evaluated according to a previously described scoring system31. The total score was given by the sum of the scores for the proximal, middle, and distal regions of the colon. For immunohistochemical analysis, fresh colon tissues were embedded in OCT compound (Sakura Finetek). Frozen sections were fixed in 4% paraformaldehyde (PFA), blocked in Blocking One (Nacalai Tesque), and incubated with rat anti-CD68 antibody (FA-11, AbD Serotec) or rat anti-B220 antibody (RA3-6B2, BD Pharmingen), followed by incubation with Alexa Fluor® series secondary antibodies (Thermo Fisher Scientific). Samples were examined using a fluorescence microscope (Fluoview FV10i, Olympus).
LPMac isolation and flow cytometric analysis
We cut the colons of euthanised mice into small pieces, which were serially incubated in 1 × HBSS containing 5% foetal bovine serum (FBS) and 2 mM EDTA, and Digestion Buffer containing 1 × HBSS supplemented with 5% FBS, collagenase VIII (1.5 mg/ml; C2139, Sigma-Aldrich), and DNase I (40 μg/ml; DN25, Sigma-Aldrich). The released cells were subjected to density gradient separation with 40% and 80% Percoll solutions (GE Healthcare). The cells located between the upper and lower phases were collected and stained with APC-conjugated anti-CD45 (30-F11), PE-conjugated anti-CD11b (M1/70), PE/Cy7-conjugated anti-CD11c (N418), FITC-conjugated anti-CD64 (X54-5/7.1), PE/Dazzle™ 594-conjugated anti-Ly6C (HK1.4), and APC/Cy7-conjugated anti-MHC-II (M5/114.15.2). All antibodies were purchased from BioLegend. We used a Zombie UV Fixable Viability Kit (BioLegend) to determine cell viability. Flow cytometric analysis was performed on a MoFlo™ XDP (Beckman Coulter), and the data were analysed with FlowJo® ver. 10 (Tree Star). If necessary, the cells of interest were sorted. Flow cytometric analysis was performed in the Stem Cell Laboratory, Medical Research Institute, TMDU.
LPL isolation and ELISA
LPLs were isolated and cultured as previously described32. Harvested supernatants were subjected to ELISA for the determination of IFN-γ, IL-4, IL-10 (BD Biosciences), and IL-17 (R&D Systems) levels.
BMDM preparation and M1/M2 polarisation
Femoral and tibial bone marrow cells were harvested from mice, and subsequently cultured in RPMI-1640 medium (R8758, Sigma-Aldrich) supplemented with 10% FBS, sodium pyruvate (Thermo Fisher Scientific), MEM NEAA (Thermo Fisher Scientific), and recombinant (r) M-CSF (40 ng/ml, BioLegend) for 6 days, with medium changes every 2 days. The differentiated BMDMs were treated with rIFN-γ (100 ng/ml; BioLegend) + LPS (50 ng/ml; L5273, Sigma-Aldrich) or rIL-4 (20 ng/ml; BioLegend) for 16 to 24 hours to achieve M1 or M2 polarisation, respectively. In order to evaluate M2 polarisation, we stained BMDMs treated with rIL-4 with PE-conjugated anti-CD11b (M1/70, BioLegend,), FITC-conjugated anti-F4/80 (BM8, eBioscience), and Alexa Fluor® 647-conjugated anti-CD206 (MR5D3, AbD Serotec), and analysed on a FACSCalibur™ cytometer (BD Biosciences).
RNA isolation and quantitative RT-PCR
Cytoplasmic mRNA was isolated with a NucleoSpin® RNA Plus Kit (Takara Bio), according to the manufacturer’s instructions. We synthesised cDNA through a reverse transcription reaction using PrimeScript™ RT Reagent Kit (Takara Bio), which we then amplified with SYBR® Premix Ex Taq™ II (Takara Bio) on an iCycler iQ™ Real-Time PCR Detection System (Bio-Rad).
Statistical analysis
Statistical comparisons were performed using unpaired two-tailed Student’s t-tests or one-way analysis of variance with post hoc Tukey’s multiple comparison tests on GraphPad Prism 7 (GraphPad Software). The results were considered statistically significant when the p-value was less than 0.05.
References
Baumgart, D. C. & Carding, S. R. Inflammatory bowel disease: cause and immunobiology. Lancet (London, England) 369, 1627–1640, https://doi.org/10.1016/s0140-6736(07)60750-8 (2007).
Maloy, K. J. & Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298–306, https://doi.org/10.1038/nature10208 (2011).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434, https://doi.org/10.1038/nature06005 (2007).
Olson, E. N. & Nordheim, A. Linking actin dynamics and gene transcription to drive cellular motile functions. Nature reviews. Molecular cell biology 11, 353–365, https://doi.org/10.1038/nrm2890 (2010).
Pipes, G. C., Creemers, E. E. & Olson, E. N. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes & development 20, 1545–1556, https://doi.org/10.1101/gad.1428006 (2006).
Record, J. et al. Immunodeficiency and severe susceptibility to bacterial infection associated with a loss-of-function homozygous mutation of MKL1. Blood 126, 1527–1535, https://doi.org/10.1182/blood-2014-12-611012 (2015).
Yu, L. et al. MRTF-A mediates LPS-induced pro-inflammatory transcription by interacting with the COMPASS complex. J Cell Sci 127, 4645–4657, https://doi.org/10.1242/jcs.152314 (2014).
Bain, C. C. & Mowat, A. M. Macrophages in intestinal homeostasis and inflammation. Immunological reviews 260, 102–117, https://doi.org/10.1111/imr.12192 (2014).
Zigmond, E. & Jung, S. Intestinal macrophages: well educated exceptions from the rule. Trends Immunol 34, 162–168, https://doi.org/10.1016/j.it.2013.02.001 (2013).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6, 498–510, https://doi.org/10.1038/mi.2012.89 (2013).
Tamoutounour, S. et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol 42, 3150–3166, https://doi.org/10.1002/eji.201242847 (2012).
Odegaard, J. I. et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 447, 1116–1120, https://doi.org/10.1038/nature05894 (2007).
Wirtz, S., Neufert, C., Weigmann, B. & Neurath, M. F. Chemically induced mouse models of intestinal inflammation. Nature protocols 2, 541–546, https://doi.org/10.1038/nprot.2007.41 (2007).
Ramprasad, M. P., Terpstra, V., Kondratenko, N., Quehenberger, O. & Steinberg, D. Cell surface expression of mouse macrosialin and human CD68 and their role as macrophage receptors for oxidized low density lipoprotein. Proceedings of the National Academy of Sciences of the United States of America 93, 14833–14838 (1996).
Rani, R., Smulian, A. G., Greaves, D. R., Hogan, S. P. & Herbert, D. R. TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol 41, 2000–2009, https://doi.org/10.1002/eji.201041135 (2011).
Shouval, D. S. et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40, 706–719, https://doi.org/10.1016/j.immuni.2014.03.011 (2014).
Feagins, L. A. Role of transforming growth factor-beta in inflammatory bowel disease and colitis-associated colon cancer. Inflammatory bowel diseases 16, 1963–1968, https://doi.org/10.1002/ibd.21281 (2010).
Franke, A. et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nature genetics 40, 1319–1323, https://doi.org/10.1038/ng.221 (2008).
Glocker, E. O. et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. The New England journal of medicine 361, 2033–2045, https://doi.org/10.1056/NEJMoa0907206 (2009).
Kuhn, R., Lohler, J., Rennick, D., Rajewsky, K. & Muller, W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75, 263–274 (1993).
Zigmond, E. et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity 40, 720–733, https://doi.org/10.1016/j.immuni.2014.03.012 (2014).
Stevceva, L., Pavli, P., Husband, A. J. & Doe, W. F. The inflammatory infiltrate in the acute stage of the dextran sulphate sodium induced colitis: B cell response differs depending on the percentage of DSS used to induce it. BMC clinical pathology 1, 3 (2001).
Lochner, M. et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. The Journal of experimental medicine 208, 125–134, https://doi.org/10.1084/jem.20100052 (2011).
Perse, M. & Cerar, A. Dextran sodium sulphate colitis mouse model: traps and tricks. Journal of biomedicine & biotechnology 2012, 718617, https://doi.org/10.1155/2012/718617 (2012).
Nobusue, H. et al. Regulation of MKL1 via actin cytoskeleton dynamics drives adipocyte differentiation. Nat Commun 5, 3368, https://doi.org/10.1038/ncomms4368 (2014).
Babaev, V. R. et al. Conditional knockout of macrophage PPARgamma increases atherosclerosis in C57BL/6 and low-density lipoprotein receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology 25, 1647–1653, https://doi.org/10.1161/01.ATV.0000173413.31789.1a (2005).
Shah, Y. M., Morimura, K. & Gonzalez, F. J. Expression of peroxisome proliferator-activated receptor-gamma in macrophage suppresses experimentally induced colitis. Am J Physiol Gastrointest Liver Physiol 292, G657–666, https://doi.org/10.1152/ajpgi.00381.2006 (2007).
Fleetwood, A. J., Lawrence, T., Hamilton, J. A. & Cook, A. D. Granulocyte-Macrophage Colony-Stimulating Factor (CSF) and Macrophage CSF-Dependent Macrophage Phenotypes Display Differences in Cytokine Profiles and Transcription Factor Activities: Implications for CSF Blockade in Inflammation. The Journal of Immunology 178, 5245–5252, https://doi.org/10.4049/jimmunol.178.8.5245 (2007).
Martinez, F. O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional Profiling of the Human Monocyte-to-Macrophage Differentiation and Polarization: New Molecules and Patterns of Gene Expression. The Journal of Immunology 177, 7303–7311, https://doi.org/10.4049/jimmunol.177.10.7303 (2006).
Weisser, S. B., van Rooijen, N. & Sly, L. M. Depletion and reconstitution of macrophages in mice. Journal of visualized experiments: JoVE, 4105, https://doi.org/10.3791/4105 (2012).
Onizawa, M. et al. Signaling pathway via TNF-alpha/NF-kappaB in intestinal epithelial cells may be directly involved in colitis-associated carcinogenesis. Am J Physiol Gastrointest Liver Physiol 296, G850–859, https://doi.org/10.1152/ajpgi.00071.2008 (2009).
Nagaishi, T. et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity 25, 769–781, https://doi.org/10.1016/j.immuni.2006.08.026 (2006).
Acknowledgements
This work was supported, in part, by grants-in aid from the Ministry of Education, Culture, Sports, Science and Technology in Japan ([JSPS] KAKENHI Grants 25293181, 15K15095, and 16H05296), and a grant from The Japan China Medical Association. This work was also supported, in part, by TMDU Presiident’s Young Reseacher Award and the Nanken-Kyoten collaboration program (TMDU).
Author information
Authors and Affiliations
Contributions
J.A. and A.K. conceived and designed the experiments. J.A., T.N., T.W., and T.K.N. performed the experiments. J.A., T.N., and M.W. analysed the data. J.A. and A.K. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, J., Nagaishi, T., Watabe, T. et al. MKL1 expressed in macrophages contributes to the development of murine colitis. Sci Rep 7, 13650 (2017). https://doi.org/10.1038/s41598-017-13629-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13629-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.