Abstract
Retinopathy of prematurity (ROP) is a vascular disorder of the developing retina in preterm infants and is a leading cause of childhood blindness. Perinatal infection plays a pathogenic role in ROP. Probiotic supplementation reduces the risk of late onset sepsis (LOS) in preterm infants but it remains to be determined whether this reduction translates into a reduction of other complications. We conducted a systematic review and meta-analysis to evaluate the possible role of probiotics in altering the risk of ROP. Eleven randomized controlled trials (4250 infants; probiotics: 2121) were included in the meta-analysis that showed a significantly decreased rate of LOS with a risk ratio (RR) of 0.807 and a 95% confidence interval (CI) of 0.705 to 0.924 (P = 0.010; fixed effects model) but could not demonstrate a significant effect of probiotics on any stage ROP (RR 1.053, 95% CI 0.903 to 1.228, P = 0.508, 4 studies), or severe ROP (RR 0.841, 95% CI 0.666 to 1.063, P = 0.148, 9 studies). Meta-regression did not show any significant association between the RR for LOS and the RR for severe ROP. In conclusion, our results suggest that infection prevention by probiotics does not affect the risk of developing ROP in preterm infants.
Similar content being viewed by others
Introduction
Retinopathy of prematurity (ROP) is a vascular disorder of the developing retina in preterm infants and is a leading cause of childhood blindness1,2,3,4,5,6,7. ROP progresses in two phases. The first phase begins with delayed retinal vascular growth after birth and partial regression of existing vessels, followed by a second phase of hypoxia-induced pathological vessel growth8. Low gestational age (GA), low birth weight (BW), and supplemental oxygen therapy following delivery have consistently been associated with ROP1,2,3,4,5,6,7. However, ROP is a multifactorial disease and multiple other modifiable clinical factors have been associated with an increased risk of ROP. These include, among others, hypoxia, hypercapnia, hyperglycaemia, exposure to blood transfusions, poor postnatal weight gain, and perinatal infection/inflammation1,2,3,4,5,6,9,10,11,12,13,14. While prevention of ROP would be best aimed at reducing preterm birth, postnatal preventive efforts are directed at reducing the other stressors that may lead to injury of the developing retinal vessels15.
In a very recent systematic review, Fang et al. analysed the effectiveness of oxygen saturation targeting, nutritional interventions, blood transfusion management, and infection prevention on the incidence of ROP1. They found that lower oxygen saturation targets reduced the risk of developing any stage ROP and severe ROP but increased mortality. In addition, aggressive parenteral nutrition reduced the risk of any stage ROP but not severe ROP. Supplementation of vitamin A, E, or inositol and breast milk feeding were beneficial but the effect was not observed in randomized controlled trials (RCTs)1. Finally, when the authors analysed the literature on infection prevention and its effect on the incidence of ROP, they only included studies on fluconazole prophylaxis of invasive fungal infections because these studies were the only reporting sufficient numbers for quantitative analysis. Despite a reduced risk of invasive fungal infection, fluconazole prophylaxis had no significant effect on the risk of developing severe ROP1.
Experimental and clinical studies support the concept that modulation of intestinal microbiota in preterm infants may alter the risk of infection, and systemic inflammatory response syndrome either directly or through immune modulation16,17,18. Probiotic bacteria are live microbial supplements that colonize the gastrointestinal tract and potentially provide benefit to the host19,20,21. Recent meta-analyses showed that probiotic supplementation reduces the time to achieve full enteral feeding and the risk of developing necrotizing enterocolitis (NEC) as well as late onset sepsis (LOS) in preterm infants22,23,24,25,26,27,28,29,30,31,32,33,34. Therefore, probiotic supplementation can be considered as a method of infection prevention in this population. In addition, some probiotic strains may have antioxidant properties35. Interestingly, a number of RCTs of probiotic supplementation in preterm infants included data on ROP as secondary outcome. Nevertheless, systematic analysis regarding the possible effect of probiotics on preventing ROP is lacking. The objective of this systematic review and meta-analysis was to study the effect of probiotic supplementation in the incidence of ROP in preterm infants.
Methods
A protocol was developed prospectively that detailed the specific objectives, criteria for study selection, the approach to assessing study quality, clinical outcomes, and statistical methodology. The study is reported according to the PRISMA checklist36.
Data Sources and Search Strategies
A comprehensive literature search was undertaken using PubMed, EMBASE and CENTRAL (the Cochrane Central Register of Controlled Trials, The Cochrane Library) from their inception to March 1, 2016. The search terms used in the three databases were (probiotic(s) OR lactobacillus OR saccharomyces OR bifidobacterium OR streptococcus) AND (retinopathy of prematurity OR sepsis OR late onset sepsis). Language was not restricted. Additional strategies to identify studies included manual review of reference lists of key articles that fulfilled our eligibility criteria, use of the “related articles” feature in PubMed, use of the “cited by” tool in Google Scholar, and manual review of reference lists of meta-analyses on probiotics in preterm infants22,23,24,25,26,27,28,29,30,31,32,33,34,37,38.
Eligibility Criteria and Study Selection
Investigators were divided into two groups (E.V.-M./E.V. and G.C./L.F.). Both groups searched the literature independently and assessed the eligibility of trials for inclusion in the review. Disagreements were settled by discussion and, if necessary, the other author (F.M.) was consulted. All titles and abstracts of papers identified by the search strategy were screened for relevance. At this stage, only clearly non-relevant articles were excluded. Full copies of all potentially relevant papers were obtained and texts were screened to assess eligibility for inclusion. Studies were included if they were RCTs involving the use of probiotics in preterm infants (GA <37 weeks) and reported results on ROP. Studies were excluded if they did not meet all of these inclusion criteria. Studies were reviewed to ensure that study populations did not overlap by checking subject sources and study time-frame. Where two or more studies reported on the same population, the most recent study was preferentially used (provided it reported data on ROP) to avoid duplicate data.
Data Extraction and Assessment of Risk of Bias
The two groups of investigators extracted the data independently by using a data collection form designed for this review. Data extracted included: GA and BW of participants, patient inclusion criteria, study design (age at the first day of intervention, duration of intervention, dosage, and type of probiotic), and outcomes of interest. We defined ROP as any stage ROP or severe ROP (including stage 3 or 4, surgical, and threshold ROP). The number of cases of ROP and the number of patients analysed in each treatment group of each trial were entered into the form. Data on LOS were also extracted.
Two reviewers (G.C. and E.V.) independently assessed risk of bias in each trial by using the Cochrane “Risk of Bias Assessment Tool”39. For each domain (allocation sequence, allocation concealment, blinding of participants and outcome assessors, incomplete outcome data, selective outcome reporting, and other potential sources of bias) the risk was assessed as low, high, or unclear. Potential discrepancies during the data extraction process and assessment of risk of bias were resolved by discussion and consensus among all reviewers.
Statistical Analysis
Studies were combined and analysed using comprehensive meta-analysis V 3.0 software (Biostat Inc., Englewood, NJ, USA). A fixed-effects model (Mantel–Haenszel; M-H) was used. However, analysis using random effects model was also conducted to ensure that the results and conclusions were not influenced by the type of model used for the meta-analysis. Effect size was expressed as risk ratio (RR) and 95% confidence interval (CI). Statistical heterogeneity was assessed with the Cochran’s Q statistic and by the I 2 statistic, which is derived from Q and describes the proportion of total variation that is due to heterogeneity beyond chance40. An I 2 value of 0% indicates no observed between-study heterogeneity, and large values show increasing between-study heterogeneity. The risk of publication bias was assessed by visual inspection of the funnel plot and using Egger test. To identify any study that may have exerted a disproportionate influence on the summary effect, we deleted studies one at a time. To explore differences between studies that might be expected to influence the effect size, we performed univariate random-effects meta-regression (method of moments)41,42. A probability value of less than 0.05 (0.10 for heterogeneity) was considered statistically significant.
Results
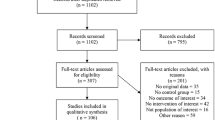
There was no substantial disagreement between the reviewers on articles for inclusion, data extraction, and risk of bias assessment. Based on the titles and abstracts of 1317 citations, we identified 58 potentially relevant studies, 11 of which met the inclusion criteria43,44,45,46,47,48,49,50,51,52,53 (Figure 1, Table 1). Two additional studies54,55 met the inclusion criteria but were excluded due to the presence of unclear or high risk of bias in more than three domains (Table 2). The main characteristics of the studies are shown in Table 1. The 11 studies included 4250 infants from which 2121 infants received probiotics. Nine studies included very preterm (GA <32 weeks) and/or very low BW (<1500 g) infants44,45,46,47,48,49,51,52,53. One study included extremely low BW preterm infants (<1000 g)43. One study included larger preterm infants (GA <37 weeks and BW <2500 g)50. The included studies randomized infants to different preparations, times of initiation and duration of therapy (Table 1). Of the 11 included studies, 1044,45,46,47,48,49,50,51,52,53 (91%) were judged to have low risk of bias for the domain of “random sequence generation,” and 744,45,46,47,48,50,51 (64%) were considered to have low risk of bias for “allocation concealment.” Details of the risk of bias analysis are depicted in Table 2. Additional data on percentage of cesarean section, use of antenatal corticosteroids, antenatal antibiotics, maternal infection, preterm rupture of membranes (PROM), and use of exclusive maternal milk are shown in Supplementary Table 1.
Although not clearly specified in the studies, it was assumed that data on ROP referred to the eye with the higher disease severity. Four studies43,45,49,50 reported data on any stage ROP and neither the individual studies nor the meta-analysis could detect a significant effect of probiotic supplementation (RR 1.053, 95% CI 0.903 to 1.228, P = 0.508, Fig. 2). The use of a random effects model instead of a fixed effect model did not significantly affect the results of the meta-analysis (RR 1.053, 95% CI 0.903 to 1.228, P = 0.508). In sensitivity analyses, excluding one study at a time, the summary RR ranged from 1.025 (95% CI 0.864–1.217, P = 0.774), when the study of Al Hosni et al.43 was excluded, to 1.206 (95% CI 0.932–1.562, P = 0.155), when the study of Costeloe et al.45 was excluded. The study of Roy et al.50 included larger infants than the other 3 studies (Table 1). However, when this study was excluded overall results were not substantially affected (RR 1.036, 95% CI 0.886–1.212, P = 0.658). Publication bias for the outcome of any stage ROP was not assessed due to the low number of studies.
Nine studies43,44,45,46,47,48,51,52,53 reported data on severe ROP and neither the individual studies nor the meta-analysis could detect a significant effect of probiotic supplementation (RR 0.841, 95% CI 0.666 to 1.063, P = 0.148, Fig. 3). The use of a random effects model instead of a fixed effect model did not significantly affect the results of the meta-analysis (RR 0.844, 95% CI 0.666 to 1.070, P = 0.160). Neither inspection of the funnel plot nor formal assessment using Egger’s test showed any evidence of publication bias in this analysis (Fig. 4). In sensitivity analyses, excluding one study at a time, the summary RR ranged from 0.808 (95% CI 0.634–1.029, P = 0.084), when the study of Al Hosni et al.43 was excluded, to 0.889 (95% CI 0.683–1.157, P = 0.380), when the study of Totsu et al.52 was excluded. In addition, exclusion of the analysis of the 3 studies43,52,53 with an unclear risk of allocation concealment bias did not substantially affect the results of the meta-analysis (RR 0.849, 95% CI 0.633–1.138, P = 0.273). Finally, exclusion of the study of Dilli et al.47, which reported a lower rate of ROP than the other studies, did not significantly affect the effect size (RR 0.849, 95% CI 0.633–1.138, P = 0.273).
All the included studies reported data on LOS and, when pooled, we observed a significant reduction of this outcome in the probiotics group (RR 0.807, 95% CI 0.705 to 0.924, P = 0.010). This significant reduction of LOS with probiotics was also observed when the 9 studies43,44,45,46,47,48,51,52,53 reporting on severe ROP were pooled (RR 0.809, 95% CI 0.692 to 0.946, P = 0.008). We performed meta-regression analyses (methods of moments) in order to investigate the possible correlation between the effect size for LOS and the effects size for severe ROP. As shown in Fig. 5, meta-regression could not detect a statistically significant correlation between the reduction in LOS produced by the probiotics and the effect size for severe ROP. In addition, meta-regression could not detect any significant effect of the type of probiotic on the effect size for severe ROP (Lactobacillus yes/no: coefficient −0.048, 95% CI −0.587 to 0.473, P = 0.856; Bifidobacterium yes/no: coefficient 0.019, 95% CI −0.519 to 0.557, P = 0.945; multi-strain products yes/no: coefficient 0.141, 95% CI −0.635 to 0.354, P = 0.577).
Other potential moderator variables tested by meta-regression were duration (in weeks) of the supplementation with probiotics (coefficient 0.040, 95% CI −0.079 to 0.158, P = 0.515), supplementation with probiotics ≤7 weeks (yes/no; coefficient −0.315, 95% CI −0.832 to 0.203, P = 0.233), mean GA of the studied population (coefficient per week: −0.181, 95% CI −0.447 to 0.087, P = 0.185), mean BW of the studied population (coefficient per 100 g: −0.154, 95% CI −0.478 to 0.170, P = 0.351), total number of included infants (coefficient per 100 infants 0.016, 95% CI −0.040 to 0.071, P = 0.583), publication year (coefficient 0.017, 95% CI −0.092 to 0.126, P = 0.754), percentage of cesarean section (coefficient −0.028, 95% CI −0.027 to 0.021, P = 0.816), percentage of PROM (coefficient −0.004, 95% CI −0.048 to 0.040, P = 0.855), percentage of use of antenatal corticosteroids (coefficient 0.006, 95% CI −0.008 to 0.020, P = 0.390), and percentage of use of antenatal antibiotics (coefficient 0.001, 95% CI −0.021 to 0.019, P = 0.929). Due to the low number of studies reporting on maternal infection and percentage of use of exclusive maternal milk (Supplementary Table 1), meta-regression for these two moderators was not performed.
Discussion
Probiotic supplementation in preterm infants is one of the most studied interventions in neonatal medicine16,19,26,29,30,31,32,33,34,56. However, to the best of our knowledge, this is the first meta-analysis assessing the effect of probiotics on the development of ROP. Despite a reduced risk of LOS, our study could not demonstrate any significant effect of probiotic supplementation on the risk of developing ROP. The validity of our meta-analysis is potentially compromised as the included trials were highly variable with regard to enrolment criteria (i.e., BW and GA), timing, dose, and formulation of probiotic used. Moreover, ROP was not the primary outcome in any of the trials and the definition of severe ROP varied among the studies. In addition, separate data on the population with the highest risk of ROP (i.e., extremely preterm or extremely low BW infants) could not be retrieved.
Increased susceptibility to infections in the preterm infant is due to functional defects of both innate and adoptive immunity combined with prolonged hospitalization, and frequent need for invasive procedures57,58. As reviewed by Lee & Dammann3, the effects of infection and inflammation in the pathogenesis of ROP may be direct, indirect, or both. Proinflammatory cytokines may exert a direct effect on retinal angiogenesis or sensitize the developing retina to the effects of oxygen, or other stressors. On the other hand, the circulatory instability and fluctuation of oxygen saturation following sepsis may affect the retinal perfusion and lead to increased retinal injury, particularly during the second phase of pathological vessel growth.
Despite the pathogenic role of sepsis in the development of ROP, there is very little literature published on reducing the risk of infection in preterm neonates and its effect on ROP incidence1. As mentioned in the introduction, only the effect of fungal infection prophylaxis with fluconazole has been systematically analyzed1. Our study is the largest meta-analysis to date investigating the effects of a strategy of infection reduction on the development of ROP. We expected that an intervention, such as probiotic supplementation, with proven efficacy in reducing LOS, would also affect the rate of ROP. In fact, when the 11 studies included in our meta-analysis were pooled, it was observed a reduction of the risk of LOS among the infants receiving probiotics (RR 0.81, 95% CI 0.71 to 0.92). Interestingly, this RR was very similar to the one reported by Rao et al. in their meta-analysis on probiotics and LOS (RR 0.86, 95% CI 0.78 to 0.94)26. It should be noted that the meta-analysis of Rao et al. included 37 RCTs (i.e., the 11 studies of the present meta-analysis plus 26 studies that did not report on ROP). Therefore, our sample of 11 studies appears to be representative of the effects of probiotics on LOS reduction in preterm infants.
We performed a meta-regression analysis in order to investigate whether the studies achieving a higher rate of protection against LOS also achieved a higher rate of protection against severe ROP. Meta-regression is a statistical technique which examines the relationship between continuous or categorical moderators and the size of effects observed in the studies41,42. Thus, meta-regression allows for the exploration of more complex questions than does traditional meta-analysis. In our study, meta-regression could not show any significant correlation between the RR for LOS and the RR for severe ROP. This suggests that the reduction in sepsis rate did not translate into a reduction of ROP. Nevertheless, it should be taken into account that a robust conclusion from meta-regression would require a larger number of included studies41,42.
Since probiotic supplementation in RCTs is not a homogeneous intervention, the choice of probiotic strain(s) is crucial and meta-analyses may be misleading with the risk that generalized conclusions are erroneously extrapolated to other probiotics16,59. Furthermore it is unclear whether multi-strain products are more effective than single strain products16. Separate meta-analyses of the effects of well-defined individual, single strain or multiple-strain probiotic preparations appears to be more appropriate but the important heterogeneity of the RCTs makes this approach unfeasible16. In the present study, meta-regression could not detect any significant effect of the type of probiotic (Lactobacillus, Bifidobacterium or multi-strain products) on the effect size for severe ROP. Of note is that the largest trial published so far45 was negative for all clinical outcomes, including ROP, but the study product (B breve BBG-001) had never been reported to have any clinical effect in neonates59. Therefore, more studies to address the optimal probiotic preparation, dosing, and duration of therapy are still needed in head to head comparative studies rather than placebo controlled trials29. These studies should compare strains that have been reported to be safe and effective in previous trials59.
It has been suggested that the type of milk (human or formula) that infants receive modifies the effect of probiotics60,61. Interestingly, in a recent meta-regression analysis, Thomas et al. showed that the effectiveness of probiotics in reducing NEC was higher in cohorts with increased rates of exclusive human milk61. Unfortunately, most studies included in the present analysis lacked information on type of feeding and, therefore, we were not able to stratify by type of milk feeding. Besides the use of human milk, other prenatal and postnatal factors such as maternal infections, delivery mode (vaginal delivery vs. cesarean section), or antibiotic use may shape the initial bacterial inoculum of the newborn and further configure the microbiome during early life62,63. This may interfere with the effects of probiotics. Thomas et al. showed, through meta-regression, that probiotics were more effective in preventing NEC in cohorts where lower proportions of mothers received antenatal corticosteroids61. In contrast, the rate of cesarean section did not significantly correlate with the effect of probiotics on NEC61. The present meta-regression analysis could not demonstrate a significant correlation between the rate of antenatal corticosteroids, antenatal antibiotics, PROM, or cesarean section and the effect of probiotics on ROP. However, as mentioned above, our meta-regression is limited by the low number of studies.
The issue of whether it is time to change practice and adopt the use of probiotics as a standard of care in preterm infants remains a hot topic for neonatologists. While some advocate a change in practice based on significant reduction in time to achieve full enteral feeding, severe NEC, LOS, and all-cause mortality17,19,26,29,38,61, others have raised concerns about the methodology of many of the published and advocate for waiting until further data on efficacy and safety in extremely preterm infants are available16,62,63,64,65. The evidence summarized in this meta-analysis suggests that, despite being effective in reducing LOS, probiotic supplementation does not affect the incidence of ROP in preterm infants. Nevertheless, further studies addressing this issue are needed to confirm our findings66,67,68.
References
Fang, J. L. et al. Interventions To Prevent Retinopathy of Prematurity: A Meta-analysis. Pediatrics 137, e20153387, https://doi.org/10.1542/peds.2015-3387 (2016).
Cavallaro, G. et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol 92, 2–20 (2014).
Lee, J. & Dammann, O. Perinatal infection, inflammation, and retinopathy of prematurity. Semin. Fetal Neonatal Med. 17, 26–29 (2012).
Hartnett, M. E. & Penn, J. S. Mechanisms and management of retinopathy of prematurity. N Engl J Med 367, 2515–2526 (2012).
Hartnett, M. E. & Lane, R. H. Effects of oxygen on the development and severity of retinopathy of prematurity. J. AAPOS. 17, 229–234 (2013).
Hartnett, M. E. Pathophysiology and mechanisms of severe retinopathy of prematurity. Ophthalmology 122, 200–210 (2015).
Soetikno, B. T. et al. Inner retinal oxygen metabolism in the 50/10 oxygen-induced retinopathy model. Sci. Rep. 5, 16752 (2015).
Chen, J. & Smith, L. E. Retinopathy of prematurity. Angiogenesis 10, 133–140 (2007).
Chen, M. et al. Infection, oxygen, and immaturity: interacting risk factors for retinopathy of prematurity. Neonatology 99, 125–132 (2011).
Hauspurg, A. K. et al. Blood gases and retinopathy of prematurity: the ELGAN Study. Neonatology 99, 104–111 (2011).
Hellstrom, A. et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics 123, e638–645 (2009).
Au, S. C., Tang, S. M., Rong, S. S., Chen, L. J. & Yam, J. C. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Sci. Rep. 5, 9091 (2015).
Chan, P. Y. et al. Association of Gestational Hypertensive Disorders with Retinopathy of prematurity: A Systematic Review and Meta-analysis. Sci. Rep. 6, 30732 (2016).
Uddin, M. I. et al. In Vivo Imaging of Retinal Hypoxia in a Model of Oxygen-Induced Retinopathy. Sci. Rep. 6, 31011 (2016).
Phelps, D. L., Lakatos, L. & Watts, J. L. D-Penicillamine for preventing retinopathy of prematurity in preterm infants. Cochrane Database Syst. Rev., CD001073 (2000).
Mihatsch, W. A. et al. Critical systematic review of the level of evidence for routine use of probiotics for reduction of mortality and prevention of necrotizing enterocolitis and sepsis in preterm infants. Clin. Nutr. 31, 6–15 (2012).
Zhou, P., Li, Y., Ma, L.-Y. & Lin, H.-C. The role of immunonutrients in the prevention of necrotizing enterocolitis in preterm very low birth weight infants. Nutrients 7, 7256–7270 (2015).
Hakansson, A. & Molin, G. Gut microbiota and inflammation. Nutrients 3, 637–682 (2011).
Ofek Shlomai, N., Deshpande, G., Rao, S. & Patole, S. Probiotics for preterm neonates: what will it take to change clinical practice? Neonatology 105, 64–70 (2013).
Hardy, H., Harris, J., Lyon, E., Beal, J. & Foey, A. D. Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutrients 5, 1869–1912 (2013).
Liu, Z., Liu, W., Ran, C., Hu, J. & Zhou, Z. Abrupt suspension of probiotics administration may increase host pathogen susceptibility by inducing gut dysbiosis. Sci. Rep. 6, 23214 (2016).
Agrawal, S., Rao, S. & Patole, S. Probiotic supplementation for preventing invasive fungal infections in preterm neonates–a systematic review and meta‐analysis. Mycoses 58, 642–651 (2015).
Deshpande, G., Rao, S., Patole, S. & Bulsara, M. Updated meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Pediatrics 125, 921–930 (2010).
Guthmann, F., Kluthe, C. & Bührer, C. Probiotics for prevention of necrotising enterocolitis: an updated meta-analysis. Klin. Padiatr. 222, 284–290 (2010).
Sawh, S. C., Deshpande, S., Jansen, S., Reynaert, C. J. & Jones, P. M. Prevention of necrotizing enterocolitis with probiotics: a systematic review and meta-analysis. PeerJ. 4, e2429 (2016).
Rao, S. C., Athalye-Jape, G. K., Deshpande, G. C., Simmer, K. N. & Patole, S. K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-analysis. Pediatrics 137, 1–16 (2016).
Baucells, B. J., Hally, M. M., Sánchez, A. T. Á. & Aloy, J. F. Probiotic associations in the prevention of necrotising enterocolitis and the reduction of late-onset sepsis and neonatal mortality in preterm infants under 1500g. A systematic review. An. Pediatr. 85, 247–255 (2016).
Billimoria, Z. C., Pandya, S., Bhatt, P. & Pandya, B. Probiotics—To Use, or Not to Use? An Updated Meta-analysis. Clin. Pediatr. (Phila). 55, 1242–1244 (2016).
AlFaleh, K. & Anabrees, J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid. Based Child Health. 2014 9, 584–671 (2014).
Lau, C. S. & Chamberlain, R. S. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: A meta-analysis. J. Pediatr. Surg. 50, 1405–1412 (2015).
Wang, Q., Dong, J. & Zhu, Y. Probiotic supplement reduces risk of necrotizing enterocolitis and mortality in preterm very low-birth-weight infants: an updated meta-analysis of 20 randomized, controlled trials. J. Pediatr. Surg. 47, 241–248 (2012).
Aceti, A. et al. Probiotics for prevention of necrotizing enterocolitis in preterm infants: systematic review and meta-analysis. Ital. J. Pediatr. 41, 89 (2015).
Olsen, R., Greisen, G., Schroder, M. & Brok, J. Prophylactic Probiotics for Preterm Infants: A Systematic Review and Meta-Analysis of Observational Studies. Neonatology 109, 105–112 (2016).
Zhang, G. Q., Hu, H. J., Liu, C. Y., Shakya, S. & Li, Z. Y. Probiotics for Preventing Late-Onset Sepsis in Preterm Neonates: A PRISMA-Compliant Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medicine (Baltimore) 95, e2581 (2016).
Mishra, V. et al. Probiotics as potential antioxidants: a systematic review. J. Agric. Food. Chem. 63, 3615–3626 (2015).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 339, b2535 (2009).
Athalye-Jape, G., Deshpande, G., Rao, S. & Patole, S. Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am. J. Clin. Nutr. 100, 1508–19 (2014).
Aceti, A. et al. Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis. Nutrients 8, 471 (2016).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 343, d5928 (2011).
Higgins, J. P. & Green, S. Cochrane handbook for systematic reviews of interventions. 2011. The Cochrane Collaboration 5 (2015).
Borenstein, M., Hedges, L. V., Higgins, J. & Rothstein, H. R. Meta‐Regression. Introduction to meta-analysis, 187-203 (2009).
Thompson, S. G. & Higgins, J. How should meta‐regression analyses be undertaken and interpreted? Stat. Med. 21, 1559–1573 (2002).
Al-Hosni, M. et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J. Perinatol. 32, 253–259 (2012).
Chou, I. C. et al. Lack of effects of oral probiotics on growth and neurodevelopmental outcomes in preterm very low birth weight infants. J. Pediatr. 156, 393–396 (2010).
Costeloe, K. et al. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 387, 649–660 (2016).
Demirel, G., Erdeve, O., Celik, I. H. & Dilmen, U. Saccharomyces boulardii for prevention of necrotizing enterocolitis in preterm infants: a randomized, controlled study. Acta Paediatr. 102, e560–565 (2013).
Dilli, D. et al. The propre-save study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J. Pediatr. 166, 545–551 e541 (2015).
Jacobs, S. E. et al. Probiotic effects on late-onset sepsis in very preterm infants: a randomized controlled trial. Pediatrics 132, 1055–1062 (2013).
Manzoni, P. et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin. Infect. Dis. 42, 1735–1742 (2006).
Roy, A., Chaudhuri, J., Sarkar, D., Ghosh, P. & Chakraborty, S. Role of Enteric Supplementation of Probiotics on Late-onset Sepsis by Candida species in Preterm Low Birth Weight Neonates: A Randomized, Double Blind, Placebo-controlled Trial. N. Am. J. Med. Sci. 6, 50–57 (2014).
Sari, F. N. et al. Do oral probiotics affect growth and neurodevelopmental outcomes in very low-birth-weight preterm infants? Am. J. Perinatol. 29, 579–586 (2012).
Totsu, S. et al. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr. Int. 56, 714–719 (2014).
Manzoni, P. et al. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 302, 1421–1428 (2009).
Fujii, T. et al. Bifidobacterium breve enhances transforming growth factor β1 signaling by regulating smad7 expression in preterm infants. J. Pediatr. Gastroenterol. Nutr. 43, 83–88 (2006).
Saengtawesin, V., Tangpolkaiwalsak, R. & Kanjanapattankul, W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J. Med. Assoc. Thai. 97, S20–25 (2014).
Li, D., Rosito, G. & Slagle, T. Probiotics for the prevention of necrotizing enterocolitis in neonates: an 8‐year retrospective cohort study. J. Clin. Pharm. Ther. 38, 445–449 (2013).
Higgins, R. D., Baker, C. J. & Raju, T. N. Executive summary of the workshop on infection in the high-risk infant. J. Perinatol. 30, 379–383 (2010).
Shah, J., Jefferies, A. L., Yoon, E. W., Lee, S. K. & Shah, P. S. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at <32 weeks’ gestation. Am. J. Perinatol. 32, 675–682 (2015).
Abrahamsson, T. R. Not all probiotic strains prevent necrotising enterocolitis in premature infants. Lancet 387, 624–625 (2016).
Samuels, N. et al. Necrotising enterocolitis and mortality in preterm infants after introduction of probiotics: a quasi-experimental study. Sci. Rep. 6, 31643 (2016).
Thomas, J. P., Raine, T., Reddy, S. & Belteki, G. Probiotics for the prevention of necrotizing enterocolitis in very‐low‐birth‐weight infants: A meta‐analysis and systematic review. Acta Paediatr (2017).
Tamburini, S., Shen, N., Wu, H. C. & Clemente, J. C. The microbiome in early life: implications for health outcomes. Nat Med 22, 713–722 (2016).
Dominguez-Bello, M. G. et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat Med 22, 250–253 (2016).
Janvier, A., Malo, J. & Barrington, K. J. Cohort study of probiotics in a North American neonatal intensive care unit. J. Pediatr. 164, 980–985 (2014).
Neu, J. Routine probiotics for premature infants: let’s be careful! J Pediatr. 158, 672–674 (2011).
Millar, M., Wilks, M., Fleming, P. & Costeloe, K. Should the use of probiotics in the preterm be routine? Arch. Dis. Child Fetal Neonatal Ed. 97, F70–F74 (2012).
Modi, N. Probiotics and necrotising enterocolitis: the devil (as always) is in the detail. Neonatology 105, 71–73 (2013).
Neu, J. Necrotizing enterocolitis: the mystery goes on. Neonatology 106, 289–295 (2014).
Author information
Authors and Affiliations
Contributions
G. Cavallaro collected data, contributed to statistical analysis and interpretation of results, and reviewed and revised the manuscript. E. Villamor-Martínez collected data, contributed to statistical analysis and interpretation of results, contributed to the drafting of the manuscript, and reviewed and revised the manuscript. L. Filippi and F. Mosca supervised data collection, contributed to interpretation of results, and reviewed and revised the manuscript. E. Villamor conceptualized and designed the study, supervised data collection, carried out statistical analyses, contributed to interpretation of results, and drafted the different versions of the manuscript. All authors approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cavallaro, G., Villamor-Martínez, E., Filippi, L. et al. Probiotic supplementation in preterm infants does not affect the risk of retinopathy of prematurity: a meta-analysis of randomized controlled trials. Sci Rep 7, 13014 (2017). https://doi.org/10.1038/s41598-017-13465-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13465-2
This article is cited by
-
Probiotics for Preterm Infants—Update 2024
Current Treatment Options in Pediatrics (2024)
-
Sex differences in the risk of retinopathy of prematurity: a systematic review, frequentist and Bayesian meta-analysis, and meta-regression
World Journal of Pediatrics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.