Abstract
Anthropogenic carbon dioxide (CO2) emissions and consequent ocean acidification (OA) are projected to have extensive consequences on marine calcifying organisms, including corals. While the effects of OA on coral calcification are well documented, the response of reproduction is still poorly understood since no information are reported for temperate corals. Here we investigate for the first time the influence of OA on sexual reproduction of the temperate azooxanthellate solitary scleractinian Leptopsammia pruvoti transplanted along a natural pCO2 gradient at a Mediterranean CO2 vent. After 3 months, future projection of pH levels did not influence the germ cell production, gametogenesis and embryogenesis in this azooxanthellate coral. These findings suggest that reproductive potential may be quite tolerant to decreasing pH, with implications for ecosystem function and services in a changing ocean.
Similar content being viewed by others
Introduction
Anthropogenic CO2 absorbed by the ocean is decreasing seawater pH and changing ocean chemistry, reducing the availability of carbonate ions (CO3 2−)1, the building blocks used by calcifying marine organisms, such as corals2. Seawater pH has already decreased by 0.1 pH units since the industrial revolution1,3 and if CO2 emissions continue under the “business-as-usual” scenario, oceanic pH levels will drop to 7.8 by the end of the century and may reach 7.4 units by 25001,3.
Several studies reveal how calcifying algae4,5, corals4,6,7 and coral reef communities, including fish8, show reduced calcification rates under low seawater pH, due to depleted carbonate saturation. Moreover, many biological processes and physiological functions independent of calcification may be impacted by decreasing pH9,10,11. Ocean acidification negatively influences the metabolism of marine invertebrates that have a low ability to compensate for disturbances to the extracellular ion and acid-base status, leading to negative effects on reproduction and behaviour12. Sexual reproduction represents a crucial process in the development and persistence of populations and its reduction threatens the resilience of species, leading to shifts in size and abundance of populations13. Early life history stages of echinoderms, mollusks and crustaceans including larval availability9,10,11,14,15,16,17,18,19 (gamete production, fertilization etc.), larval development9,10,11,14,15,16,20 and growth14,20, larval settlement9,20, post-settlement growth11 and survival11 seem to be very sensitive to increasing pCO2. The few available studies on the effects of ocean acidification on coral sexual reproduction were conducted in tropical species and only under laboratory conditions19,21,22,23,24, thus lacking information on the complex natural environment25.
Naturally acidified areas (i.e. CO2 vents) mimic future ocean conditions and can be used as natural laboratories to investigate the effects of increasing pCO2 on marine ecosystems in situ 6,26,27,28,29. The Panarea CO2 vent (Tyrrhenian Sea, Italy) is shallow, and characterized by almost pure CO2 emissions at ambient temperature, with no toxic compounds, making this an ideal site for ocean acidification studies in a natural setting28,29. The pCO2 gradient is characterized by extremely low pH values on the rim of the crater that exceed the most pessimistic scenarios for the coming century, representing a more distant scenario (25001,3) and by progressively higher values that match near future scenarios (2100) all the way to the periphery where normal pH conditions are re-established.
This study investigated for the first time at the Panarea CO2 vents the effects of future pH scenarios on coral reproductive efficiency. The model species is a solitary, azooxanthellate, gonochoric and brooding30 Mediterranean scleractinian, Leptopsammia pruvoti, Lacaze-Duthiers31 (Supplementary Fig. S1). A previous study on reproductive cycle of this species showed that its spermatogenesis follows an annual cycle, while the oogenesis is characterized by a 2-year cycle. From November to January the size of reproductive elements increases, fertilization takes place from January to April, and planulation occurs in May and June32.
Previous studies on L. pruvoti reported that sea surface temperature and solar radiation do not affect its skeletal bulk density33, skeletal porosity33, population abundance34 and structure stability35, net calcification rate36 and reproduction37 along an 850-km latitudinal gradient on the west coast of Italy. Specimens of L. pruvoti transplanted along the same pCO2 gradient as in the present study show a decrease in net calcification rates with decreasing pH, and a significant increase in polyp mortality rate, but only when average temperatures is high38.
Results
Gametogenetic polyps together with sexually inactive individuals (without germ cells), were found in both, the gonadal development and fertilization periods (Supplementary Table S1).
Size/frequency distribution of oocytes and maturation stage/frequency distribution of spermaries were significantly different between gonadal development period and fertilization period, in all sites (Kolmogorov-Smirnov test, oocyte: p < 0.001 for site 1 vs 1 and for site 3 vs 3, p = 0.002 for site 2 vs 2, p = 0.009 for site 4 vs 4, spermary: p < 0.001 for all sites; Fig. 1a,b).
Oocyte size and spermary maturation stage distributions. (a) Distribution of oocyte size during gonadal development (blue line) and fertilization (red line) periods. n = number of polyps/oocytes. (b) Distribution of the five maturation stages of spermaries during gonadal development (blue histogram bars) and fertilization (red histogram bars) periods. n = number of polyps/spermaries.
Gonadal development period
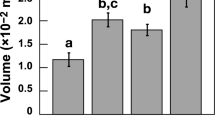
Oogenesis was characterized by a cohort of small oocytes in all sites, without difference in size/frequency distribution among sites (PERMANOVA, p = 0.293; Fig. 1a). Spermatogenesis showed small spermaries with the mode in developmental stage III at all sites and homogeneous distributions among sites (PERMANOVA, p = 0.443; Fig. 1b). Abundance, gonadal index and diameter of both, oocyte and spermary, did not show differences among sites (Kruskal-Wallis test with Monte Carlo estimate, oocyte abundance p = 0.486 and gonadal index p = 0.795, spermary abundance p = 0.152 and gonadal index p = 0.061; PERMANOVA, oocyte diameter p = 0.892 and spermary diameter p = 0.051; Tables 1 and 2; Fig. 2a,b
).
Oocyte and spermary reproductive parameters. Mean ± standard deviations of abundance, gonadal index and diameter of (a) oocytes and (b) spermaries in the gonadal development (blue dots) and fertilization (red dots) periods. Number of samples and mean values for each site and period are listed in Table 1 for oocytes and in Table 2 for spermaries.
Fertilization period
Most of the oocytes were smaller than 300 µm at all sites without differences in size/frequency distribution among sites (PERMANOVA, p = 0.676; Fig. 1a). Spermatogenesis was characterized by advanced maturation stages in all sites and no differences were found among sites (PERMANOVA, p = 0.379; Fig. 1b). Oocyte and spermary abundance, gonadal index and diameter were homogeneous among sites (Kruskal-Wallis test with Monte Carlo estimate, oocyte abundance p = 0.112 and gonadal index p = 0.267, spermary abundance p = 0.159 and gonadal index p = 0.176; PERMANOVA, oocyte diameter p = 0.192 and spermary diameter p = 0.521; Tables 1 and 2; Fig. 2a,b). During this period, female polyps containing embryos in the coelenteric cavity were found at all sites, and fertility, embryonal index and embryo diameter did not differ along the gradient (Kruskal-Wallis test with Monte Carlo estimate, p = 0.819 for fertility and p = 0.796 for embryonal index; PERMANOVA, p = 0.083 for embryo diameter; Table 3; Fig. 3
).
Embryo reproductive parameters. Mean ± standard deviations of fertility, embrional index and embryo diameter in the fertilization period. Samples number and mean values for each site are listed in Table 3.
Discussion
This is the first detailed study concerning the effect of high pCO2 on the reproductive output of corals transplanted along a natural pCO2 gradient.
Increasing pCO2 showed no effects on the production of male and female germ cells, as suggested by the oocyte and spermary abundances, which were homogenous along the gradient in both periods analyzed, and by the presence of embryos in female corals during the fertilization period at all sites.
During both, gonadal development and fertilization period, the development of male and female germ cells did not follow trends associated with increasing pCO2 (Fig. 1a,b), and all reproductive parameters of oocytes and spermaries seemed unrelated to decreasing pH, suggesting that this species could be quite tolerant to acidified conditions (Fig. 2a,b). These results are in agreement with previous studies performed in aquaria. After 6 months under pH levels projected for the end of this century, the tropical zooxanthellate coral Montipora capitata showed no effect on gametogenesis and gamete production22. The same result was found in Oculina patagonica after 12 months under similar pH conditions21. A negative effect was found in the sea urchin Strongylocentrotus droebachiensis, which after 60 days under high pCO2 conditions (average pH 6.98), shows a significant decrease in gonadal development17.
During the fertilization period, the detection of embryos at all sites suggested that the fertilization process had started at all sites. Moreover, no differences were found in the embryo production and embryo size, indicating that after three months under experimental conditions, also the fertilization process and embryogenesis of L. pruvoti were unaffected by future pCO2 levels.
Influence of decreasing pH on fertilization has been well studied through aquarium experiments in marine invertebrates, such as echinoderms, mollusks, crustaceans and tropical corals. Many experiments show negative responses, especially if sperm concentration is low and limited11,19,20,39. Fertilization success of the tropical coral Acropora palmata decreases with increasing pCO2, with a reduction (averaged across all sperm concentrations) of 13% at the CO2 level expected for the end of this century23. At the contrary, the fertilization process of A. tenuis and A. millepora is unaffected by elevated pCO2 projected for the end of this century, alone or in combination with temperature40, but only if sperm concentration is high41.
Moreover, experimental laboratory studies show that increasing pCO2 negatively affects embryo growth and development in mollusks9,14, echinoderms15,16,39,42 and crustaceans43, but not significant effect was detected on the timing of embryonic development in the tropical corals A. palmata under seawater pH levels expected for the end of this century (pH 7.744).
The solitary non-zooxanthellate L. pruvoti seemed quite tolerant to future pH levels, showing normal reproduction along the gradient. The lack of effects on gametogenesis and embryogenesis in L. pruvoti under decreasing pH may be explained in different ways.
The gametogenesis of L. pruvoti extends over the year, in fact, male germ cells takes approximately 12 months to mature while female germ cells need ~24 months32. Considering the short-term exposure to experimental conditions in this study (3 months), spermatogenesis was exposed to high pCO2 levels for a quarter of its time, while oogenesis was exposed for one eighth of its length, possibly explaining the low sensitivity observed with future pH levels.
Another explanation concern the channeling of available energy into reproduction at the expense of other metabolic processes. The high investment of energy that L. pruvoti places to keep the gametogenesis constant along the pH gradient may leave little energy for the coral to sustain net calcification in the face of ocean acidification45. Confirming this hypothesis, a parallel study on L. pruvoti transplanted along the same natural pCO2 gradient shows decreased net calcification rate with decreasing pH38, even under pH values projected for the end of this century. Thus, under future pH conditions, L. pruvoti may allocate more energy to maintain constant the reproduction process, at the expense of net calcification, which significantly decreases under acidified conditions.
In conclusion, the reproductive process of L. pruvoti seems that will be fine in coming decades, showing no effects on gametogenesis, spermatogenesis and embryogenesis along the pH gradient. However, this study did not consider the post-fertilization process, including settlement success, larval survival and development and juvenile growth, even if other studies have reported significant impact of ocean acidification on tropical corals.
This study considered the short-term effect of pH (~3 months), but further investigations are needed to understand if L. pruvoti is capable of maintaining a constant reproductive output, fertilization success and embryo growth under a long-term exposure to future pH levels.
Materials and Methods
Study site
The experimental site, which has been previously described in detail by Goffredo et al.28, is located near Panarea Island (Mediterranean Sea, Aeolian Archipelago, Italy, 38°38′16″N 15°06′37″E), where an underwater crater (20 × 14 m) at 10 m depth, generates a stable and continuous column of bubbles (98–99% CO2 28,29,46) at ambient temperature, creating a natural pH gradient. Along this gradient, four sampling sites were selected: the control site (site 1: mean Total Scale (TS) pHTS 8.07), located about 34 m away from the center of the crater, two intermediate pH sites (site 2 and 3: mean pHTS respectively 7.87 and 7.74), representing the intermediate and the most pessimistic IPCC scenario for the end of this century and the extreme pH site (site 4: mean pHTS 7.40), situated in proximity of the vents, representing the projection for 2500. The experimental site has stable hydrothermal–chemical properties and only pCO2 concentration differed significantly among the four sites (see Supplementary information of 38 for detailed seawater carbonate chemistry in each transplantation site).
Ethics statement
This study was carried out following the fundamental ethical principles. According to the European normative, there is no active conservation measure for the Mediterranean scleractinian species under study (L. pruvoti). The species is not protected in Italy, nor is it subject to any regulations. Thus, no permit was needed to sample specimens. For this study, sampling was limited strictly to the number necessary for the described analyses and performed where the species has high population density to minimize the impact of removing individuals and preserve both the demographic and genetic structure of the natural populations.
Sampling and field transplantation
Sexually mature L. pruvoti specimens (length > 3 mm, the size at sexual maturity of this species32) of similar size (average length 7 mm) were sampled at ~6 m depth by SCUBA diving in an area ~2 km away from the vent area and transplanted to the four sites in six experimental periods.
The same number of corals was randomly assigned to each of the four sites and each polyp was considered as a replicate (n = 4–6 polyps per site, per experimental period; Supplementary Table S1). Polyps were glued with a bicomponent epoxy coral glue (Milliput, Wales, UK) onto ceramic tiles and placed upside-down under plastic cages to mimic their natural orientation in overhangs and caves. Corals were exposed to experimental conditions during six different transplantation periods (September 2010–November 2010; November 2010–March 2011, March–June 2011, June–July 2011, July–December 2011 and April–June 2012) to identify the key moments and the seasonality of reproductive processes32. At the end of each transplantation period, samples were fixed in a formaldehyde fixative solution (10% formaldehyde and 90% seawater, saturated with calcium carbonate) to preserve the sample tissue for further histological analysis.
Biometric and cyto-histometric analyses
Polyp length (L, maximum axis of the oral disk), width (w, minimum axis of the oral disk), and height (h, oral-aboral diameter) were measured for each specimen with a pair of calipers (±0.05 mm). The body volume (V) was estimated using the equation:
Biometric measurements were used to calculate the reproductive parameters (see paragraph below).
Polyps were post-fixed in Bouin solution. After decalcification in EDTA and dehydration in a graded alcohol series from 80 to 100%, polyps were embedded in paraffin, and serial transverse sections were cut at 7 µm intervals along the oral-aboral axis, from the oral to the aboral poles. Tissues were stained with Mayer’s hematoxylin and eosin32.
Cytometric analyses were performed with a light microscope NIKON Eclipse 80i using an image analysis software: NIKON NIS-Elements D 3.1. The maximum and minimum diameters of oocytes in nucleated sections, and spermaries, classified in five maturation stages in accordance with previous studies on gametogenesis of this species30,32,37, were measured. The maximum and minimum diameters of embryos found in the gastrovascular cavity were measured.
Reproductive parameters
Reproductive output was defined by six parameters: 1) oocyte and spermary abundance, both defined as the number of reproductive elements per body volume unit (100 mm3); 2) gonadal index, defined as the percentage of body volume occupied by oocytes and spermaries; 3) reproductive element size, defined as the average of the maximum and minimum diameter of spermaries and oocytes in nucleated section; 4) fertility, defined as the number of embryos per body volume unit (100 mm3); 5) embryonal index, defined as the percentage of body volume occupied by embryos; 6) embryo size, defined as the average of the maximum and minimum diameter of embryos.
Based on the developing stages of oocytes and spermaries found in the analyzed samples and on the presence/absence of embryos in the coelenteric cavity of female polyps, samples from the six transplantation periods were grouped in two gonadal activity periods: 1) gonadal development period, characterized by the beginning of spermary and oocytes development, and 2) fertilization period, characterized by a little stock of big mature oocytes not yet fertilized, a bigger stock of oocytes that will be fertilized in the following reproductive year, advanced maturation stages of spermaries and presences of embryos in the coelenteric cavity of females.
Statistical analyses
Levene’s test was used to test homogeneity of variance and one-sample Kolmogorov-Smirnov’s test was used to test normality of data distribution. When the sample size was lower than 2000, the Shapiro-Wilk test was used. The two-sample Kolmogorov-Smirnov’s test was used to do a pairwise comparison of the distribution of oocyte size and spermary maturation stages between the two periods. The Monte Carlo method47 solves problems in the non-parametric test for small samples, estimating the P-value by taking a random sample from the reference set and studies its permutations48 (10,000 random permutations in this study). This method was used to estimate the significance of the Kruskal-Wallis test when comparing the reproductive parameters (oocyte and spermary abundance and gonadal index; fertility and embryonal index) among sites.
The analyses were computed using PASW Statistics 22.0.
A one-way permutation multivariate analysis of variance (PERMANOVA)49 based on Euclidean distances was performed with 999 permutation, to test differences in oocyte size distribution, spermary maturation stage distribution and in oocyte, spermary and embryo diameter among sites, using the software Primer 6 (Quest Research Limited).
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
References
Caldeira, K. & Wickett, M. E. Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J. Geophys. Res. Oceans (1978–2012). 110, C9 (2005).
Hoegh-Guldberg, O. et al. Coral Reefs under rapidclimate change and ocean acidification. Science. 318, 1737–1742 (2007).
Collins, M. & Knutti, R. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker, T. F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S. K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P. M.)(Cambridge Univ. Press, 2013).
Anthony, K. R., Kline, D. I., Diaz-Pulido, G., Dove, S. & Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 105, 17442–17446 (2008).
Kuffner, I. B., Andersson, A. J., Jokiel, P. L., Ku’ulei, S. R. & Mackenzie, F. T. Decreased abundance of crustose coralline algae due to ocean acidification. Nat. Geosci. 1, 114–117 (2008).
Rodolfo-Metalpa, R. et al. Coral and mollusc resistance to ocean acidification adversely affected by warming. Nature Clim. Change. 1, 308–312 (2011).
Strahl, J. et al. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 184, 179–186 (2015).
Langdon, C. et al. Effect of calcium carbonate saturation state on the calcification rate of an experimental coral reef. Global Biogeochem. Cy. 14, 639–654 (2000).
Kurihara, H. Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284 (2008).
Parker, L. M., Ross, P. M. & O’Connor, W. A. The effect of ocean acidification and temperature on the fertilization and embryonic development of the Sydney rock oyster Saccostrea glomerata (Gould 1850). Global Change Biol. 15, 2123–2136 (2009).
Byrne, M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Annu. Rev. 49, 1–42 (2011).
Pörtner, H. O. Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar. Ecol. Prog. Ser. 373, 203–217 (2008).
Roth, L., Koksal, S. & van Woesik, R. Effects of thermal stress on key processes driving coral population dynamics. Mar. Ecol. Prog. Ser. 411, 73–87 (2010).
Kurihara, H., Shimode, S. & Shirayama, Y. Sub-lethal effects of elevated concentration of CO2 on planktonic copepods and sea urchins. J. Oceanogr. 60, 743–750 (2004).
Kurihara, H. & Shirayama, Y. Effects of increased atmospheric CO2 on sea urchin early development. Mar. Ecol. Prog. Ser. 274, 161–169 (2004).
Kurihara, H. & Shirayama, Y. Effects of increased atmospheric CO2 and decreased pH on sea urchin embryos and gametes. In Echinoderms: München (eds Heinzeller & Nebelsick) 31-36 (Taylor & Francis Group, ISBN, 2004).
Siikavuopio, S. I., Mortensen, A., Dale, T. & Foss, A. Effect of carbon dioxide exposure on feed intake and gonad growth in green sea urchin, Strongylocentrotus droebachiensis. Acquaculture. 266, 97–101 (2007).
Morita, M. et al. Ocean acidification reduces sperm flagellar motility in broadcast spawning reef invertebrates. Zygote. 18, 103–107 (2009).
Reuter, K. E., Lotterhos, K. E., Crim, R. N., Thompson, C. A. & Harley, C. D. Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Global Change Biol. 17, 163–171 (2011).
Uthicke, S. et al. Impacts of ocean acidification on early life-history stages and settlement of the coral-eating sea star Acanthaster planci. PLoS ONE. 8, e82938 (2013).
Fine, M. & Tchernov, D. Scleractinian coral species survive and recover from decalcification. Science. 315, 1811 (2007).
Jokiel, P. L. et al. Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs. 27, 473–483 (2008).
Albright, R., Mason, B., Miller, M. & Langdon, C. Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc. Natl. Acad. Sci. USA 107, 20400–20404 (2010).
Albright, R. Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J. Mar. Biol. 2011 (2011).
Meron, D., Buia, M. C., Fine, M. & Banin, E. Changes in microbial communities associated with the sea anemone Anemonia viridis in a natural pH gradient. Microb. Ecol. 65, 269–276 (2013).
Hall-Spencer, J. M. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature. 454, 96–99 (2008).
Fabricius, K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim. Change 1, 165–169 (2011).
Goffredo, S. et al. Biomineralization control related to population density under ocean acidification. Nature Clim. Change. 4, 593–597 (2014).
Fantazzini, P. et al. Gains and losses of coral skeletal porosity changes with ocean acidification acclimation. Nat. Commun. 6, 7785 (2015).
Goffredo, S., Radetić, J., Airi, V. & Zaccanti, F. Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia, Dendrophylliidae) in the Mediterranean. 1. Morphological aspects of gametogenesis and ontogenesis. Mar. Biol. 147, 485–495 (2005).
Lacaze-Duthiers, H. Faune du Golfe du Lion. Coralliaires, Zooanthaires, Scle′roderme′s. Arch. Zool. Exp. Ge′n. 5, 1–249 (1897).
Goffredo, S., Airi, V., Radetić, J. & Zaccanti, F. Sexual reproduction of the solitary sunset cup coral Leptopsammia pruvoti (Scleractinia, Dendrophylliidae) in the Mediterranean. 2. Quantitative aspects of the annual reproductive cycle. Mar. Biol. 148, 923–932 (2006).
Caroselli, E. et al. Environmental implications of skeletal micro-density and porosity variation in two scleractinian corals. Zoology. 114, 255–264 (2011).
Caroselli, E. et al. Inferred calcification rate of a Mediterranean azooxanthellate coral is uncoupled with sea surface temperature along an 8° latitudinal gradient. Frontiers Zool. 9, 32 (2012).
Goffredo, S., Caroselli, E., Pignotti, E., Mattioli, G. & Zaccanti, F. Variation in biometry and population density of solitary corals with solar radiation and sea surface temperature in the Mediterranean Sea. Mar. Biol. 152, 351–361 (2007).
Caroselli, E. et al. Growth and demography of the solitary scleractinian coral Leptopsammia pruvoti along a sea surface temperature gradient in the Mediterranean Sea. PLoS ONE. 7, e37848 (2012).
Airi, V. et al. Reproductive output of a non-zooxanthellate temperate coral is unaffected by temperature along an extended latitudinal gradient. PLoS ONE. 12, e0171051 (2017).
Prada, F. et al. Ocean warming and acidification synergistically increase coral mortality. Sci. Rep. 7, 40842 (2017).
Gonzalez-Bernat, M. J., Lamare, M., Uthicke, S. & Byrne, M. Fertilisation, embryogenesis and larval development in the tropical intertidal sand dollar Arachnoides placenta in response to reduced seawater pH. Mar. Biol. 160, 1927–1941 (2013).
Chua, C. M., Leggat, W., Moya, A. & Baird, A. H. Temperature affects the early life history stages of corals more than near future ocean acidification. Mar. Ecol. Prog. Ser. 475, 85–92 (2013).
Albright, R. & Mason, B. Projected near-future levels of temperature and pCO2 reduce coral fertilization success. PLoS ONE. 8, e56468 (2013).
Kurihara, H., Yin, R., Nishihara, G. N., Soyano, K. & Ishimatsu, A. Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus. Aquatic Biol. 18, 281–292 (2013).
Arnold., K. E., Findlay, H. S., Spicer, J. I., Daniels, C. L. & Boothroyd, D. Effect of CO2-related acidification on aspects of the larval development of the European lobster, Homarus gammarus (L). Biogeosciences. 6, 1747–54 (2009).
Medina-Rosas, P., Szmant, A. M. & Whitehead, R. F. CO2 enrichment and reduced seawater pH had no effect on the embryonic development of Acropora palmata (Anthozoa, Scleractinia). Invertebr. Reprod. Dev. 57, 132–141 (2013).
Cohen, A. L. & Holcomb, M. Why corals care about ocean acidification: uncovering the mechanism. Oceanography. 22, 118–127 (2009).
Capaccioni, B., Tassi, F., Vaselli, O., Tedesco, D. & Poreda, R. Submarine gas burst at Panarea Island (southern Italy) on 3 November 2002: A magmatic versus hydrothermal episode. J. Geophys. Res. Solid Earth (1978–2012). 112, B5 (2007).
Gabriel, K. R. & Lachenbruch, P. A. Non-parametric ANOVA in small samples: a Monte Carlo study of the adequacy of the asymptotic approximation. Biometrics. 25, 593–6 (1969).
Senchaudhuri, P., Mehta, C. R. & Patel, N. R. Estimating exact pvalues by the method of control variates, or Monte Carlo rescue. J. Am. Stat. Assoc. 90, 640–8 (1995).
Anderson, M. J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 26, 32–46 (2001).
Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement n° [249930-CoralWarm: Corals and global warming: the Mediterranean versus the Red Sea]. The Scientific Diving School (www.sdseducational.org) gave logistical support for fieldwork. F. Sesso and B. Basile gave previous support for fieldwork on Panarea Island. The experiment complied with current Italian law.
Author information
Authors and Affiliations
Contributions
S.G., G.F. and Z.D. conceived the experiment; S.G., F.G., E.C. and F.P. performed the fieldwork; S.G., F.G., L.d.M., V.A., E.C. and F.P. analyzed the data; S.G., F.G., L.d.M., V.A., E.C. and F.P. wrote the manuscript; S.G. supervised the research. All authors discussed the results and participated to the scientific discussion.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gizzi, F., de Mas, L., Airi, V. et al. Reproduction of an azooxanthellate coral is unaffected by ocean acidification. Sci Rep 7, 13049 (2017). https://doi.org/10.1038/s41598-017-13393-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13393-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.