Abstract
Grain yield is a complicated trait, which is highly associated with biomass productivity. The cell wall is a central element of biomass, and its biogenesis contributes to plant architecture and development. However, the genetic link between cell-wall property and grain yield is largely unclear. Here, we report on identification of quantitative trait loci (QTLs) for grain yield-related traits and cell-wall composition with a set of chromosomal segment substitution lines (CSSLs) that were generated by using 9311, an indica cultivar as donor, and Nipponbare, a japonica cultivar as recipient. Nipponbare and 9311 showed significant differences in grain yield-related traits and cell-wall composition. Genotyping with molecular markers, 125 lines covering 95.6% of the whole genome of 9311 were employed for phenotypic and chemical examinations. Thirty-seven QTLs for grain yield-related traits and nineteen QTLs for cell-wall composition have been identified. In addition to correlation analysis, we found overlapped and closely linked QTLs for two sets of traits. Fine-mapping further narrowed a QTL for cellulose content together with HD17, a known QTL for heading date and grain yield, suggesting that plants may regulate cell wall biogenesis and grain yield via related means. Our study provided genetic clues for cloning QTLs for both complicated traits.
Similar content being viewed by others
Introduction
Cell walls, consisting of a complex polysaccharide network, encase plant cells and are characteristic plant cellular structures. These structures are enriched in cellulose, hemicelluloses and pectins, which represent the most abundant natural biopolymers on Earth1. Without cell walls, plant cells loose their shape, indicating fundamental roles on plant morphogenesis and development. Land plants generally possess at least 35 types of cells that are surrounded by different wall structure and properties compatible with their functions in various tissues and across developing stages.
It has been estimated that cell wall synthesis and integration involve more than 1000 genes2. Based on characterization of mutants that showed defects in plant growth and mechanical strength, multiple glycosyltransferases (GTs), glycosyl hydrolases (GHs), and other proteins have been found implicated in wall synthesis. It is well known that cellulose synthesis is catalyzed by cellulose synthase subunits belonging to GT2 family at the plasma membrane3,4. Non-cellulosic polysaccharides are synthesized by multiple cellulose synthase-like proteins (CSLs) and GTs, including GT8, GT43, GT47, and GT61 family members, etc5,6,7,8,9,10. Korrigan (KOR), a GH9 member, and Chitinases1 (CTL1), a GH18/19 protein, are also required for cellulose biosynthesis11,12. Besides GT and GH, other proteins, such as COBRA, affect cellulose synthesis and deposition in Arabidopsis and rice13,14. Enzymes involved in substrate synthesis and transportation are also needed for cell-wall biosynthesis15,16. Two classes of proteins, xyloglucan endotransglycosylases (XET) and expansins, modify the cell wall and integrate new polysaccharides during cell expansion17,18. As some components and newly synthesized polysaccharides need to be secreted to the plasma membrane and apoplast, proteins related to cytoskeleton dynamics and vesicle trafficking are required19,20,21,22. In addition to cell-wall metabolic processes, transcriptional regulation constitutes another level to modulate cell-wall biosynthesis23,24,25. Upstream of the transcriptional regulatory network, receptor-like kinases acting as sensors of internal and external stimuli are responsible for perception and transduction of signals to attune downstream processes26,27. Therefore, control of cell wall biogenesis requires the involvement of multiple metabolic and regulatory processes. Cell-wall composition is indeed a highly complex trait that possibly can be dissected as quantitative trait loci (QTL). QTLs for cell-wall properties, such as cell-wall digestibility, lignin content, and sugar composition, have been characterized in several plant species28,29,30. CSLF6 is the first cloned cell-wall QTL6. As characterization of cell-wall properties is laborious, the wall-related genes identified by QTL mapping are very limited.
Nevertheless, QTL cloning is a powerful approach for identifying major genes for agronomic complex traits, such as grain yield. Rice (Oryza sativa L.) is a major staple food crop feeding about three billion people, and the most important trait in rice production is grain yield. Gn1a, a major QTL controlling grain number, was cloned based on natural allelic variations31. DEP1, the locus that governs the panicle architecture and grain number was isolated by QTL positional cloning32. Grain size and grain weight are also key determinants for grain yield. Till now, some QTLs for grain size (GS) and grain weight (GW) control have been cloned. For example, GS3 encodes a transmembrane protein, GW2 encodes an E3 ligase, GW8 encodes SPL16, and Grain length3.1 (GL3.1) is a phosphatase, suggesting that cell-cycle or cell-size regulators are required for grain yield control33,34,35,36. Plant height (PH) and heading date (HD) are other determinants of grain yield. The most famous gene for plant height control is sd1, which greatly enhanced grain productivity by improving lodging resistance in the 1960s37. But the first cloned QTLs for plant height and heading date are PH1 and HD1 38,39, respectively. Moreover, these agronomic traits are often correlated. For example, GS2/GL2 that controls grain size also affects grain weight40,41. Ghd7, a QTL for HD, controls plant height and grain number42,43. In addition to these major loci, numerous minor loci also contribute to grain yield, which might be more useful in genetic breeding. Due to the small effects on genetic variability, the connection between genotype and phenotype is hard to bridge, which impedes cloning of the minor QTLs.
Metabolites that act as either causes or effects of complex traits may build a bridge between genotype and phenotype44. As the technical advances progress on high throughput examination of metabolites, comprehensive metabolic profiling and large-scale gene characterization by association mapping have been achieved45,46. Discovery of genetic links between metabolic and phenotypic variation becomes implementable. Correlation analysis of fruit metabolic loci and morphological genes for yield-associated traits in tomato introgression lines has revealed the links between nutritional metabolites and organoleptic traits47. By using the same approach, lignin precursors identified by genome wide association study (GWAS) are correlated with plant height45. A major locus controlling the trigonelline level identified by metabolic GWAS in 489 rice varieties is identical to a QTL for grain width48. Recently, two loci (Lin5 and SSC11.1) regulating sugar content and tomato flavor were found negatively correlated to fruit size49. Therefore, discovery of the metabotype-phenotype linkage is a useful approach for interactive functional genomics, especially for cloning minor QTLs for complex traits.
Cell wall is the central element of biomass. It is well known that extended heading date and increased plant height lead to more biomass and consequently promote grain yields. Hence, cell wall synthesis may be a valuable metabolic marker associated with grain yields. However, genetic links between cell-wall composition and grain yield-related traits are unclear.
Here we report on generation of a chromosome segment substitution lines (CSSL) by using Nipponbare (NP), a representative cultivar for japonica, as recipient parent and 9311, a representative cultivar for indica, as donor parent. Based on the variations between the two rice subspecies indica and japonica, parallel studies on six grain yield-related traits and cell-wall composition were performed in the CSSLs, and the corresponded QTLs for these traits were mapped. The overlapped QTLs for the two sets of traits have been identified. Fine-mapping of a QTL for cellulose content provided further evidence for the genetic connection between both sets of traits. Our study thus sheds lights on dissecting the machinery on cell-wall biosynthesis and grain-yield improvement in rice.
Results
Analysis of phenotypic variations between NP and 9311
Among the morphological and physiological differences between NP and 9311, biomass abundance is the fundamental one. Indica variety 9311 generally has more biomass than japonica variety NP, which is in agreement with that 9311 has increased heading date, indicating as a longer vegetative growth period, increased plant height, larger panicles, and more grains than NP (Fig. 1A and B). Another morphological difference between 9311 and NP is the grain shape. Variety 9311 has long and slender grains whereas those of NP are short and wide (Fig. 1C and D). Hence, the ratio of grain length to width is significantly increased in 9311, resulting in increased thousand-grain weight compared to NP (Fig. 1D). To address this difference at a cellular level, we investigated cell shape on glumes by scanning electronic microscopy. Surprisingly, cells in 9311 glumes were shorter in length but wider than those in NP (Fig. 1E and F). Quantification of cell numbers in glumes showed that 9311 had more cells at the longitudinal direction, but fewer cells at the latitudinal direction (Fig. 1F). Therefore, all these differences are likely derived from varied ability of biomass productivity between NP and 9311.
Phenotypic difference between NP and 9311. (A) The mature plants of NP and 9311. (B) Quantification of the morphological phenotypes in NP and 9311. Error bars represent the mean value ± SD (n ≥ 9 plants). (C) Grain shape of NP and 9311. (D) Measurement of the grain size and grain weight of NP and 9311. Error bars represent the mean value ± SD (n ≥ 50 grains harvested from at least 10 plants). Error bars of thousand-grain weight is the mean value ± SD (n = 3 replicates). (E) Scanning electron microscopic photographs of the outer surface of a glume. The right panels are the enlarged view circled by the rectangles. (F) Quantification of cell length, cell width, and cell number at the longitudinal and latitudinal direction in glume. Error bars represent the mean value ± SD (n ≥ 500 cells from 12 seeds). ***P < 0.001, **P < 0.01, and *P < 0.05 by two-tailed t-test. Bar = 15 cm in (A), 1 cm in (C), and 1 mm for left panel and 250 μm for right panel in (E).
NP and 9311 have different cell-wall composition
As cell walls represent the major element of plant biomass, we investigated whether variations on cell-wall composition exist in the two varieties. Because internodes constitute a central source of rice biomass and contribute directly to plant height and grain traits by linking source and sink, internodes were subjected to cell-wall composition examination. The content of cellulose and xylose, the two major components of cell wall, was significantly increased in 9311 (Fig. 2A). In contrast, galactose and glucose that are often present in hemicellulose or amorphous cellulose were reduced in 9311 (Fig. 2A). As arabinosyl residues (Ara) present as side chains on xylans in rice, the ratio of xylose to arabinose (XA) reflects the xylan substitution level. Although the arabinose content was not significantly changed, more xylose content caused higher XA ratios in 9311 (Fig. 2B), indicating that 9311 has lower arabinosyl substitution on xylan compared to NP. Due to a very low level of pectin presenting in rice cell walls, the pectic sugars, rhamnose and fucose, were hard to be detected (Fig. 2C). Therefore, NP and 9311 have different cell-wall composition in the internodes.
Measurement of the cell-wall composition in NP and 9311. (A) The content of major neutral sugars and crystalline cellulose in wall residues from internodes. (B) The ratio of xylose and arabinose content. (C) The content of minor neutral sugars in wall residues from internodes. Error bars represent the means ± SD (n = 4 replicates using AIR from 10 mature plants. **P < 0.01 and *P < 0.05 by two-tailed t-test.
Construction of CSSLs
To identify QTL for cell-wall composition and grain yield, we constructed a CSSL population by backcrossing the previously reported 57 CSSLs50 with NP and followed by self-crossing. With the aid of molecular marker-assisted selection, a total of 351 CSSLs were obtained. These CSSLs were further genotyped by 357 polymorphic molecular markers that were evenly distributed across the 12 chromosomes. Subsequently, 138 core lines that may cover the whole 9311 genome were selected and subjected to re-sequencing.
Consequently 125 lines that had convincing re-sequencing data were used to construct a graphical genotypic map. As shown in Supplemental Fig. 1, each CSSL contains one to seven substituted segments. In more detail, 53 lines harbored only one substituted segment, 44 lines contained two introgressed segments, and 28 lines had more than two segments (Supplemental Fig. 1). The total length of the 353 substituted segments was around 1999.76 Mb, which is five times of the 9311 genome. Re-sequencing analysis revealed that the length of individual substituted segments ranged from 0.02 to 24.6 Mb, with an average length of 5.6 Mb. Bin mapping showed that five chromosomes, Chr. 2, 3, 6, 7 and 9, were fully covered by the substituted segments, whereas the remaining chromosomes had 81.5% to 98.5% coverage. The uncovered regions, shown as seven small gaps, are about 4.4% of the whole 9311 genome. Therefore, this CSSL population covers ~95.6% of the 9311 genome, which is suitable for genome-wide surveys.
The CSSLs display a wide range of variation in grain yield-related traits and cell-wall composition
We investigated six grain yield-related traits, including PH, HD, and grain size/weight in the CSSLs and the two parents. PH, HD, and grain length to width ratio (GLWR) were continuous within the range of two parents, and a few lines were superior to their parents (Supplemental Fig. 2). However, for GL, grain width (GWD), and thousand-grain weight (TGW), more lines were superior to their parents, indicating that loci-interacting effects might exist in these traits (Supplemental Fig. 2). The wide range of phenotypic variation suggested complexity in these agronomic traits.
We also analyzed cell-wall composition of the mature culms from the CSSLs. To guarantee accuracy of examination, we performed variation analysis on the content of four major sugars and cellulose, as that of rhamnose, fucose and mannose is too low to be precisely measured. As shown in Supplemental Fig. 3, the content of the four sugars and cellulose showed a continuous distribution among the CSSLs with transgressive segregation on both sides. The ratio of xylose to arabinose, reflecting arabinoxylan structure, was also continuously distributed. Therefore, cell-wall composition belongs to a complex trait.
Cell-wall composition is correlated to grain yield-related traits
To determine whether cell-wall composition is correlated with the grain yield-related traits, we performed correlation analysis. The content of cellulose and four sugars and XA ratio showed varied correlation coefficient against six grain yield-related traits (Table 1). PH, one of the most important traits for crop architecture and grain yield, was significantly associated with cellulose content and XA ratio. Similarly, HD that indicates vegetative growth period was associated with XA ratio and the content of cellulose, xylose and galactose (Table 1), demonstrating that biosynthesis of cellulose and xylan is correlated with PH and HD. GWD, GL and TGW are direct determinants of grain yields. Interestingly, XA ratio displayed a high correlation with TGW and GL, while the glucose and galactose content were significantly correlated with TGW and GWD (Table 1). The highest correlation coefficient between cell-wall composition (XA ratio) and grain yield-related traits (TGW) was 0.44, indicating that xylan structure may contribute to TGW and grain yield. As positive controls, 0.85 or 0.6 correlation coefficients were found between the abundance of arabinose and galactose or xylose due to the existence of arabinogalactan and arabinoxylan in rice cell walls. Similarly, around 0.6 correlation coefficients were showed between PH and HD and between GL and TGW (Table 1), which is in agreement with previous reports51.
Taken together, the cell-wall compositions are correlated with grain yield-related traits in rice.
QTL analysis for grain yield-related traits and cell-wall composition
QTL mapping for the grain yield-related traits and cell-wall composition was carried out based on Bin-map converted from physical map52. We found 56 QTLs in total. In detail, nine QTLs for PH were found located on five chromosomes with positive and negative additive effects. The contribution from 9311 indicates a positive additive effect, and that from NP is negative. The variance explained by these QTLs varied from 4.7% to 31.1%. qPH3-1 and qPH8 with positive effects were major loci and located on Chr. 3 and Chr. 8, whilst qPH1-2 with negative effect was located on Chr. 1 (Table 2). We further found that qPH1-2 and qPH8 included the known important loci for plant height, such as sd1 37 and Ghd8/DTH8 53,54, suggesting that the mapping data are reliable. Among eleven QTLs for HD, three major loci were mapped on Chr.3 and Chr.8, which explained 17.72% to 30.81% phenotypic variance (Table 2). The 30.81% variation is likely due to the presence of DTH8/Ghd8 53,54, a major QTL for heading date, in the mapping region. The remaining QTLs had minor effects, in which qHD6-1 included Hd3a 55 and Hd17 56, and qHD6-2 included Hd1 38 (Table 2). For grain size or weight control, many minor QTLs with 6–11% explanation were found distributed on seven chromosomes (Table 2). Meanwhile, six major QTLs for GL, TGW, and GLWR located on Chr.2, 3 and 5 and explained 15.31% to 48.9% variance were also identified (Table 2). Within the mapping regions of qGL3-2 and qGWD5, GS3, GL3.2 and GW5, the known QTLs for grain size or weight control36,57,58, are located.
Using the same approach, a total of nineteen QTLs for the content of cellulose and four major neutral sugars and XA ratio were identified on nine chromosomes. Probably because cellulose biosynthesis is modulated by multiple genes with small effects, only one QTL for cellulose content (qCel6) with positive additive effect was mapped and explained 10.9% phenotypic variance (Table 3). Three QTLs for xylose content were detected, of which qXyl7 and qXyl10 were of negative effect and concentrated on Chr. 7 and 10, whilst qXyl8 that had positive effect was located on Chr. 8. The variance explained by these QTLs varied from ~8% to 13.6% (Table 3). Four QTLs for the arabinose content showing positive effect were mapped on Chr. 4, 7 and 8 with around 6.4%-12.5% variance (Table 3). Similarly, four QTLs for XA ratio were targeted onto Chr. 7 and 8, in which qXA7-1 and qXA7-2 are major QTLs as they explained 59.2% and 17.7% phenotypic variance (Table 3). Within five QTLs for the glucose content, qGlc1-1 and qGlc11 explained 17.7% and 15.6% phenotypic variance (Table 3). Finally, two QTLs for the galactose content were mapped on Chr. 4 and 8, respectively (Table 3).
By using this CSSL population, QTLs for six grain yield-related traits and cell-wall composition have been parallelly mapped on eleven rice chromosomes with the exception of Chr. 9.
QTLs for cell-wall composition and grain yield are overlapped
Physical mapping of the above loci further showed nicely co-localized QTLs. Firstly, several grain yield-related QTLs were co-localized. QTLs for PH and GLWR overlapped on Chr. 1 (Supplemental Fig. 4). The overlapping could extend to PH and HD on Chr. 3, 6 and 8, and to HD and TGW on Chr. 8, suggesting that plant height and heading date are correlated to grain yield. Within the nineteen QTLs for cell-wall composition, QTLs for the arabinose content were co-localized with XA ratio on Chr. 7 and 8, and with the galactose and xylose content on Chr. 8 (Supplemental Fig. 5), in agreement with the existence of arabinoxylan and arabinogalactan in the rice cell-wall polymers.
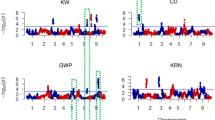
Interestingly, QTLs for the cellulose (qCel6), glucose (qGlc1-2), and galactose (qGal8) content, and XA ratio (qXA7-2) were overlapped with those for PH (qPH6-1 and qPH1-1), TGW (qTGW8) and HD (qHD6-1 and qHD7) (Fig. 3). qAra7 and qXA7-1 were adjacent to qGL7 and qTGW7. Similar relationship was found between qAra8-1 and qPH8. This finding indicated that certain genes required for cell-wall biosynthesis may affect agronomic traits. To explore evidence underlying the connection, we investigated the genotypes and phenotypes in the corresponding CSSLs. qCel6 and qPH6-1/qHD6-1 were co-targeted within a 2.46-Mb region on Chr. 6. Five substitution lines containing and not containing the 9311 fragment showed consistent tendency in cellulose content and plant height. In detail, the CSSLs possessing the 9311 fragment had increased cellulose content and plant height (Fig. 4A). qXA7-2, a major QTL for XA ratio, was mapped at the end of Chr. 7, where qHD7 is located. Line 88 (N88) containing the 9311 fragment displayed higher XA ratio and longer heading date than NP and N57 that do not harbor the substitution fragment (Fig. 4B). Within the mapping region on Chr. 8, QTLs for thousand-grain weight and qGal8 were colocated. The substitution lines harboring the 9311 fragment showed increased galactose content and slightly reduced thousand-grain weight (Fig. 4C). Taken together our data suggest that these regions are very likely to harbor QTLs synchronously controlling cell-wall biosynthesis and grain yield.
Verification of the overlapped QTLs for cell-wall composition and grain yield-related traits by the CSSLs. (A) The co-localized QTLs on Chr. 6 and examining the cellulose content and plant height in the CSSLs. (B) The co-localized QTLs on Chr. 7 and examining the XA ratio and heading date in the CSSLs. (C) The co-localized QTLs on Chr. 8 and examining the galactose content and thousand-grain weight in the CSSLs. The black and white rectangles in this figure represent 9311 and NP genomic region, respectively. ***P < 0.001, **P < 0.01, and *P < 0.05 by two-tailed t-test.
Fine-mapping of qCel6
To find a further support for this conclusion, we generated a mapping population by backcrossing N62, a line containing a 9311 fragment and contributing to high cellulose level, with NP. Based on genotyping of more than 3500 BC8F2 plants and examining the cellulose content in the internodes of plants that possess the corresponding genotypes, qCel6 was narrowed down to a ~800 kb region, in which HD3a, HD3b and HD17 controlling heading date and grain yields are located55,56 (Fig. 5A and B). Natural variations on these genes between NP and 9311 or the substitution lines were investigated by DNA sequencing. Surprisingly, we have not found any variations in the coding and promoter regions of HD3a and HD3b. Seven single nucleotide polymorphisms (SNPs) that cause five amino acid substitutions in HD17 have been identified (Fig. 5C), indicating that the increased cellulose content is likely due to variations on HD17. Co-segregation of qCel6 and HD17 in the fine-mapping assay provided further evidence for the genetic correlation between cell-wall composition and an agronomic trait.
Fine-mapping of qCel6. (A) Mapping of qCel6 in the backcross population. The numbers on chromosome 6 indicate the physical locations (Mb). S1-S7 indicate molecular markers. (B) Genotyping the segregation lines (left) and measuring the cellulose level in the internodes of corresponding lines (right) shown in left. n = 4. ***P < 0.001, **P < 0.01, and *P < 0.05 by two-tailed t-test. The black, white and grey rectangles indicate 9311, NP and heterozygous genomic region, respectively. The red bar and red dash lines indicate the QTL mapping and narrowed region of qCel6. The red triangle indicates the place HD17 located. (C) HD17 gene structure and allelic variations between 9311 and NP. The numbers indicate the place of SNPs located. The arrows indicate the changes of amino acid (aa).
Discussion
CSSLs that contain one or a few donor segments in each line are widely used for QTL mapping. Since the CSSLs possess the same genetics background, QTL mapping with this population is of high accuracy and could be dissected as single Mendelian factors59 although preparation of CSSLs is time-consuming. Here we generated CSSLs using NP as receptor and 9311 as donor. Both parents are representative varieties for two rice subspecies and show a great genetic variance suitable for QTL association analysis. Rice is one of Earth’s major staple crops. Improvement of grain yield is the most important issue in rice production. Due to the complexity of grain yield control, a great number of genes have been inferred to be involved. However, although some major QTLs for grain size/weight have been identified33,34,35,36,60, the cloned loci with smaller effects are very limited. Recent works have revealed that metabotype-phenotype links are powerful to clone minor QTLs for complex phenotypic traits44,45,47. As cell walls are regarded as basic materials for plant growth, relevant metabolites could be annotated as causes or effects2. Therefore, combining QTL mapping for two sets of traits may provide a comprehensive view to understand the mechanism of grain yield control and assigning the biological functions to cell-wall synthesizing genes.
In this study, six phenotypic traits, named as grain yield-related traits, were investigated in the CSSLs. Plant height and heading date are widely studied traits and associated with crop yield37,53. We identified nine QTLs for PH and eleven QTLs for HD, which not only included several previously identified QTLs, such as SD1 37, HD1 38, HD3a 55, HD17 56, and DTH8/GHD8 53,54, according to the mapping region of qPH1-2, qHD6-1, qHD6-2, and qHD8-1/qPH8, but also contained several new loci, such as qPH1-1, qHD3-2 and qHD5. Similarly, QTLs for grain size and grain weight were investigated, which identified some known QTLs. For example, qGL3-2/qGLWR3 included GS3 36 and GL3.2 57, and qGWD5 contained GW5 58. qGL7-2 and qGWD2-1 were adjacent to the previously reported GL7 and GW2, respectively33,61, indicating that our mapping results are reliable. Notably, several unidentified loci were also found, such as qGL3-1 and qTGW7. Although the mapping population was derived from the 57 CSSLs, three previously reported minor QTLs for grain weight50 have not being found here, suggesting that mapping minor loci for complex traits is of great challenge.
We parallelly analyzed cell-wall composition in the CSSLs. Consequently, nineteen QTLs for six cell-wall content characteristics were identified. Only one QTL for the cellulose content, qCel6 that explained 10.9% of phenotypic variation, was found, indicating that cellulose property is affected by many minor loci, consistent with the notion that cellulose synthesis is very complicated and required the involvement of multiple genes2,3,4. Interestingly, qCel6 is very likely to be an uncharacterized cell-wall synthesis related gene, because within the mapping region on Chr. 6, none of known cellulose synthesizing or regulatory genes has been reported. Arabinoxylan is the second most abundant component next to cellulose in rice cell wall. Xylose content and XA ratio are the most important parameters reflecting arabinoxylan abundance and structure. In this study, we identified three QTLs for xylose content and four QTLs for XA ratio. Previous genetic evidence demonstrated that GT43 and GT47 family members are xylan synthases8,62,63. However, we did not find any GT43 and GT47 members located in, or adjacent to, the xylose QTLs, suggesting that these QTLs may represent unknown genes influencing xylan biosynthesis. It is worth to point out that the mapping region of qXyl10 includes CESA7, a key gene for secondary wall cellulose synthesis, in agreement with the elucidated correlation relationship between cellulose and xylan biosynthesis8. OsUAM3, encoding a putative uridine diphosphate-arabinose mutase, was adjacent to qXA7-1, a major QTL for XA ratio that explained up to 59.22% phenotype variation. OsUAM3 may be a candidate for this locus. Moreover, the nineteen QTLs identified here were different from the previously reported cell-wall QTLs mapped by a CSSL population containing a common wild rice, Yuanj introgressed segment(s) in an indica cultivar, Teqing30. The distinction not only indicated the complexity of cell wall biogenesis, but also prompted us to investigate the biological functions behind these genetic clues.
Correlation analysis between different traits has been widely used to ascertain a QTL with pleiotropic functions. Several studies have reported positive correlation between PH and HD, and between GL and TGW/HD40,41,43,51. In this study, we found that the mapping regions for qPH1-2 and qGLWR1 and for qHD8-1 and qTGW8 were overlapped, in agreement with the previous reports that Ghd7 and DTH8/Ghd8 are a pleiotropic QTL, which simultaneously regulates flowering time, plant height and grain productivity42,54. Bridging genetic links across metabolites and phenotypic traits by paralleled QTL mapping is effective for annotation of the biological function of a metabolite, and for cloning QTLs with minor effects44. Through establishing metabolic profile and GWAS analysis in rice, maize and tomato, several metabolic loci controlling lignin content, trigonelline level, and sugar amount have been cloned and assigned with biological functions, based on the impacts on plant height45, grain width48 and fruit size49. In this study, we adopted the same strategy to determine the genetic connection between cell-wall biosynthesis and grain yield-related traits. Correlation coefficient analysis revealed that all the observed cell-wall composition showed varied degrees of correlation with grain yield-related traits. The cellulose and galactose content was correlated with PH and HD and with TGW and GWD, respectively. Meanwhile, XA ratio was associated with PH and HD, as well as with GL and TGW. The combined QTL analysis revealed several overlapped loci for both sets of traits, providing genetic clues to the association. The inferred genetic links were strengthened by exploring the correlated phenotypes between cell-wall composition (qCel6, qXA7-2 and qGal8) and plant height (qPH6-1), heading date (qHD7) or grain weight (qTGW8) in the corresponding CSSLs. Fine-mapping assay narrowed qCel6 down together with HD3a, HD3b and HD17, QTLs for heading date55,56, within ~800 kb region. Investigation of allelic variations in these genes found seven SNPs in HD17 between 9311 and NP, which introduce five nonsynonymous mutations, indicating HD17 as a candidate of qCel6. Further studies, such as QTL cloning and transformation verification, are required to ensure the authentic effect of HD17 on cellulose content. Our study thus provides genetic connection for both sets of traits, which may benefit for understanding the molecular basis of grain yield control, and for assigning biological functions to cell wall synthesizing genes.
Methods
Plant materials
To develop the CSSLs, the previously reported 57 CSSLs50 generated by using NP as recipient and 9311 as donor were subjected to a series of backcross with NP and combined with self-crosses. In total, 351 CSSLs were obtained. After genotyping with molecular markers, 138 core lines were chosen and subjected to re-sequencing. Based on the resequencing data, 125 CSSLs that cover 95.6% the 9311 genome were chosen for further phenotyping and QTL mapping.
Field planting
The two parents and 125 lines of CSSLs were grown in the experimental fields located at the campus of Yangzhou University and at the Jiudian County of Yangzhou, Jiangsu Province, China, respectively. N62 and the related backcross population were grown in the experimental fields located at the Jiudian County of Yangzhou, Jiangsu Province and at the Lingshui County of Sanya, Hainan Province, China, respectively. Eighty plants (8 × 10) of two parents and each CSSL were planted in the plots at 25 cm interval in the horizontal direction and at 15 cm interval in the longitudinal direction.
Measurement of grain yield-related traits and data analysis
Heading date of 125 lines and two parents were evaluated by counting the dates from sowing to flowering. Heading date of two parents and each line was recorded when more than 50% individual plants started heading. After maturation, ten representative plants of two parents and each line were subjected to measure the height from ground to the tallest panicle. One month later after seed harvest, seeds derived from 16 individual plants of two parents and each line were pooled and subjected to measure grain size and grain weight. Grain length, grain width, and grain length to width ratio were measured by a camera and analyzed with Smart Grain equipped (Version 1.1). Thousand-grain weight was measured by weighing 200 seeds from two parents and each line for three biological replicates. The data was analyzed using an independent sample t test program (SPSS version 15.0). Correlation coefficient between different traits was analyzed by SPSS software (version 15.0).
Scanning electronic microscopy
The outer surface of glumes from mature seeds was sprayed with gold particles and observed with a scanning electron microscope (S-3000N, Hitachi) at an acceleration voltage of 10 kV. At least 500 cells from 12 individual seeds of two parents were measured and quantified.
Cell-wall composition analysis
The cell-wall composition was analyzed as described previously64. In brief, the mature 2nd internodes of two parents and CSSLs were ground into powder and treated with 70% ethanol and a mixture of chloroform and methanol (1:1 v/v) twice to prepare alcohol insoluble residues (AIRs). The de-starched AIRs were obtained by treatment with pullulanase M1 (Megazyme) and α-amylase (Sigma) in 0.1 M sodium acetate buffer (pH 5.0) for at least 24 h. After hydrolyzed in 2 M trifluoroacetic acid (TFA) at 121 °C for 90 min, the released sugars were reduced with sodium borohydride (10 mg mL−1 in 1 M ammonium hydroxide). Samples were analyzed by an Agilent 7890 series GC equipped with a 5975 C MS detector (Agilent). Meanwhile, crystalline cellulose content was quantified by anthrone assay65 using the remains after TFA treatment. The data were presented as the mean of four biological repeats.
Molecular markers
Molecular markers developed for reconstruction of CSSLs in this study were classified into two types. Rice microsatellite (RM) markers were selected from rice microsatellite maps. The sequence and location information of these markers are available from the Gramene website (http://www.gramene.org). Sequence tagged site (STS) markers were developed by using PRIMER PREMIER 5.0 software according to the publicly available rice genome sequence (http://www.ncbi.nlm.nih.gov/). The polymorphic markers that were validated in the two parents were used for marker assistant selection.
Resequencing of the CSSLs
Five microgram of genomic DNA from each sample was randomly fragmented by sonication and size-fractionated using electrophoresis. DNA fragments of ~500 bp were then purified. Adapter ligation and DNA clustering were performed according to the procedure from manufacturer. The fragmental samples were sequenced by Illumina HiSeq. 2000 according to the manufacturer instructions (lllumina, San Diego, California, USA). Read sequences were aligned with the reference pseudomolecules (MSU Rice Genome Annotation Project, http://rice.plantbiology.msu.edu/) and applied for SNP detection.
QTL mapping
ICIMapping 4.0 software was used to detected the QTLs by the aid of constructing Bin-map in which the genotype of 9311 and NP was assigned as 2 and 0, respectively66. The QTL loci were determined by a likelihood ratio test based on single point analysis. QTLs with logarithm of the odds ratio (LOD) larger than 2.5 were considered as real QTLs. The allelic effect was calculated as the mean effect of replacing NP alleles by 9311 alleles at the QTL. The trait value and QTL located chromosome number were indicated. Mapchart (version 2.2) was applied to locate the QTLs on the Bin-map.
Construction of Bin-map
To locate the donor segments in the CSSLs for QTL mapping, Bin-map was constructed based on the above SNP genotyping analyses. The detected SNPs were placed onto the chromosomes according to the physical location. The recombination breakpoints and donor segments were determined using a sliding window approach according to the method described50. Finally, 357 Bins were generated. The physical length of bins ranges from 0.01 to 8.92 Mb.
References
Cosgrove, D. J. Growth of the plant cell wall. Nat Rev Mol Cel Bio 6, 850–861 (2005).
Somerville, C. et al. Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211 (2004).
Pear, J. R., Kawagoe, Y., Schreckengost, W. E., Delmer, D. P. & Stalker, D. M. Higher plants contain homologs of the bacterial celA genes encoding the catalytic subunit of cellulose synthase. Proc. Natl. Acad. Sci. USA 93, 12637–12642 (1996).
Arioli, T. et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720 (1998).
Dhugga, K. S. et al. Guar seed β-mannan synthase is a member of the cellulose synthase super gene family. Science 303, 363–366 (2004).
Burton, R. A. et al. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1, 3; 1, 4)-ß-D-glucans. Science 311, 1940–1942 (2006).
Cocuron, J. C. et al. A gene from the cellulose synthase-like C family encodes a β-1,4 glucan synthase. Proc Natl Acad Sci USA 104, 8550–8555 (2007).
Brown, D. M. et al. Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J 52, 1154–1168 (2007).
Pena, M. J. et al. Arabidopsis irregularxylem8 and irregular xylem9: implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19, 549–563 (2007).
Anders, N. et al. Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci USA 109, 989–993 (2012).
Robert, S. et al. An Arabidopsis endo-1,4-β-D-glucanase involved in cellulose synthesis undergoes regulated intracellular cycling. Plant Cell 17, 3378–3389 (2005).
Zhong, R., Kays, S. J., Schroeder, B. P. & Ye, Z. H. Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. Plant Cell 14, 165–179 (2002).
Schindelman, G. et al. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes & Deveopment 15, 1115–1127 (2001).
Li, Y. H. et al. BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15, 2020–2031 (2003).
Seifert, G. J. Nucleotide sugar interconversions and cell-wall biosynthesis: how to bring the inside to the outside. Curr Opin Plant Biol 7, 277–284 (2004).
Zhang, B. et al. Golgi nucleotide sugar transporter modulates cell-wall biosynthesis and plant growth in rice. Proc Natl Acad Sci USA 108, 5110–5115 (2011).
Chanliaud, E., De Silva, J., Strongitharm, B., Jeronimidis, G. & Gidley, M. J. Mechanical effects of plant cell wall enzymes on cellulose/xyloglucan composites. Plant J 38, 27–37 (2004).
McQueen-Mason, S. & Cosgrove, D. J. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proc Natl Acad Sci USA 91, 6574–6578 (1994).
Zhong, R., Burk, D. H., Morrison, W. H. 3rd & Ye, Z. H. A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell 14, 3101–3117 (2002).
Gu, Y. et al. Identification of a cellulose synthase-associated protein required for cellulose biosynthesis. Proc Natl Acad Sci USA 107, 12866–12871 (2010).
Oda, Y., Iida, Y., Kondo, Y. & Fukuda, H. Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr Biol 20, 1197–1202 (2010).
Endler, A. et al. A mechanism for sustained cellulose synthesis during salt stress. Cell 162, 1353–1364 (2015).
Zhao, Q. Lignification: flexibility, biosynthesis and regulation. Trends Plant Sci 21, 713–721 (2016).
Zhong, R., Lee, C. & Ye, Z. H. Evolutionary conservation of the transcriptional network regulating secondary cell-wall biosynthesis. Trends Plant Sci 15, 625–632 (2010).
Zhong, R. et al. Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors. Plant Cell Physiol 52, 1856–1871 (2011).
Hematy, K. et al. A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17, 922–931 (2007).
Xu, S. L., Rahman, A., Baskin, T. I. & Kieber, J. J. Two leucine-rich repeat receptor kinases mediate signaling, linking cell-wall biosynthesis and ACC synthase in Arabidopsis. Plant Cell 20, 3065–3079 (2008).
Krakowsky, M., Lee, M. & Coors, J. Quantitative trait loci for cell wall components in recombinant inbred lines of maize (Zea mays L.) II: leaf sheath tissue. Theor Appl Genet 112, 717–726 (2006).
Ranjan, P. et al. Bioinformatics-based identification of candidate genes from QTLs associated with cell wall traits in Populus. BioEnergy. Research 3, 172–182 (2010).
Zhang, S. J. et al. Identification of quantitative trait loci affecting hemicellulose characteristics based on cell-wall composition in a wild and cultivated rice species. Mol Plant 5, 162–175 (2012).
Ashikari, M. et al. Cytokinin oxidase regulates rice grain production. Science 309, 741–745 (2005).
Huang, X. et al. Natural variation at the DEP1 locus enhances grain yield in rice. Nat Genet 41, 494–497 (2009).
Song, X. J., Huang, W., Shi, M., Zhu, M. Z. & Lin, H. X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39, 623–630 (2007).
Wang, S. K. et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat Genet 47, 949–954 (2015).
Qi, P. et al. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1;3. Cell Res 22, 1666–1680 (2012).
Fan, C. H. et al. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor Appl Genet 112, 1164–1171 (2006).
Monna, L. et al. Positional cloning of rice semidwarfing gene, sd-1: Rice “Green revolution gene” encodes a mutant enzyme involved in gibberellin synthesis. DNA Research 9, 11–17 (2002).
Yano, M. et al. Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12, 2473–2483 (2000).
Ishimaru, K., Ono, K. & Kashiwagi, T. Identification of a new gene controlling plant height in rice using the candidate-gene strategy. Planta 218, 388–395 (2004).
Duan, P. G. et al. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat Plants 2, 15203 (2016).
Che, R. H. et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat Plants 2, 15195 (2016).
Xue, W. Y. et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40, 761–767 (2008).
Weng, X. Y. et al. Grain number, plant height, and headingdate7 is a central regulator of growth, development, and stress response. Plant Physiol 164, 735–747 (2014).
Saito, K. Phytochemical genomics–a new trend. Curr Opin Plant Biol 16, 373–380 (2013).
Riedelsheimer, C. et al. Genome-wide association mapping of leaf metabolic profiles for dissecting complex traits in maize. Proc Natl Acad Sci USA 109, 8872–8877 (2012).
Chen, W. et al. Genome-wide association analyses provide genetic and biochemical insights into natural variation in rice metabolism. Nat Genet 46, 714–721 (2014).
Schauer, N. et al. Comprehensive metabolic profiling and phenotyping of interspecific introgression lines for tomato improvement. Nat Biotechnol 24, 447–454 (2006).
Chen, W. et al. Comparative and parallel genome-wide association studies for metabolic and agronomic traits in cereals. Nat Commun 7, 12767 (2016).
Tieman, D. et al. A chemical genetic roadmap to improved tomato flavor. Science 355, 391–394 (2017).
Zhang, H. et al. Development and high-throughput genotyping of substitution lines carring the chromosome segments of indica 9311 in the background of japonica Nipponbare. J Genet Genomics 38, 603–611 (2011).
Yuan, Y. et al. Identification and fine mapping of qPH6, a novel major quantitative trait locus for plant height in rice. Mol Breeding 35, 56 (2015).
Xu, J. J. et al. Developing high throughput genotyped chromosome segment substitution lines based on population whole-genome re-sequencing in rice (Oryza sativa L.). Bmc Genomics 11, 1–14 (2010).
Wei, X. J. et al. DTH8 suppresses flowering in rice, influencing plant height and yield potential simultaneously. Plant Physiol 153, 1747–1758 (2010).
Yan, W. H. et al. A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4, 319–330 (2011).
Monna, L., Lin, H. X., Kojima, S., Sasaki, T. & Yano, M. Genetic dissection of a genomic region for a quantitative trait locus, Hd3, into two loci, Hd3a and Hd3b, controlling heading date in rice. Theor Appl Genet 104, 772–778 (2002).
Matsubara, K. et al. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol 53, 709–716 (2012).
Xu, F. et al. Variations in CYP78A13 coding region influence grain size and yield in rice. Plant Cell Environ 38, 800–811 (2015).
Weng, J. F. et al. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res 18, 1199–120 (2008).
Nadeau, J. H. & Frankel, W. N. The roads from phenotypic variation to gene discovery: mutagenesis versus QTLs. Nat Genet 25, 381–384 (2000).
Mao, H. L. et al. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. P Natl Acad Sci USA 107, 19579–19584 (2010).
Wang, Y. X. et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat Genet 47, 944–948 (2015).
Wu, A. M. et al. The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J 57, 718–731 (2009).
Faik, A. Xylan biosynthesis: news from the grass. Plant Physiol 153, 396–402 (2010).
Huang, D. B. et al. A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in Rice. Plant Cell 27, 1681–1696 (2015).
Updegraff, D. M. Semimicro determination of cellulose in biological materials. Anal Biochem 32, 420–424 (1969).
Meng, L., Li, H., Zhang, L. & Wang, J. QTL IciMapping: Integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3, 269–283 (2015).
Acknowledgements
We thank Yanbao Tian (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for help with scanning electronic microscope analysis and Prof. Staffan Persson (University of Melbourne) for comments on the manuscript. This study was supported by the National Natural Science Foundation of China (31530051, 31561143008, and 91535105), CAS grants for XDA08010103, and the State Key Laboratory of Plant Genomics (2017A0407-18), and the Jiangsu PAPD and 333 projects.
Author information
Authors and Affiliations
Contributions
Y.Z. and Q.L. conceived the study; Z.X., S.L., C.Z., and K.Z. performed the experiments and analyzed the data; B.Z. reviewed and edited the article; Y.Z. and Z.X. wrote the article.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Z., Li, S., Zhang, C. et al. Genetic connection between cell-wall composition and grain yield via parallel QTL analysis in indica and japonica subspecies. Sci Rep 7, 12561 (2017). https://doi.org/10.1038/s41598-017-12903-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12903-5
This article is cited by
-
Characterization of qPL5: a novel quantitative trait locus (QTL) that controls panicle length in rice (Oryza sativa L.)
Molecular Breeding (2022)
-
QTL mapping of biomass and forage quality traits measured using near-infrared reflectance spectroscopy (NIRS) in switchgrass
Euphytica (2021)
-
MYB61 is regulated by GRF4 and promotes nitrogen utilization and biomass production in rice
Nature Communications (2020)
-
Characterization and fine-mapping of qHd2-1, a minor quantitative locus that affects heading date under long-day conditions in rice (Oryza sativa L.)
Molecular Breeding (2020)
-
Identification and fine mapping of qGN1c, a QTL for grain number per panicle, in rice (Oryza sativa)
Molecular Breeding (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.