Abstract
Despite intense research the optimal endogenous biomarker for glomerular filtration rate (GFR) estimation has not been identified yet. We analyzed if ß-trace protein (BTP) improved GFR estimation in elderly. 566 participants aged 70+ from the population-based Berlin Initiative Study were included in a cross-sectional validation study. BTP, standardized creatinine and cystatin C were measured in participants with iohexol clearance measurement as gold standard method for measured GFR (mGFR). In a double logarithmic linear model prediction of mGFR by BTP was assessed. Analyses with BTP only and combined with creatinine and cystatin C were performed. Additionally, performance of GFR estimating equations was compared to mGFR. We found that the combination of all three biomarkers showed the best prediction of mGFR (r2 = 0.83), whereat the combination of creatinine and cystatin C provided only minimally diverging results (r2 = 0.82). Single usage of BTP showed worst prediction (r2 = 0.67) within models with only one biomarker. Subgroup analyses (arterial hypertension, diabetes, body mass index ≤23 and >30) demonstrated a slight additional benefit of including BTP into the prediction model for diabetic, hypertensive and lean patients. Among BTP-containing GFR equations the Inker BTP-based equation showed superior performance. Especially the use of cystatin C renders the addition of BTP unnecessary.
Similar content being viewed by others
Introduction
Glomerular filtration rate (GFR) is considered the best indicator of kidney function1. It can either be estimated (eGFR) using mathematical equations based on endogenous biomarkers or it can be measured (mGFR) using invasive methods based on the injection of exogenous markers. At present, in clinical routine the majority of health care providers use serum creatinine levels to calculate eGFR. Recently, it has been shown that, especially in older adults and children, adding cystatin C as another renal biomarker to creatinine level for assessing GFR increases the accuracy of eGFR as compared to the use of single-biomarker eGFR2,3,4. A rising number of novel biomarkers have emerged as alternative to creatinine and cystatin C5,6, one of them ß-trace protein (BTP), a heterogeneous low molecular weight glycoprotein produced in the central nervous system7. Concentrations in serum however are found to be lower8. Since BTP’s discovery about 20 years ago research has focused on its possible ability to shed additional light on assessing kidney function beyond serum creatinine (Scr) and serum cystatin C (SCys)9. With regard to its renal metabolism, BTP has been shown to be freely filtered through the glomerular basement membrane with little if any tubular reabsorption or non-renal elimination7. Also, ß2-microglobulin (B2M), another low molecular weight protein, has been found to be highly correlated with measured GFR (mGFR) and like BTP, is less affected by age and sex10,11. Other research groups have investigated the approach of “biomarker-panels” using several, still unspecified, markers simultaneously, although these markers are not yet established for standard laboratory analysis12.
For the estimation of GFR it is important to know whether the renal marker in use performs equally well in different patient populations, such as children, older adults, individuals with liver cirrhosis or muscle wasting, and transplanted patients. Factors that are known to influence a renal biomarker’s potential to correctly estimate GFR are called the non-GFR determinants (e.g. muscle mass, age, gender, and diet). Although creatinine-based GFR equations give reliable estimates in adult patients with reduced kidney function, it is known that both, for children and for older adults, continuous change in muscle mass leads to unreliable Scr levels for GFR estimation13,14. In this respect, BTP has been shown to have potentially superior properties for assessing kidney function, especially in renal transplant recipients15,16. Whether BTP may increase the accuracy of GFR estimates when combined with existing markers applied to older adults is still unknown.
In the present study we hypothesized that in old age adding BTP to single or the combination of current biomarkers improved GFR estimation compared to using Scr or Scys alone. In a double logarithmic linear model prediction of mGFR by BTP only and combined with creatinine and cystatin C was assessed. Additionally, we evaluated current BTP-containing eGFR equations against measured GFR and in comparison to combined creatinine/cystatin C-based equations in a population of older adults.
Results
In the BIS iohexol population with measured BTP (Table 1), mean age (±SD) was 78.5 (6.2) years, 43% were female, one quarter had diabetes, more than three quarters had hypertension, and nearly one third was overweight (body mass index ≥30 kg/m2). Median serum concentration of BTP in the total cohort was 0.68 mg/l with 0.73 mg/l in males and 0.62 mg/l in females. For creatinine it was 0.91 mg/dl with 1.02 in males and 0.79 in females and for cystatin C it was 1.05 mg/dl with 1.1 mg/dl in males and 0.99 mg/dl in females. Figure 1 shows an increase of BTP concentration by age category: in 70–75 year old patients mean BTP was 0.57 mg/L in females and 0.67 mg/dl in males as compared to age >90 years with mean BTP of 0.80 mg/L in females and 0.92 mg/L in males, corresponding to a 14% increase of BTP concentration between the lowest and the highest age stratum for male and females.
Median BTP concentration by age and gender in the BIS iohexol population (n = 566). Age strata 70–75 years up to ≥90 years, males are in grey and females in black. Boxes indicate medians (line inside box), quartiles (upper and lower margins of box). Antennae are defined by the rule upper-lower box margin ± 1.5x interquartile range. Circles indicate outliers. Exact median BTP concentrations per age stratum for males and females are given in mg/l next to the boxplots.
Performance of BTP alone and in combination with creatinine and cystatin C and compared to mGFR
All filtration markers were negatively correlated with measured GFR (mGFR) (Supplement Table 1) ranging from −0.78 (creatinine) to −0.78 (BTP) and −0.87 (cystatin C). Comparison of renal markers showed the highest correlation between BTP and cystatin C (0.83) followed by creatinine and cystatin C (0.82) and the lowest between BTP and creatinine (0.77). In the regression model adjusted for age, gender and body mass index (BMI) (Table 2) the single usage of BTP showed the worst prediction (r2 = 0.632) within the models with only one biomarker. The age, gender, and BMI-adjusted combination of BTP, creatinine and cystatin C showed the best prediction of mGFR (r2 = 0.833), whereat the adjusted combination of creatinine and cystatin C provided only minimally diverging results (r2 = 0.828). Additionally, the combination of creatinine and BTP (r2 = 0.789) did not outperform the combination of creatinine and cystatin C.
Validation of BTP-based eGFR equations
Mean mGFR (range) was 60.4 (16–117) ml/min/1.73 m2 and compared to the estimated values using the equations described in the methods section we found rather heterogeneous results: the highest mean estimated GFR (eGFR) (range) of 86 (14–163) ml/min/1.73 m2 was found with the White(BTP/Crea) equation compared to less biased BTP-based eGFR results of 58 (15–105), 63 (13–112), and 64 (15–117) for the Inker(BTP), the Pöge(BTP/Crea) and the Pöge(BTP) equations, respectively. Figure 2a shows the boxplot of mGFR and eGFR, according to all four BTP-based equations. Compared to the median mGFR value of 61 ml/min/1.73 m2 the Inker(BTP) equation showed the closest estimate with the smallest interquartile range. Both equations by Pöge slightly overestimated median mGFR. The estimate of the White(BTP/Crea) equation largely overestimated mGFR and showed the widest interquartile range. Figure 2b shows the boxplot of mGFR and eGFR, according to the three creatinine/cystatin C-based equations with the best results for the BIS2 equation. The boxplots of change shows the difference between eGFR and mGFR and reconfirms these results for all seven eGFR equations (Supplement Fig. 1).
(a) Comparison of mGFR with the four BTP-based GFR equations. (b) Comparison of mGFR with combined creatinine/cystatin C GFR equations applicable in older adults. (a,b) Boxes indicate medians (line inside box), quartiles (upper and lower margins of box). Antennae are defined by the rule upper-lower box margin ± 1.5x interquartile range. Circles, squares and triangles indicate outliers. For estimating equations, refer to material section. mGFR = measured glomerular filtration rate; eGFR = estimated glomerular filtration rate; BIS = Berlin Initiative Study; CKD-Epi = Chronic Kidney Disease Epidemiology Collaboration, FAS = Full Age Spectrum.
Performance of BTP based estimating equations and comparison with established equations
Table 3 shows that for the BTP-based equations, bias and accuracy was best for the Inker(BTP) equation and worst for the White(BTP/Crea) equation. When comparing the new BTP-based equations with the established creatinine/cystatin C-based CKD-EPI(Crea/CysC), BIS2 and FAS equations it was surprising that the Inker(BTP) equation showed a better performance of bias (−2 versus 7 ml/min/1.73 m2) and accuracy (P10 of 45% versus 40% and P30 of 91% versus 88%) than the CKD-EPI(Crea/CysC) equation. All in all the BIS2 showed the best performance, which is not surprising since it was developed from the BIS dataset, and the FAS was relatively equal to Inker(BTP) and superior to CKD-EPI(Crea/CysC). Both Pöge equations did not outperform the established creatinine/cystatin C-based equations.
Comparison of BTP-based and creatinine/cystatin C-based equations
Figure 3a illustrates the performance of the four BTP-based GFR equations versus mGFR whereas Fig. 3b illustrates the three combined creatinine/cystatin C-based equations versus mGFR using Bland-Altman analysis. Also, both figures show that all GFR equations perform inferior at higher levels of GFR. Finally, exact McNemar significance probability of respective eGFR equations using P30 criterion can be found in Supplement Table 3a and b.
(a) Bland and Altman of BTP-based eGFR-equations versus mGFR. (b) Bland and Altman of creatinine-/cystatin C-based eGFR-equations versus mGFR. (a) and (b) Bland and Altman plots of BTP-based and combined creatinine/cystatin C-based eGFR-equations versus mGFR (n = 566). The bias is represented by the dashed middle line. The horizontal grey bar represents the area between the upper and lower limits of the interval of agreement. mGFR = measured glomerular filtration rate; eGFR = estimated glomerular filtration rate; BIS = Berlin Initiative Study; CKD-Epi = Chronic Kidney Disease Epidemiology Collaboration, FAS = Full Age Spectrum. For details about the GFR estimating equations please refer to the material section.
Subgroup analysis in individuals with arterial hypertension, diabetes mellitus, low or high body mass index demonstrated a slight additional benefit of including BTP into the prediction model for patients with arterial hypertension and diabetes as well as lean individuals (Supplement Table 2a,b).
Discussion
Improving the accuracy of glomerular filtration rate (GFR) estimating equations has been a long-standing aim in order to optimize clinical decision making with regard to diagnosing chronic kidney disease (CKD), medication dose-adjustment, contrast media application, initiating renal replacement therapy, evaluation of potential organ donors as well as prognostication of CKD-associated CV risk - all scenarios very relevant in the elderly.
In the present study we investigated ß-trace protein (BTP), a relatively novel filtration marker for estimating kidney function, for the first time exclusively in older adults. This was motivated by the fact that introduction of cystatin C into current GFR estimating equations a couple of years ago has been shown to increase accuracy and precision especially in older adults2,3,4,17 most probably due to its independence of height, age, gender and muscle mass. While the appropriate renal biomarker in old age has yet to be established, this raised the question whether the addition of BTP could further optimize GFR assessment in elder patients to improve patient management in geriatric nephrology.
Our analysis was performed in 566 individuals aged 70 years and above with iohexol plasma clearance measurements. We demonstrated for the first time that when BTP was included into measured GFR (mGFR) predicting regression models, neither the single biomarker with or without age and gender was superior to creatinine or cystatin C, nor did BTP in combination with creatinine and/or cystatin C increase the regression model’s fit significantly.
When comparing all BTP-containing GFR equations to mGFR the Inker(BTP) 18 equation showed the best performance. No additional benefit was found when evaluating the performance of BTP-based GFR estimating equations15,16,18 in our population of older adults compared to BIS2 and FAS(Crea/Cys) equations2,3,4. Therefore, using the two “established” standardized biomarkers creatinine and cystatin C for estimating GFR in individuals above the age of 70 is still superior to the currently available BTP-based equations. When comparing the Inker(BTP) to the creatinine/cystatin C-based CKD-EPI(Crea/Cys) 2 equation we found that the Inker(BTP) equation showed a substantially smaller bias and better precision. This was very surprising to us since in our mGFR predicting regression models we found that the combination of creatinine and cystatin C was clearly superior to BTP as a single biomarker in older adults. Therefore, we would have expected the established CKD-EPI(Crea/Cys) equation to outperform the Inker(BTP) equation. One possible explanation why this was not the case might be that the development population of both equations were not the same: amongst others, the Inker(BTP) study population included individuals with a higher mean age and reported a diabetes prevalence of 21%, both of which corresponds more closely to the characteristics of our BIS population compared to the CKD-EPI study population. Also, one prominent non-GFR determinant in old age certainly is inflammation by which BTP is known to be less influenced compared to cystatin C11. This could be another possible explanation why the Inker(BTP) equation performed better than the combined creatinine/cystatin C- based equation.
Interestingly, in contrast to former analyses that found no independent association between age and BTP19, our data showed that mean BTP concentration increases by 14% between the age of 70 and the age of 90 years and above, leading to a higher prevalence of GFR <60 ml/min/1.73 m2 in the higher age strata. The same phenomenon has been shown for creatinine and cystatin C20, and has been interpreted as a sign of physiological kidney senesence21. Cystatin C is said to be more sensitive to earlier GFR decrease22 in contrast to creatinine, which detects changes in kidney function not before a decrease of at least 50%. Priem et al. as well as Filler et al. have found the same advantage for BTP with regard to the “creatinine-blind” range23,24; in our population of elderly we did not find such a correlation (data not shown).
We further investigated BTP for assessing kidney function in hypertensive, diabetic, lean or obese elderly patients and found a slight additional benefit of including BTP into the prediction model for patients with arterial hypertension and diabetes as well as lean individuals compared to the combination of creatinine and cystatin C alone. All in all our data did not show that BTP, in addition to creatinine and cystatin C, contains significant additional information on kidney function enabling a better distinction between disease-related and age-related “physiological” change of kidney function. Besides, the accuracy of BTP measurement is lower compared to creatinine and cystatin C25,26 since a certified assay standardization has not yet been successfully established27, a fact that may also contribute to the rather disappointing results.
In 2016, Foster and colleagues have shown that BTP may contribute valuable risk prediction information beyond the current biomarkers creatinine and cystatin C28. As a purely cross-sectional analysis our focus exclusively lay on GFR prediction.
Several limitations of our study deserve mentioning. First, BTP has been shown to perform superior in combination with beta-2-microglobulin (B2M)18. Since in our population we did not measure B2M our observations are limited to BTP only or in combination with creatinine and/or cystatin C. Secondly, our study explicitly focused on older adults and BTP’s potential benefit in estimating GFR in this selected population. Even if we cannot simply expand our results to other populations they support former findings where BTP performed neither superior in children13,29 nor in adults30,31 when compared to creatinine and cystatin C. Also we cannot comment on the impact of race, since we investigated a purely Caucasian population. Thirdly, the BTP-based equation by Pöge16 and White15 were derived in and developed for adult kidney transplant recipients; these equations were expected to perform inferior in our elderly non-transplanted study population.
In summary, BTP alone or the addition of BTP does not outperform current biomarkers such as creatinine and cystatin C for GFR estimation in older adults. Especially the use of cystatin C renders the addition of BTP unnecessary. A slight improvement might be present in diabetic, hypertensive and lean individuals, whether this is clinically relevant remains to be proven. Further research is necessary to evaluate whether BTP is a useful renal marker in risk prediction for older adults and whether it outperforms creatinine and/or cystatin C in this respect.
Methods
Study participants
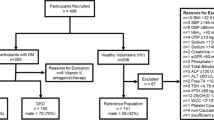
The study includes a subpopulation (n = 570) of the longitudinal population-based Berlin Initiative Study (BIS) cohort (n = 2,069). Participants, all living in Berlin, were recruited through one of the largest German statutory health care insurance company (AOK Nordost – Die Gesundheitskasse). Details on inclusion and exclusion criteria as well as study design and goals can be found elsewhere32. In the iohexol subpopulation of 570 out of 2,069 BIS baseline participants we performed iohexol plasma clearance for exact measurement of GFR. Out of these 570 participants we had 566 with measured serum ß-trace protein (BTP) as well as the two common renal biomarkers creatinine and cystatin C; in four patients BTP analysis was not performed due to lack of biobank material. The methodology used to measure GFR has been described elsewhere3,33. Briefly, an eight time point plasma iohexol measurement was performed over a five hour time period after i.v. injection. Iohexol was analyzed with high performance liquid chromatography (HPLC) method. Measured GFR (mGFR) was calculated with the area under the receiver-operating characteristic curve of plasma concentration over time. External quality control was provided by Equalis (Equalis AB, Uppsala, Sweden). Iohexol results were indexed for body surface area according to the DuBois and DuBois formula34. All procedures involving participants and data were in accordance with the revised Helsinki Declaration of 2000, concerning ethical principles for medical research involving human subjects. We confirm that the study protocols and patient information were approved by the local’s ethics committee of the Charité University.
Laboratory measures
BTP measurement was done from frozen samples (−80 °C) at Labor Limbach Heidelberg, Germany, using the particle enhanced nephelometric (PENIA) N Latex® assay on the BN™ II System (Siemens Health Care Diagnostics, ex-Dade-Behring, Marburg, Germany) in 2015. The interassay coefficients of variation for serum BTP levels were 3.45% at concentrations of 1.76 mg/L.
Cystatin C measurements were performed from frozen samples at Charité laboratory, Labor Berlin, Germany, using the particle-enhanced nephelometric assay on the BN ProSpect nephelometer (Siemens Healthcare) in 2011. The interassay coefficient of variation for serum cystatin C levels were 1.5%, 3.5% and 2.4% at mean concentrations of 0.8, 2.3, 7.4 mg/L, respectively. The manufacturer’s reference interval for healthy subjects is 0.59–1.05 mg/L, after standardization of cysC according to ERM – DA 471/IFCC for BN Systems. All reagents and control material were provided by Siemens Health Care Diagnostics.
All creatinine samples were analyzed directly after the study visit at the Synlab MVZ Laboratory, Heidelberg, using the standardized isotope dilution mass spectrometry-traceable enzymatic method (CREA plus, Roche Diagnostics, Mannheim, Germany) on a Roche modular analyzer P-Module between 2010 and 2011. The interassay coefficient of variation for serum creatinine were 2.3% and 3.4% at mean concentrations of 0.99 mg/dL and 3.75 mg/dL, respectively.
Equations that were used for validation of estimated GFR (eGFR)
The performance of the four currently published GFR estimating equations (including either serum BTP alone or in combination with creatinine) was calculated:
-
(1)
Inker(BTP) equation18: eGFR = 55 × BTP −0.695 × 0.998 age (×0.899 if female)
-
(2)
Pöge(BTP) equation16: eGFR = 47.17 × BTP −0.7933
-
(3)
Pöge(BTP/Crea) equation16: eGFR = 974.31 × BTP −0.2594 × creatinine −0.6
-
(4)
White(BTP/Crea) equation15: eGFR = 167.8 × BTP −0.758 × creatinine −0.204 (×0.871 if female).
For comparison purposes we also calculated eGFR values with the combined creatinine and cystatin C based (5) BIS23, (6) CKD-EPI(Crea/CysC) 2 and (7) FAS(Crea/CysC) 4 equations.
Statistical analysis
Descriptive analysis includes means, SDs, ranges or medians for continuous variables; absolute frequencies and percentages for categorical variables. We assessed Pearson and Spearman correlation coefficients of the log of each marker to the log of mGFR. In double logarithmic linear models prediction of mGFR (reference standard) by BTP only and in combination with serum creatinine and/or cystatin C were performed (including analyses adjusted for age, gender, and BMI). Subgroup analyses were performed in hypertensive (AHT), diabetic (DM), lean (BMI ≤ 23), and obese (BMI > 30) elderly individuals. We calculated the coefficient of determination (corrected R2) to quantify to which extent the inclusion of BTP would improve the fit of the model of mGFR. To investigate whether interaction between endogenous biomarkers and BMI was significant we defined three BMI categories (BMI ≤ 23, 23 < BMI < 30, BMI ≥ 30) and included them and their interaction terms with respective biomarkers into regression models adjusted for age and gender.
Additionally, confidence limits for R2 and Root Residual Mean Square Error (RMSE) were calculated via bootstrap resampling.
To examine how well the four current BTP-based GFR estimating equations performed in our population of older adults we assessed bias as the mean and median difference between eGFR and mGFR, with positive values indicating an overestimation of mGFR. Precision was assessed as interquartile range for the difference and SD of the bias, and accuracy as the percentage of estimates within 10% and 30% of mGFR (P10, P30). Mc Nemar test were applied to compare P30 values between equations.
Additionally, for comparison of estimated GFR versus measured GFR, we used Bland-Altman analysis including limits of agreement taking into account random measurement errors35.
The analysis was done using STATA (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical approval and informed consent
We confirm that informed consent was obtained from all participants and/or their legal guardian/s.
Change history
09 May 2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Smith, H. Comparative physiology of the kidney. In: Smith, H ed. The Kidney: Structure and function in health and disease, 520–74 (New York: Oxford University Press, 1951).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367, 20–29, https://doi.org/10.1056/NEJMoa1114248 (2012).
Schaeffner, E. S. et al. Two novel equations to estimate kidney function in persons aged 70 years or older. Ann Intern Med 157, 471–481, https://doi.org/10.7326/0003-4819-157-7-201210020-00003 (2012).
Pottel, H. et al. Estimating glomerular filtration rate for the full age spectrum from serum creatinine and cystatin C. Nephrol Dial Transplant, https://doi.org/10.1093/ndt/gfw425 (2017).
Astor, B. C. et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 59, 653–662, https://doi.org/10.1053/j.ajkd.2011.11.042 (2012).
Ferguson, M. A. & Waikar, S. S. Established and emerging markers of kidney function. Clin Chem 58, 680–689, https://doi.org/10.1373/clinchem.2011.167494 (2012).
White, C. A., Ghazan-Shahi, S. & Adams, M. A. beta-Trace protein: a marker of GFR and other biological pathways. Am J Kidney Dis 65, 131–146, https://doi.org/10.1053/j.ajkd.2014.06.038 (2015).
Filler, G., Kusserow, C., Lopes, L. & Kobrzynski, M. Beta-trace protein as a marker of GFR–history, indications, and future research. Clin Biochem 47, 1188–1194, https://doi.org/10.1016/j.clinbiochem.2014.04.027 (2014).
Hoffmann, A., Nimtz, M. & Conradt, H. S. Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology 7, 499–506 (1997).
Filler, G., Witt, I., Priem, F., Ehrich, J. H. & Jung, K. Are cystatin C and beta 2-microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem 43, 1077–1078 (1997).
Foster, M. C. et al. Non-GFR Determinants of Low-Molecular-Weight Serum Protein Filtration Markers in the Elderly: AGES-Kidney and MESA-Kidney. Am J Kidney Dis., https://doi.org/10.1053/j.ajkd.2017.03.021 (2017).
Sekula, P. et al. A Metabolome-Wide Association Study of Kidney Function and Disease in the General Population. J Am Soc Nephrol 27, 1175–1188, https://doi.org/10.1681/ASN.2014111099 (2016).
Filler, G., Guerrero-Kanan, R. & Alvarez-Elias, A. C. Assessment of glomerular filtration rate in the neonate: is creatinine the best tool? Curr Opin Pediatr 28, 173–179, https://doi.org/10.1097/MOP.0000000000000318 (2016).
Fliser, D. & Ritz, E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis 37, 79–83 (2001).
White, C. A. et al. Estimating GFR using serum beta trace protein: accuracy and validation in kidney transplant and pediatric populations. Kidney Int 76, 784–791, https://doi.org/10.1038/ki.2009.262 (2009).
Poge, U. et al. Beta-trace protein-based equations for calculation of GFR in renal transplant recipients. Am J Transplant 8, 608–615, https://doi.org/10.1111/j.1600-6143.2007.02117.x (2008).
Fan, L. et al. Comparing GFR Estimating Equations Using Cystatin C and Creatinine in Elderly Individuals. J Am Soc Nephrol, https://doi.org/10.1681/ASN.2014060607 (2014).
Inker, L. A. et al. GFR Estimation Using beta-Trace Protein and beta2-Microglobulin in CKD. Am J Kidney Dis 67, 40–48, https://doi.org/10.1053/j.ajkd.2015.07.025 (2016).
White, C. A. et al. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis 54, 922–930, https://doi.org/10.1053/j.ajkd.2009.06.003 (2009).
Ebert, N. et al. Prevalence of reduced kidney function and albuminuria in older adults: the Berlin Initiative Study. Nephrol Dial Transplant, https://doi.org/10.1093/ndt/gfw079 (2016).
Glassock, R., Delanaye, P. & El Nahas, M. An Age-Calibrated Classification of Chronic Kidney Disease. JAMA, https://doi.org/10.1001/jama.2015.6731 (2015).
Shlipak, M. G., Praught, M. L. & Sarnak, M. J. Update on cystatin C: new insights into the importance of mild kidney dysfunction. Curr Opin Nephrol Hypertens 15, 270–275, https://doi.org/10.1097/01.mnh.0000222694.07336.92 (2006).
Filler, G. et al. Beta-trace protein, cystatin C, beta(2)-microglobulin, and creatinine compared for detecting impaired glomerular filtration rates in children. Clin Chem 48, 729–736 (2002).
Priem, F. et al. Beta-trace protein in serum: a new marker of glomerular filtration rate in the creatinine-blind range. Clin Chem 45, 567–568 (1999).
Stevens, L. A. & Stoycheff, N. Standardization of serum creatinine and estimated GFR in the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 51, S77–82, https://doi.org/10.1053/j.ajkd.2008.01.001 (2008).
Grubb, A. et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med 48, 1619–1621, https://doi.org/10.1515/CCLM.2010.318 (2010).
White, C. A. et al. beta-Trace Protein Assays: A Comparison Between Nephelometric and ELISA Methodologies. Am J Kidney Dis 69, 866–868, https://doi.org/10.1053/j.ajkd.2017.02.371 (2017).
Foster, M. C. et al. Serum beta-Trace Protein and beta2-Microglobulin as Predictors of ESRD, Mortality, and Cardiovascular Disease in Adults With CKD in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis 68, 68–76, https://doi.org/10.1053/j.ajkd.2016.01.015 (2016).
Filler, G., Alvarez-Elias, A. C., Westreich, K. D., Huang, S. S. & Lindsay, R. M. Can the new CKD-EPI BTP-B2M formula be applied in children? Pediatr Nephrol 31, 2175–2177, https://doi.org/10.1007/s00467-016-3493-3 (2016).
Priem, F., Althaus, H., Jung, K. & Sinha, P. Beta-trace protein is not better than cystatin C as an indicator of reduced glomerular filtration rate. Clin Chem 47, 2181 (2001).
Huber, A. R. & Risch, L. Recent developments in the evaluation of glomerular filtration rate: is there a place for beta-trace? Clin Chem 51, 1329–1330, https://doi.org/10.1373/clinchem.2005.053389 (2005).
Schaeffner, E. S. et al. The Berlin initiative study: the methodology of exploring kidney function in the elderly by combining a longitudinal and cross-sectional approach. Eur J Epidemiol 25, 203–210 (2010).
Ebert, N. et al. Iohexol plasma clearance measurement in older adults with chronic kidney disease-sampling time matters. Nephrol Dial Transplant 30, 1307–1314, https://doi.org/10.1093/ndt/gfv116 (2015).
DuBois, D. D. E. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med 17, 863–871 (1916).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Acknowledgements
The authors thank their colleagues at the 13 study sites in Berlin for supporting their work and providing the necessary infrastructure for the study and Jaqueline Herrmann for her support with the biobank samples. We are indebted to the participants of the BIS for their participation and commitment. We also thank the health insurance fund AOK Nordost-Die Gesundheitskasse for their technical support and their cooperation. This study was funded by the Kuratorium für Dialyse und Nierentransplantation (KfH) Foundation for Preventive Medicine, Neu-Isenburg, Germany and the Dr. Werner Jackstädt Foundation.
Author information
Authors and Affiliations
Contributions
N.E. and E.S. are PIs of the BIS and wrote the main manuscript text, K.S. and P.M. prepared Figs 1–2b and reviewed the tables. J.B. performed BTP analysis, M.V.D.G., M.S. and M.T. performed iohexol analyses, N.M., C.K., M.K. and J.G. were part of the study recruiting team. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ebert, N., Koep, C., Schwarz, K. et al. Beta Trace Protein does not outperform Creatinine and Cystatin C in estimating Glomerular Filtration Rate in Older Adults. Sci Rep 7, 12656 (2017). https://doi.org/10.1038/s41598-017-12645-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12645-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.