Abstract
Lipid mediators play pivotal roles in colorectal cancer and colitis, but only a limited member of the phospholipase A2 (PLA2) subtypes, which lie upstream of various lipid mediators, have been implicated in the positive or negative regulation of these diseases. Clinical and biochemical evidence suggests that secreted PLA2 group III (sPLA2-III) is associated with colorectal cancer, although its precise role remains obscure. Here we have found that sPLA2-III-null (Pla2g3 −/−) mice are highly resistant to colon carcinogenesis. Furthermore, Pla2g3 −/− mice are less susceptible to dextran sulfate-induced colitis, implying that the amelioration of colonic inflammation by sPLA2-III ablation may underlie the protective effect against colon cancer. Lipidomics analysis of the colon revealed significant reduction of pro-inflammatory/pro-tumorigenic lysophosholipids as well as unusual steady-state elevation of colon-protective fatty acids and their oxygenated metabolites in Pla2g3 −/− mice. Overall, our results establish a role of sPLA2-III in the promotion of colorectal inflammation and cancer, expand our understanding of the divergent roles of multiple PLA2 enzymes in the gastrointestinal tract, and point to sPLA2-III as a novel druggable target for colorectal diseases.
Similar content being viewed by others
Introduction
Colorectal cancer is a frequent form of malignancy and a major cause of death in the Western hemisphere. Although physiologic levels of inflammation are protective and promote tissue repair, excessive inflammation is deleterious and lies at the basis of inflammatory bowel disease (IBD) that are ultimately linked to the development of colorectal cancer1. When commensal bacteria breach the colonic epithelial barrier, they trigger a state of chronic inflammation, which leads to neoplastic transformation of the overlying colorectal epithelium2,3. Continuous production of cytokines, growth factors, matrix proteases, angiogenic factors, and reactive oxygen species promote tumorigenesis by creating a microenvironment favoring colonic epithelial proliferation, survival, and invasiveness.
Lipid mediators represent a group of bioactive molecules that have detrimental or beneficial impacts on colorectal inflammation and cancer. Clinical use of non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenases (COXs) and thereby block the biosynthesis of prostaglandins (PGs) from ω6 arachidonic acid (AA; C20:4), is associated with a decreased risk of colorectal cancer4,5. This effect of NSAIDs is attributable mainly to reduced production of PGE2, since genetic deletion of biosynthetic enzymes or receptors for PGE2 prevents colonic carcinogenesis6,7,8. Conversely, ablation of the PGD2 or PGI2 pathway accelerates colitis or colitis-associated cancer9,10,11. ω3 Polyunsaturated fatty acids (PUFAs), such as eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6), are protective against inflammation and cancer in general, partly through conversion to pro-resolving lipid mediators such as resolvins and protectins12,13,14,15. Lysophospholipid mediators, such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), have also been implicated in colorectal homeostasis and disease16,17,18,19,20. Moreover, medium- to long-chain saturated fatty acids have aggravating effects on intestinal and systemic immunological responses, while long-chain unsaturated or short-chain fatty acids counteract these processes toward homeostatic maintenance21,22.
Phospholipase A2 (PLA2) is a group of enzymes that hydrolyze phospholipids to liberate fatty acids and lysophospholipids, representing the first rate-limiting step in the biosynthesis of a variety of lipid mediators. The mammalian genome encodes more than 30 PLA2s or related enzymes, which are classified into several subfamilies23. Of these, group IVA cytosolic PLA2 (cPLA2α) is coupled with the production of a large pool of colorectal PGE2, which promotes colorectal cancer through its receptor, EP2, and prevents colitis through another receptor, EP47,8,24,25,26. Group IIA secreted PLA2 (sPLA2-IIA), an intestinal Paneth cell-derived sPLA2 also known to be a genetic modifier for tumor multiplicity in mice27, reduces susceptibility to intestinal tumorigenesis possibly by altering the differentiation and function of intestinal stem cells, by mobilization of eicosanoids, or by other mechanisms28. Group X sPLA2 (sPLA2-X), a major sPLA2 expressed in colonic epithelial and goblet cells, releases ω3 PUFAs, thus attenuating colitis24 and colorectal cancer28. These observations in mouse models appear to corroborate the inverse correlation between the expression levels of sPLA2-IIA and -X and the malignancy of gastrointestinal cancers in humans29,30. However, the entire picture of the PLA2-driven lipid pathways that are positively or negatively linked to colon pathophysiology is still not fully understood.
Group III sPLA2 (sPLA2-III), an atypical sPLA2 with unique structural and functional features31,32, has been proposed as a candidate biomarker for human colon cancer33. Implantation of sPLA2-III-transfected colon cancer cells into nude mice leads to increased growth of tumor xenografts34. Higher expression of sPLA2-III in human colorectal cancer is positively correlated with a higher rate of lymph node metastasis and shorter survival35. Moreover, polymorphisms in the human PLA2G3 gene are significantly associated with a higher risk of colorectal cancer36. In the present study, to gain further insights into the role of sPLA2-III in colorectal diseases, we utilized sPLA2-III-deficient (Pla2g3 −/−) mice37,38. The results we obtained suggest that sPLA2-III promotes colonic cancer and colitis at least partly through mobilization of pro-inflammatory/pro-tumorigenic lysophospholipids. Thus, an agent that specifically inhibits this atypical sPLA2 could be useful for treatment of patients with colon disorders.
Results
sPLA2-III is expressed in the colorectal epithelium
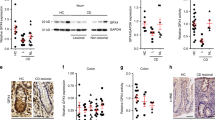
Quantitative RT-PCR of C57BL/6 mouse tissues revealed high expression of Pla2g3 mRNA in the colon and skin, followed in order by the stomach, lung, and immune organs (Fig. 1a). Within the gut tissues, Pla2g3 was expressed throughout the proximal to distal colon, with a tendency for higher expression in the proximal than distal areas, and the expression was markedly higher than in the small intestine (duodenum, jejunum and ileum) (Fig. 1a, Inset). In the colon, Pla2g3 expression was enriched in Cd326 (Epcam)-positive colonic epithelial cells (CECs) (Fig. 1b). Immunohistochemistry of the wild-type (WT; Pla2g3 +/+) mouse colon showed that sPLA2-III protein was expressed mainly in CECs, particularly those facing the colonic lumen (Fig. 1c). No such staining was evident in the colon of Pla2g3 −/− mice, confirming the specificity of the anti-sPLA2-III antibody used.
Expression of sPLA2-III in mouse colon. (a) Quantitative RT-PCR of Pla2g3 in C57BL/6 mouse tissues (8-weeks-old males), with Gapdh normalization (n = 3). Inset, expression of Pla2g3 in distinct portions of the small and large intestines (n = 3). BAT and WAT; brown and white adipose tissues, respectively. (b) Quantitative RT-PCR of Pla2g3 and Cd326 in CECs and non-CECs in WT colon (n = 3). Mean ± SEM, *P < 0.05 and **P < 0.01. (c) Immunohistochemistry of sPLA2-III in Pla2g3 +/+ and littermate Pla2g3 −/− colons (bar, 100 μm). Boxed areas are magnified. Results are from one (a,b) and three (c) experiments.
Pla2g3 −/− mice are protected from colorectal cancer
To assess the role of sPLA2-III in colonic cancer, we applied a colon carcinogenesis model induced by azoxymethane (AOM), a procarcinogen that – upon metabolic activation in the liver and distal colon – induces the formation of O 6-methyl-guanine39, in Pla2g3 −/− mice and also littermate Pla2g3 +/+ mice for comparison. Pla2g3 +/+ and Pla2g3 −/− mice were intraperitoneally administered AOM once a week for 6 weeks and then sacrificed 28 weeks after the last treatment (Fig. 2a). AOM treatment induced the development of multiple tumors in the middle to distal colon of Pla2g3 +/+ mice, whereas tumor development was markedly attenuated in Pla2g3 −/− mice (Fig. 2b–d). sPLA2-III deletion decreased the total tumor burden in the colon after AOM treatment, the number of large (>2 mm in diameter) and small (<2 mm) tumors being markedly lower in Pla2g3 −/− mice than in Pla2g3 +/+ mice (Fig. 2b,c). Histologically, tumors in Pla2g3 −/− mice were rather smaller than those in Pla2g3 +/+ mice (Fig. 2d). Thus, consistent with previous cell biological and clinical studies33,34,35,36, our present results lend further support to the notion that sPLA2-III contributes to exacerbation of colon cancer.
Pla2g3 −/− mice are protected against colorectal cancer. (a) Schematic representation of the procedure for AOM-induced colon cancer. (b) Number of total, small (<2 mm in diameter) and large (>2 mm) polyps per mouse in the colons of AOM-treated Pla2g3 −/− in comparison with littermate Pla2g3 −/− mice (n = 19–38). (c) Representative photographs of colon tissues from Pla2g3 +/+ and Pla2g3 −/− mice. Polyps are indicated by arrows. (d) Representative hematoxylin and eosin staining of the colon tissues from Pla2g3 +/+ and Pla2g3 −/− mice. Bar: 200 µm. (e) Microarray gene profiling of the whole descending colons of Pla2g3 −/− (KO) and littermate Pla2g3 +/+ (WT) mice with AOM or vehicle treatment. Equal amounts of total RNA (pooled from four mice for each genotype) were subjected to one-color gene expression microarray analysis. Data were processed using Agilent’s Feature Extraction Software and analyzed using GeneSpring Software. Fold changes (KO relative to WT) on the microarray are listed, and genes showing a >2-fold increase (red) or decrease (blue) in expression are highlighted. (f) Quantitative RT-PCR of Pla2g3 and several inflammatory genes in the colons of Pla2g3 +/+ and Pla2g3 −/− mice after treatment with or without AOM (n = 5–8). (g) Quantitative RT-PCR of genes for M2 macrophages, regulatory T cells, and angiogenesis in the colons of AOM-treated Pla2g3 +/+ and Pla2g3 −/− mice (n = 5). (h) Number of polyps per mouse in the small and large intestines of Pla2g3 +/+ Apc Min/+ and Pla2g3 −/− Apc Min/+ mice (4-month-old males) (n = 27–45). Mean ± SEM, *P < 0.05 and **P < 0.01. Results are from one experiment (e) or complied from at least two experiments (b,f–h).
Microarray gene profiling of the colon revealed notable changes in the expression of a panel of genes related to epithelial homeostasis and inflammation in Pla2g3 −/− mice, relative to Pla2g3 +/+ mice (Fig. 2e). In the vehicle-treated control group, the expression levels of Il6, Il17a and Il22, but not Il23, were increased in Pla2g3 −/− mice relative to Pla2g3 +/+ mice. These cytokines regulate Th17-type immune responses, which on the one hand help to maintain the intestinal barrier, defense and repair, but can also cause immunopathology particularly in the presence of IL-2340,41,42,43,44,45. Consistently, expression of the IL-22-inducible anti-microbe genes Reg3g and Defa3, as well as the adherence junction protein Dsg1, was upregulated in the Pla2g3 −/− colon relative to the Pla2g3 +/+ colon (Fig. 2e), suggesting enhancement of colorectal defense and barrier function in the null mice under steady-state conditions. In contrast, following AOM treatment, the expression levels of many genes related to pro-inflammatory or Th17-related cytokines (e.g. Il1b, Il6, Tnfa, Ifng, Il17a, Il22 and Il23), lipid mediators (e.g. Ptgs2 and Ptges), immune cells (e.g. Emr1 and Tbx21), epithelial defense (e.g. Reg3g, S100a8, S100a9 and Defa3), and adherence junctions (e.g. Dsg4, Cdh13 and Cldn4) were reduced in the Pla2g3 −/− colon relative to the Pla2g3 +/+ colon (Fig. 2e), implying that sPLA2-III deficiency protects mice from tumor-associated inflammation and homeostatic perturbation in the colon.
In support of these observations, quantitative RT-PCR revealed that the expression of inflammatory genes (Il1b, Il23, Ptgs2 and Ptges) was robustly increased in the Pla2g3 +/+ colon following AOM challenge, whereas these disease-associated changes were scarcely evident in the Pla2g3 −/− colon (Fig. 2f). The decreased induction of Ptgs2 (encoding COX-2) and Ptges (encoding PGE2 synthase (mPGES-1)) in Pla2g3 −/− mice relative to Pla2g3 +/+ mice implies that the tumor-associated production of PGE2, a pro-tumorigenic prostanoid6,7,8, is reduced by the lack of sPLA2-III. Il22 expression was elevated in the basal state and decreased after AOM treatment in Pla2g3 −/− mice relative to Pla2g3 +/+ mice (Fig. 2f), thus validating the microarray data (Fig. 2e). In line with the crucial role of sPLA2-III in mast cell maturation38, AOM-induced robust expression of mast cell markers (Mcpt1 and Mcpt2) was nearly absent in Pla2g3 −/− colon (Fig. 2f). Furthermore, in the AOM-treated group, the expression of markers for M2 macrophages (Arg1 and Ym1) and regulatory T cells (Foxp3), which facilitate tumor growth by counteracting anti-tumor immunity46,47, was lower in Pla2g3 −/− colon than in Pla2g3 +/+ colon, although that of the angiogenic marker Vegfa was comparable between the genotypes (Fig. 2g). Unlike the situation in human colorectal cancer33,34,35,36, however, colorectal Pla2g3 expression was decreased in this colon cancer model (Fig. 2f), probably reflecting tumor heterogeneity or species difference. Nevertheless, these results collectively suggest that sPLA2-III promotes tumorigenesis and attendant inflammation in AOM-induced colon cancer.
To address the role of sPLA2-III in colon cancer further, we crossed Pla2g3 −/− mice with Apc Min/+ mice, a model of human familial adenomatous polyposis in which the oncogenic Wnt/β-catenin signal is hyperactivated due to a mutation in the Apc gene, leading to spontaneous development of intestinal cancer, particularly in the small intestine48. Although polyposis in the small intestine was barely affected by sPLA2-III depletion, the number of larger polyps was significantly lower in the colon of Pla2g3 −/− Apc Min/+ mice than in that of Pla2g3 +/+ Apc Min/+ mice (Fig. 2h). This colon-specific effect might be attributable to the fact that sPLA2-III is expressed mainly in the colon, but only minimally in the small intestine (Fig. 1a). Thus, sPLA2-III plays an exacerbating role in colonic tumorigenesis in two distinct models.
Pla2g3 −/− mice are less sensitive to colitis
Given that all sporadic colon cancers exhibit some aspects of inflammation and that the pathogenesis of some types of colon cancer is associated with IBD1, we hypothesized that the protection against colon cancer in Pla2g3 −/− mice might be based on an ameliorated inflammatory response. To investigate this possibility, we next performed a model of acute colitis induced by dextran sulfate sodium (DSS), a sulfated polysaccharide known to be toxic to the colonic epithelium49,50. The DSS study is applicable to a model of IBD cancer (see below).
We administered 1.5% (w/v) DSS in water to Pla2g3 −/− and littermate Pla2g3 +/+ mice for 4 days and then allowed the animals to recover with clean drinking water for an additional 5 days (Fig. 3a). Pla2g3 +/+ mice were susceptible to this regimen, beginning to lose body weight on day 5 and losing ~15% of their initial body weight by day 8 (Fig. 3b). This body weight change was preceded by progressive elevation of the clinical score (as monitored by fecal bleeding plus diarrhea), which began to increase on day 1 and peaked on days 6–8 (Fig. 3c). In contrast, the body weight of Pla2g3 −/− mice remained nearly constant throughout the experimental period (Fig. 3b). Although the clinical score was gradually increased in Pla2g3 −/− mice, it was less severe than that in Pla2g3 +/+ mice (Fig. 3c). On day 9, necropsy revealed drastic shortening of the colon length in Pla2g3 +/+ mice, whereas Pla2g3 −/− mice were protected from this severe sign of colitis (Fig. 3d). Histologically, the Pla2g3 +/+ colon showed a progressive increase in crypt abscesses, mucosal inflammation with leukocyte infiltration, and enlargement of the muscularis propria with loss of the colonic epithelium and crypt structure by day 6, followed by apparent recovery from these symptoms by day 9 (Fig. 3e). On the other hand, these histopathological features were fairly mild in the Pla2g3 −/− colon (Fig. 3e). On day 4, Ki67-positive epithelial cells were more numerous in the Pla2g3 −/− colon than in the Pla2g3 +/+ colon (Fig. 3f), suggesting that the lack of sPLA2-III had allowed rapid recovery from epithelial injury.
Pla2g3 −/− mice are protected against DSS-induced colitis. (a) Schematic representation of the procedure for DSS-induced colitis. (b,c) Daily monitoring of body weight loss (b) and clinical score (c) in Pla2g3 −/− and littermate Pla2g3 +/+ mice (8-week-old males) with or without DSS treatment (n = 16). (d,e) Gross appearance (d) and histology (e) of the colon in Pla2g3 +/+ and Pla2g3 −/− mice after treatment with DSS. Bar, 100 μm. (f) Scoring of proliferating CEC cells per section as determined by Ki67 staining of Pla2g3 +/+ and Pla2g3 −/− colons on day 4 (n = 3–5). (g) Quantitative RT-PCR of Pla2g3 and several inflammatory or epithelial barrier genes in the colon of Pla2g3 +/+ and Pla2g3 −/− mice after DSS treatment (n = 3–5). Mean ± SEM, *P < 0.05 and **P < 0.01. Data are from one experiment (f,g) or compiled from four experiments (b,c).
In Pla2g3 +/+ mice, expression of the pro-inflammatory genes Il1b, Il6, Tnfa, Ifng, Il17a, and Mmp9 was markedly induced, whereas that of the epithelial markers Cldn1 and Muc2, which are crucial for epithelial barrier function51,52, was decreased, over days 4–9 after DSS challenge, with a peak on day 6 (Fig. 3g). In contrast, expression of these genes was affected only modestly in Pla2g3 −/− mice (Fig. 3g). The kinetic expression profile of Pla2g3 in the WT colon was similar to that of Cldn1 and Muc2, consistent with its localization in the colorectal epithelium, which collapsed during DSS-induced injury. These results suggest that, in contrast to cPLA2α and sPLA2-X, which exert protective effects against DSS-induced colitis24, sPLA2-III has a promoting role in this disease model. Immunohistochemistry of DSS-treated WT colon revealed that, in addition to collapsing epithelial cells, some infiltrating inflammatory cells were sporadically stained with anti-sPLA2-III antibody (Supplementary Fig. 1). Therefore, it is possible that during colitis, sPLA2-III released from these particular immune cell populations might participate in the progress of the disease.
To further assess the relationship between the ameliorating effect of sPLA2-III deficiency on colitis (Fig. 3) and that on colorectal cancer (Fig. 2), we next subjected Pla2g3 −/− and control Pla2g3 +/+ mice to chronic DSS treatment (Fig. 4a). With this regimen, Pla2g3 +/+ mice progressively succumbed to the repeated DSS challenges, around 75% having died by the end point, whereas Pla2g3 −/− mice were more resistant (Fig. 4b). Quantitative RT-PCR of the colon revealed markedly reduced expression of pro-inflammatory or pro-tumorigenic genes (Arg1, Ptgs2, Il1b and Il6) in Pla2g3 −/− mice relative to Pla2g3 +/+ mice (Fig. 4c). Moreover, following the AOM + DSS regimen, in which DSS-induced chronic inflammation leads to the development of colon cancer53, the colon of surviving Pla2g3 −/− mice had a lower burden of large tumors (>2 mm in diameter) than did replicate Pla2g3 +/+ mice (Fig. 4d,e). Taken together, these results indicate that the inflammatory microenvironment created by sPLA2-III enhances the development of tumors.
Pla2g3 −/− mice are protected against colitis-induced colorectal cancer. (a) Schematic representation of the procedure for the AOM + DSS-induced colon cancer model. (b) Monitoring of survival rates of Pla2g3 −/− and littermate Pla2g3 +/+ mice following repeated DSS challenges. A representative result of three experiments, starting from 12 and 14 Pla2g3 +/+ and Pla2g3 −/− mice, respectively, is shown. (c) Quantitative RT-PCR of inflammation-associated genes in the colons of DSS-treated Pla2g3 +/+ and Pla2g3 −/− mice on day 56 (n = 3 and 9 for Pla2g3 +/+ and Pla2g3 −/− mice, respectively, in one experiment). (d) Gross appearance of the colons in Pla2g3 +/+ and Pla2g3 −/− mice after treatment with AOM + DSS on day 56. (e) Number of large (>2 mm) polyps per mouse in the colons of AOM + DSS-treated Pla2g3 +/+ and Pla2g3 −/− mice on day 56 (n = 23–29; compiled from two experiments). Mean ± SEM, *P < 0.05 and **P < 0.01.
Colonic sPLA2-III mobilizes lysophospholipids
Under in vivo conditions, lipid mobilization by sPLA2 depends not only on its intrinsic substrate specificity, but also on the spatiotemporal availability or phospholipid composition of target membranes in a given tissue microenvironment, which explains why distinct sPLA2s exert specific functions with different lipid profiles in distinct settings23. For instance, colorectal sPLA2-X preferentially releases anti-inflammatory ω3 PUFAs, thereby exerting a protective effect against DSS-induced colitis24. We reasoned that the pro-inflammatory and thereby pro-tumorigenic actions of sPLA2-III might rely on a unique form of lipid metabolism possibly differing from that driven by sPLA2-X. With this possibility in mind, we reevaluated the in vitro enzymatic action of recombinant sPLA2-III on tissue-extracted natural membranes by lipidomics analysis using electrospray ionization mass spectrometry (ESI-MS)54,55. Incubation of bulk phospholipids extracted from mouse colon with recombinant sPLA2-III resulted in dose-dependent increases of AA and DHA in preference to other fatty acids (Fig. 5a), as well as increases in various lysophospholipid species bearing a saturated or monounsaturated fatty acid (Fig. 5b). These results confirmed that sPLA2-III has the capacity to hydrolyze phospholipids with a tendency for sn-2 PUFA preference without apparent polar head group selectivity in vitro 23.
In vitro enzymatic activity of sPLA2-III on phospholipids extracted from the colon. Release of fatty acids (a) and lysophospholipids (b) from colon-extracted phospholipids (10 μM) after incubation for 30 min with the indicated concentrations of recombinant sPLA2-III (n = 4). Individual lysophospholipid and fatty acid species were evaluated by ESI-MS. Values are mean ± SEM. LPE, lysophosphatidylethanolamine; LPS, lysophosphatidylserine; LPG, lysophosphatidylglycerol. Representative results of two experiments are shown.
Having established the enzymatic property of sPLA2-III, we next performed lipidomics analysis of the colon from Pla2g3 +/+ and Pla2g3 −/− mice to identify particular lipid products (fatty acids, lysophospholipids and their metabolites) that were altered by sPLA2-III deficiency in vivo, under the assumption that the sPLA2-III-driven lipid products would be decreased in the null mice. For this purpose, we chose a condition of acute DSS-induced colitis, since chronic inflammation and cancer would be expected to be associated with global alteration in lipid metabolism (see below), making precise assessment of the sPLA2-III-intrinsic action difficult. In Pla2g3 +/+ mice, the colorectal levels of free fatty acids and their metabolites were elevated to various degrees following DSS treatment (Fig. 6a), as expected from our previous study24. However, none of these lipids were decreased in Pla2g3 −/− colon relative to Pla2g3 +/+ colon regardless of DSS challenge; in fact, they were unexpectedly elevated in the null mice, particularly under steady-state conditions (Fig. 6a). These lipids included fatty acids [oleic acid (OA, 18:1), linoleic acid (LA, 18:2), AA, EPA and DHA] as well as their oxygenated metabolites including 9-hydroxyoctadecaenoic acid (9-HODE), PGE2, 12-hydroxyheptadecatrenoic acid (12-HHT), lipoxin A4 (LXA4) and resolvin D1 (RvD1), which have been reported to facilitate colorectal barrier function, defense and repair, and prevent colonic inflammation14,26,56,57. After DSS treatment, these differences between the genotypes were masked by overall elevation of fatty acids and their metabolites (except for OA, which was significantly greater in the null mice) (Fig. 6a).
Lipidomics analysis of the colons of Pla2g3 +/+ and Pla2g3 −/− mice. ESI-MS analysis of fatty acids and their metabolites (n = 7–8) (a) and lysophospholipids (n = 5–7) (b) in the colons of Pla2g3 −/− and littermate Pla2g3 +/+ mice with (+) or without (−) DSS treatment for 4 days. Mean ± SEM, *P < 0.05 and **P < 0.01. Representative results of two experiments are shown.
Considering that the steady-state increase of fatty acids appeared to reflect a secondary, rather than direct, effect of sPLA2-III deficiency, we compared the expression of a wide array of lipid-metabolic genes in Pla2g3 +/+ and Pla2g3 −/− mice using the microarray. However, the steady-state expression levels of most lipid-metabolic genes were not profoundly affected by the lack of sPLA2-III (Supplementary Table 1). A few exceptions included substantial elevation of Lpcat2 (lysophosphatidylcholine (LPC) acyltransferase 2) and Aloxe3 (epidermal lipoxygenase 3) in Pla2g3 −/− mice relative to Pla2g3 +/+ mice, but these alterations could not fully account for the overall elevation of free fatty acids and their metabolites in the null mice. Therefore, we speculate that the steady-state elevation of fatty acid levels in the Pla2g3 −/− colon might result from post-transcriptional modifications of some lipid-metabolic enzymes or from an alteration in colorectal microbiota that produce or consume fatty acids. Nevertheless, the steady-state increases of colon-protective fatty acid metabolites in Pla2g3 −/− mice could have contributed, at least partly, to protection against colitis. As expected, the expression levels of many lipid-metabolic genes were altered in AOM-treated Pla2g3 −/− mice compared with Pla2g3 +/+ mice (Supplementary Table 1), likely as a result of the marked attenuation of colon cancer in the null mice. For instance, the reduced expression of Pla2g2d corroborates the decrease of tumor-promoting M2-like macrophages (Fig. 2g)58, the reduced expression of Plcg1 and Plcg2 could explain the decreased growth factor signaling59, and the altered expression of several lipogenic, lipolytic and β-oxidation genes might reflect cancer-associated metabolic reprogramming60, in AOM-treated Pla2g3 −/− mice relative to Pla2g3 +/+ mice.
Intriguingly, under DSS-treated (but not steady-state) conditions, the colonic levels of several, if not all, classes of lysophospholipids, including LPC, LPA, and lysophosphatidylinositol (LPI) bearing a saturated fatty acid, which are typical PLA2 reaction products, were significantly lower in Pla2g3 −/− than in Pla2g3 +/+ mice (Fig. 6b). A large body of evidence suggests that these lysophospholipid species participate in the promotion of colitis or colonic cancer16,17,18,19,61. It is thus likely that sPLA2-III mobilizes a pool of these pro-inflammatory and/or pro-tumorigenic lysophospholipids from DSS-damaged, rather than intact, epithelial membranes, thus contributing to the exacerbation of colonic inflammation and subsequent progression to colonic cancer.
Discussion
The proposed connection of sPLA2-III with colorectal cancer has arisen from the findings that overexpression of sPLA2-III in colon cancer cells enhances proliferation both in culture and in nude mice34, that higher expression of sPLA2-III is significantly correlated with more aggressive metastasis and poorer prognosis in patients with colorectal cancer35, and that human PLA2G3 polymorphisms are significantly associated with a higher risk of colon cancer36. Our present study employing Pla2g3 −/− mice provides compelling evidence for the exacerbating role of sPLA2-III in colorectal cancer. Given that Pla2g3 −/− mice are also protected from colitis, the pro-tumorigenic function of sPLA2-III may rely, at least in part, on the ability of this extracellular lipolytic enzyme to propagate colorectal inflammation, which is now well recognized as a key mechanism underlying colorectal carcinogenesis1,2,3.
Currently, only a few PLA2s have been firmly assigned to colonic pathophysiology. cPLA2α, an AA-specific intracellular PLA2 that is activated by Ca2+ and phosphorylation following diverse stimuli62,63, mobilizes a large pool of colonic PGE2, which is protective against acute injury but accelerates chronic colitis and cancer, most likely by acting on distinct PGE2 receptors spatiotemporally expressed in different cells6,7,8,24,25. Thus, mice lacking EP4 display more severe acute colitis, whereas those lacking EP2 are protected from colonic cancer8,26. Genetic absence of sPLA2-IIA and -X in the small and large intestines improves recovery from intestinal inflammation but predisposes mice to colorectal cancer, and this trade-off effect has been proposed to involve sPLA2 receptor-dependent activation of cPLA2α and thereby production of PGE2 28. Using knockout mouse lines for various sPLA2s, we have recently shown that sPLA2-X preferentially mobilizes anti-inflammatory ω3 PUFAs including DHA and EPA, which block harmful Th17-type immune responses and thereby attenuate colitis partly through the fatty acid receptor GPR12024. Our present results suggest that colorectal sPLA2-III drives another arm of the lipid pathways, namely the production of pro-inflammatory/pro-tumorigenic lysophosholipids, which may be eventually linked to the exacerbation of colonic inflammation and cancer. These findings accommodate an emerging concept that distinct PLA2s act on different membranes, thereby spatiotemporally mobilizing specific lipid products that have variable impacts on distinct stages of homeostasis and diseases even in the same tissue23.
In DSS-treated Pla2g3 −/− colon, there are significant decreases in LPC, LPA and LPI species with a saturated fatty acid, suggesting that they are produced by sPLA2-III. LPA, a pluripotent lysophospholipid mediator with diverse functions, is produced either indirectly by conversion of phosphatidylcholine (PC) to LPC by PLA2 (or PLA1) and then to LPA by autotaxin (lysophospholipase D) or directly by PLA2 (or PLA1)-mediated conversion of phosphatidic acid (PA) to LPA64. A growing body of evidence suggests that LPA promotes cancer cell proliferation, motility and metastasis65,66,67,68. This view appears to be reminiscent of the potential association of sPLA2-III with colon cancer malignancy and metastasis in humans35,36. Indeed, the attenuated colonic inflammation and cancer observed in Pla2g3 −/− mice are strikingly similar to those reported for mice lacking one of the LPA receptors, LPA2 17,69. Although the in vivo roles of LPI, a PLA2-hydrolyzed product of phosphatidylinositol (PI), are less clear, genetic or pharmacological ablation of GPR55, an LPI receptor, has been reported to alleviate colitis18,61. Since sPLA2-III has the capacity to hydrolyze all classes of phospholipids without apparent polar head group specificity, we speculate that this sPLA2 may be accessible to certain membrane compartments rich in the precursor phospholipids (PC, PA and PI) within a particular colonic microenvironment, leading to generation of a pool of the pathogenetic lysophospholipid species that may act on specific receptors such as LPA2 and GPR55. Notably, lysophospholipid production by sPLA2-III is evident in DSS-treated, but not steady-state, colon, suggesting that sPLA2-III acts on labile or damaged epithelial membranes or on certain infiltrating leukocyte membranes in this disease setting.
Contrary to our prediction, the steady-state levels of unsaturated fatty acids and their metabolites (prostanoids, lipoxins and resolvins) were found to be noticeably elevated (rather than decreased) in Pla2g3 −/− colon relative to Pla2g3 +/+ colon. Given the biological actions of these fatty acid metabolites14,26,56,57, this alteration could contribute to homeostatic protection against epithelial damage and excessive inflammation in Pla2g3 −/− mice. Indeed, the steady-state increase of PGE2 could account for that of IL-22, an epithelial cytokine crucial for intestinal homeostasis70, in Pla2g3 −/− mice, since this AA metabolite, by acting on its receptor EP4, promotes gut barrier function through driving IL-22 production by type 3 innate immune cells (ILC3s)71. AA-derived LXA4 and DHA-derived RvD1 are now well recognized as anti-inflammatory lipid mediators that sequester inflammatory responses in general72. Moreover, long-chain fatty acids can shape the host-microbiome interface by modulating homeostatic inflammasome signaling in CECs followed by IL-22 expression by ILC3s, which in turn promotes the secretion of mucins and antimicrobial peptides from CECs and the regeneration of colonic stem cells42,73,74.
The unusual increase of fatty acids and their metabolites in the Pla2g3 −/− colon in the normal state could be explained by a compensatory mechanism involving an increase of lipid synthesis or a reduction of lipid consumption. However, our microarray analysis showed that the steady-state expression levels of a wide array of lipid-metabolic genes were largely unaffected by sPLA2-III deficiency. Since our present study suggests that the Pla2g3 −/− colon shows signs of improved epithelial barrier function under steady-state conditions, it is tempting to speculate that certain homeostatic stimuli such as innate or adaptive immune responses triggered by commensal bacteria or nutrients, which exert various effects on intestinal physiology42,75, might post-transcriptionally modulate the activity, stability or localization of some lipid-metabolizing enzymes, leading to constitutive elevation of free fatty acid levels. Alternatively, the absence of sPLA2-III might alter colorectal microflora that produce or consume fatty acids. In this context, sPLA2-III constitutively secreted from CECs might affect the functions or populations of colonic stem cells, ILC3s or other cells, or even the microbiome, by mobilizing certain unidentified lipid metabolites or acting directly on microbial membranes, a possibility that remains to be investigated. Apart from the steady-state increase of PGE2, the absence of sPLA2-III markedly attenuates the tumor-associated induction of PGE2-biosynthetic enzymes (COX-2 and mPGES-1), implying the reduced biosynthesis of pro-tumorigenic PGE2 in the colon cancer state.
sPLA2-III secreted from mast cells promotes their maturation and accompanying anaphylaxis through a microenvironmental PGD2-mediated paracrine circuit38. Indeed, we have shown that the expression levels of mast cell markers are markedly lower in Pla2g3 −/− colon than in Pla2g3 +/+ colon, confirming that the null mice have mast cell insufficiency38. Several lines of evidence suggest that mast cells often influence the pathology of colitis and cancer76,77,78, raising the possibility that sPLA2-III in mast cells may have a role in colorectal diseases. However, recent findings suggesting that mast cell-derived PGD2 prevents, rather than promotes, colon inflammation and cancer10 and that mast cells facilitate recovery, rather than injury, of the epithelium in DSS-induced colitis77 argue against this idea. Future analysis using mice with conditional Pla2g3 deletion in CECs, mast cells, or even other cell types will provide further insight into the mechanistic action of sPLA2-III.
Lastly, given that sPLA2-III, an atypical sPLA2, is insensitive to classical sPLA2 inhibitors and that no protein structurally homologous to sPLA2-III is encoded in the human genome, a new agent that specifically inhibits this unique sPLA2 may be useful for the treatment of patients with IBD and colorectal cancer.
Methods
Mice
Heterozygous Pla2g3 +/- mice (C57BL/6 × 129 Sv) were backcrossed to the C57BL/6 background for three generations, and then male and female heterozygotes were intercrossed to obtain Pla2g3 −/− mice and littermate Pla2g3 +/+ mice37,38. Probably because of this genetic background, littermate Pla2g3 +/+ mice were more susceptible to colitis and colonic tumorigenesis than C57BL/6 mice, as the presence of the 129/Sv genetic background increases the sensitivity to these models79,80,81,82. Apc Min/+ mice were purchased from Jackson Laboratory and crossed with Pla2g3 −/− mice. C57BL/6 mice were obtained from SLC Japan. All mice were housed in climate-controlled (23 °C) specific pathogen-free facilities with a 12-h light-dark cycle, with free access to standard diet CE2 (CLEA Japan) and water. All procedures involving animals were approved by the Institutional Animal Care and Use Committees of the Tokyo Metropolitan Institute of Medical Science, in accordance with the Standards Relating to the Care and Management of Experimental Animals in Japan.
Histology and immunohistochemistry
Formalin-fixed tissues were embedded in paraffin, sectioned, mounted on glass slides, deparaffinized in xylene, and rehydrated in ethanol with increasing concentrations of water. The tissue sections (4 μm thick) were incubated with 20 μg/ml proteinase K (Invitrogen) in phosphate-buffered saline (PBS) for antigen retrieval as required, incubated for 10 min with 3% (v/v) H2O2, washed 3 times with PBS for 5 min each, incubated with 5% (w/v) skim milk in PBS for 30 min, washed 3 times with PBS for 5 min each, and incubated with rabbit antibody against human sPLA2-III83 or Ki67 (Novus Biologicals) or with control antibody (Abcam) at 1:500 dilution in PBS overnight at 4 °C. The sections were then treated with a CSA system staining kit (Dako) with diaminobenzidine substrate, followed by counterstaining with hematoxylin.
Quantitative RT-PCR
Total RNA was extracted from tissues or cells using TRIzol reagent (Invitrogen). First-strand cDNA synthesis was performed using a High Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems). PCR was carried out using the TaqMan Gene Expression Assay (Applied Biosystems) on the ABI7700 Real Time PCR system (Applied Biosystems). The probe/primer sets used are listed in Supplementary Table 2.
DSS-induced colitis
Mice (8 weeks old, male) were orally administered 1.5% (w/v) DSS (average molecular weight 36,000–50,000) (MP Biomedicals, Solon, OH) in drinking water for 4 days and then allowed to recover with free access to DSS-free drinking water for an additional 5 days. Changes in body weight were evaluated every day. To assess the severity of colitis, body weight, stool consistency, and occult blood in the stools were monitored daily24. Diarrhea was scored as follows: 0, normal; 2, loose stools; 4, watery diarrhea. Occult blood was scored as follows: 0, normal; 2, hemoccult positive; 4, gross bleeding. On the last day of the experiments, the colon was removed for histological and biochemical analyses.
AOM-induced colorectal cancer
Mice (8 weeks old, male) were injected intraperitoneally with AOM (Wako) at a dose of 10 mg/kg body weight once a week for 6 weeks. Mice were sacrificed 28 weeks after the last injection of AOM. On the last day of the experiments, the colon was removed for histological and biochemical analyses.
Colitis-associated colorectal cancer
Colitis-associated colorectal cancer was induced in mice (8 weeks old, male) by intraperitoneal injection with AOM at a dose of 10 mg/kg on day -5. On day 0, the mice were orally treated with 1.5% DSS for 4 days, followed by regular drinking water until day 21. The DSS treatment was repeated for two additional cycles. On day 56, the colon was removed for histological and biochemical analyses.
Separation of colonic epithelial and non-epithelial cells
The colon was removed, opened longitudinally, washed with PBS, and incubated with PBS containing 5 mM EDTA with shaking for 30 min at 37 °C. The tissue was separated into CECs and non-CECs under a stereomicroscope, and the cells were washed with PBS before use.
Microarray analysis
Total RNA extracted from mouse colons was purified using the RNeasy Mini Kit (Qiagen). The quality of the RNA was assessed with a 2100 Bioanalyzer (Agilent Technologies). cRNA targets were synthesized and hybridized with a Whole Mouse Genome Microarray in accordance with the manufacturer’s instructions (Agilent Technologies). The array slides were scanned using a SureScan Microarray Scanner (Agilent Technologies). Microarray data were analyzed with Agilent’s Feature Extraction Software. The GEO accession number for the microarray is GSE102389.
Lipidomics analysis
ESI-MS was performed in accordance with our current protocol55. In brief, for detection of phospholipids, tissues were soaked in 10 volumes of 20 mM Tris-HCl (pH 7.4) and homogenized with a Polytron homogenizer. Lipids were extracted from the homogenates by the method of Bligh and Dyer84. MS analysis was performed using a 4000Q-TRAP quadrupole-linear ion trap hybrid mass spectrometer (AB Sciex) with liquid chromatography (LC; NexeraX2 system; Shimazu). The samples were applied to a Kinetex C18 column (1 × 150 mm i.d., 1.7 μm particle) (Phenomenex) coupled to ESI-MS/MS. The samples injected by an autosampler (10 μl) were separated by a step gradient with mobile phase A (acetonitrile/methanol/water = 1:1:1 [v/v/v] containing 5 μM phosphoric acid and 1 mM ammonium formate) and mobile phase B (2-propanol containing 5 μM phosphoric acid and 1 mM ammonium formate) at a flow rate of 0.2 ml/min at 50 °C. For detection of fatty acids and their oxygenated metabolites, tissues were soaked in 10 volumes of methanol and then homogenized with a Polytron homogenizer. After overnight incubation at −20 °C, water was added to the mixture to give a final methanol concentration of 10% (v/v). The samples in 10% methanol were applied to Oasis HLB cartridges (Waters), washed with 10 ml of hexane, eluted with 3 ml of methyl formate, dried under N2 gas, and dissolved in 60% methanol. The samples were then applied to a Kinetex C18 column (1 × 150 mm i.d., 1.7 μm particles) (Phenomenex) coupled to ESI-MS/MS as described above. The samples injected by an autosampler (10 μl) were separated using a step gradient with mobile phase C (water containing 0.1% acetic acid) and mobile phase D (acetonitrile/methanol = 4:1; v/v) at a flow rate of 0.2 ml/min at 45 °C. Identification was conducted using multiple reaction monitoring (MRM) transition and retention times, and quantification was performed based on the peak area of the MRM transition and the calibration curve obtained with an authentic standard for each compound. As internal standards, d5-labeled EPA and 17:0 LPC (1 nmol; Cayman Chemicals) were added to each sample.
PLA2 enzyme assay using natural membranes
Total phospholipids were extracted from mouse colon and further purified by straight-phase chromatography. The samples extracted in chloroform were applied to a Sep-Pak Silica Cartridge (Waters), washed sequentially with acetone and chloroform/methanol (9/1; v/v), eluted with chloroform/methanol (3/1; v/v), and dried under N2 gas. The membrane mimic composed of tissue-extracted lipids (10 μM) was sonicated for 5 min in 100 mM Tris-HCl (pH 7.4) containing 4 mM CaCl2 and then incubated for appropriate periods with the mature form of recombinant human sPLA2-III protein83 (1–5 ng/ml) at 37 °C for 30 min. After incubation, the lipids were mixed with internal standards, extracted, and subjected to LC-MS for detection of fatty acids and lysophospholipids, as noted above.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance of differences between groups was evaluated by two-tailed Student’s t test or one-way ANOVA at a significance level of P < 0.05.
References
Clevers, H. At the crossroads of inflammation and cancer. Cell 118, 671–674, https://doi.org/10.1016/j.cell.2004.09.005 (2004).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545, https://doi.org/10.1016/S0140-6736(00)04046-0 (2001).
Karin, M., Jobin, C. & Balkwill, F. Chemotherapy, immunity and microbiota–a new triumvirate? Nat. Med. 20, 126–127, https://doi.org/10.1038/nm.3473 (2014).
Janne, P. A. & Mayer, R. J. Chemoprevention of colorectal cancer. N. Engl. J. Med. 342, 1960–1968, https://doi.org/10.1056/NEJM200006293422606 (2000).
Rothwell, P. M. et al. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet 377, 31–41, https://doi.org/10.1016/S0140-6736(10)62110-1 (2011).
Chulada, P. C. et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 60, 4705–4708 (2000).
Sonoshita, M. et al. Acceleration of intestinal polyposis through prostaglandin receptor EP2 in Apc Δ716 knockout mice. Nat. Med. 7, 1048–1051, https://doi.org/10.1038/nm0901-1048 (2001).
Ma, X., Aoki, T., Tsuruyama, T. & Narumiya, S. Definition of prostaglandin E2-EP2 signals in the colon tumor microenvironment that amplify inflammation and tumor growth. Cancer Res. 75, 2822–2832, https://doi.org/10.1158/0008-5472.CAN-15-0125 (2015).
Park, J. M. et al. Hematopoietic prostaglandin D synthase suppresses intestinal adenomas in Apc Min/+ mice. Cancer Res. 67, 881–889, https://doi.org/10.1158/0008-5472.CAN-05-3767 (2007).
Iwanaga, K. et al. Mast cell-derived prostaglandin D2 inhibits colitis and colitis-associated colon cancer in mice. Cancer Res. 74, 3011–3019, https://doi.org/10.1158/0008-5472.can-13-2792 (2014).
Sasaki, Y. et al. Genetic-deletion of cyclooxygenase-2 downstream prostacyclin synthase suppresses inflammatory reactions but facilitates carcinogenesis, unlike deletion of microsomal prostaglandin E synthase-1. Sci. Rep. 5, 17376, https://doi.org/10.1038/srep17376 (2015).
Arita, M. et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci. USA 102, 7671–7676, https://doi.org/10.1073/pnas.0409271102 (2005).
Jia, Q. et al. Reduced colitis-associated colon cancer in Fat-1 (n-3 fatty acid desaturase) transgenic mice. Cancer Res. 68, 3985–3991, https://doi.org/10.1158/0008-5472.CAN-07-6251 (2008).
Bento, A. F., Claudino, R. F., Dutra, R. C., Marcon, R. & Calixto, J. B. Omega-3 fatty acid-derived mediators 17(R)-hydroxy docosahexaenoic acid, aspirin-triggered resolvin D1 and resolvin D2 prevent experimental colitis in mice. J. Immunol. 187, 1957–1969, https://doi.org/10.4049/jimmunol.1101305 (2011).
Kunisawa, J. et al. Dietary ω3 fatty acid exerts anti-allergic effect through the conversion to 17,18-epoxyeicosatetraenoic acid in the gut. Sci. Rep. 5, 9750, https://doi.org/10.1038/srep09750 (2015).
Shida, D. et al. Cross-talk between LPA1 and epidermal growth factor receptors mediates up-regulation of sphingosine kinase 1 to promote gastric cancer cell motility and invasion. Cancer Res. 68, 6569–6577, https://doi.org/10.1158/0008-5472.CAN-08-0411 (2008).
Lin, S. et al. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136, 1711–1720, https://doi.org/10.1053/j.gastro.2009.01.002 (2009).
Stancic, A. et al. The GPR55 antagonist CID16020046 protects against intestinal inflammation. Neurogastroenterol. Motil. 27, 1432–1445, https://doi.org/10.1111/nmo.12639 (2015).
Kargl, J. et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 173, 142–154, https://doi.org/10.1111/bph.13345 (2016).
Suh, J. H. & Saba, J. D. Sphingosine-1-phosphate in inflammatory bowel disease and colitis-associated colon cancer: the fat’s in the fire. Transl Cancer Res 4, 469–483, https://doi.org/10.3978/j.issn.2218-676X.2015.10.06 (2015).
Haghikia, A. et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43, 817–829, https://doi.org/10.1016/j.immuni.2015.09.007 (2015).
Oishi, Y. et al. SREBP1 contributes to resolution of pro-inflammatory TLR4 signaling by reprogramming fatty acid metabolism. Cell Metab. 25, 412–427, https://doi.org/10.1016/j.cmet.2016.11.009 (2017).
Murakami, M., Sato, H., Miki, Y., Yamamoto, K. & Taketomi, Y. A new era of secreted phospholipase A2. J. Lipid Res. 56, 1248–1261, https://doi.org/10.1194/jlr.R058123 (2015).
Murase, R. et al. Group X secreted phospholipase A2 releases ω3 polyunsaturated fatty acids, suppresses colitis, and promotes sperm fertility. J. Biol. Chem. 291, 6895–6911, https://doi.org/10.1074/jbc.M116.715672 (2016).
Montrose, D. C. et al. The role of PGE2 in intestinal inflammation and tumorigenesis. Prostaglandins Other Lipid Mediat. 116–117, 26–36, https://doi.org/10.1016/j.prostaglandins.2014.10.002 (2015).
Kabashima, K. et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J. Clin. Invest. 109, 883–893, https://doi.org/10.1172/JCI14459 (2002).
MacPhee, M. et al. The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of Apc Min-induced intestinal neoplasia. Cell 81, 957–966 (1995).
Schewe, M. et al. Secreted phospholipases A2 are intestinal stem cell niche factors with distinct roles in homeostasis, inflammation, and cancer. Cell stem cell 19, 38–51, https://doi.org/10.1016/j.stem.2016.05.023 (2016).
Leung, S. Y. et al. Phospholipase A2 group IIA expression in gastric adenocarcinoma is associated with prolonged survival and less frequent metastasis. Proc. Natl. Acad. Sci. USA 99, 16203–16208, https://doi.org/10.1073/pnas.212646299 (2002).
Hiyoshi, M. et al. The expression of phospholipase A2 group X is inversely associated with metastasis in colorectal cancer. Oncol. Lett. 5, 533–538, https://doi.org/10.3892/ol.2012.1067 (2013).
Valentin, E., Ghomashchi, F., Gelb, M. H., Lazdunski, M. & Lambeau, G. Novel human secreted phospholipase A2 with homology to the group III bee venom enzyme. J. Biol. Chem. 275, 7492–7496 (2000).
Murakami, M. et al. Cellular arachidonate-releasing function of novel classes of secretory phospholipase A2s (groups III and XII). J. Biol. Chem. 278, 10657–10667, https://doi.org/10.1074/jbc.M211325200 (2003).
Mounier, C. M. et al. Distinct expression pattern of the full set of secreted phospholipases A2 in human colorectal adenocarcinomas: sPLA2-III as a biomarker candidate. Br. J. Cancer 98, 587–595, https://doi.org/10.1038/sj.bjc.6604184 (2008).
Murakami, M. et al. Cellular distribution, post-translational modification, and tumorigenic potential of human group III secreted phospholipase A2. J. Biol. Chem. 280, 24987–24998, https://doi.org/10.1074/jbc.M502088200 (2005).
Kazama, S. et al. Phospholipase A2 group III and group X have opposing associations with prognosis in colorectal cancer. Anticancer Res. 35, 2983–2990 (2015).
Hoeft, B. et al. Polymorphisms in fatty-acid-metabolism-related genes are associated with colorectal cancer risk. Carcinogenesis 31, 466–472, https://doi.org/10.1093/carcin/bgp325 (2010).
Sato, H. et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 120, 1400–1414, doi:https://doi.org/10.1172/JCI40493 (2010).
Taketomi, Y. et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 14, 554–563, https://doi.org/10.1038/ni.2586 (2013).
Pegg, A. E., Scicchitano, D. & Dolan, M. E. Comparison of the rates of repair of O 6-alkylguanines in DNA by rat liver and bacterial O 6-alkylguanine-DNA alkyltransferase. Cancer Res. 44, 3806–3811 (1984).
Zenewicz, L. A. et al. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957, https://doi.org/10.1016/j.immuni.2008.11.003 (2008).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263, https://doi.org/10.1038/nature11535 (2012).
Lindemans, C. A. et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 528, 560–564, https://doi.org/10.1038/nature16460 (2015).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738, https://doi.org/10.1016/j.immuni.2015.09.003 (2015).
Kumar, P. et al. Intestinal interleukin-17 receptor signaling mediates reciprocal control of the gut microbiota and autoimmune inflammation. Immunity 44, 659–671, https://doi.org/10.1016/j.immuni.2016.02.007 (2016).
Kanellopoulou, C. & Muljo, S. A. Fine-tuning Th17 cells: to be or not to be pathogenic? Immunity 44, 1241–1243, https://doi.org/10.1016/j.immuni.2016.06.003 (2016).
Condeelis, J. & Pollard, J. W. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263–266, https://doi.org/10.1016/j.cell.2006.01.007 (2006).
Kryczek, I. et al. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res. 69, 3995–4000, https://doi.org/10.1158/0008-5472.CAN-08-3804 (2009).
Taketo, M. M. & Edelmann, W. Mouse models of colon cancer. Gastroenterology 136, 780–798 (2009).
Kitajima, S., Takuma, S. & Morimoto, M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp. Anim. 48, 137–143 (1999).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241, https://doi.org/10.1016/j.cell.2004.07.002 (2004).
Tsukita, S. & Furuse, M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 9, 268–273 (1999).
Lu, P. et al. Colonic gene expression patterns of mucin Muc2 knockout mice reveal various phases in colitis development. Inflamm. Bowel Dis. 17, 2047–2057, https://doi.org/10.1002/ibd.21592 (2011).
Dupaul-Chicoine, J. et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 32, 367–378, https://doi.org/10.1016/j.immuni.2010.02.012 (2010).
Yamamoto, K. et al. Therole of group IIF-secreted phospholipase A2 in epidermal homeostasis and hyperplasia. J. Exp. Med. 212, 1901–1919, https://doi.org/10.1084/jem.20141904 (2015).
Yamamoto, K. et al. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 583, 101–117, https://doi.org/10.1016/bs.mie.2016.09.007 (2017).
Iizuka, Y. et al. Protective role of the leukotriene B4 receptor BLT2 in murine inflammatory colitis. FASEB J. 24, 4678–4690, https://doi.org/10.1096/fj.10-165050 (2010).
Gewirtz, A. T. et al. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J. Immunol. 168, 5260–5267 (2002).
Miki, Y. et al. Dual roles of group IID phospholipase A2 in inflammation and cancer. J. Biol. Chem. 291, 15588–15601, https://doi.org/10.1074/jbc.M116.734624 (2016).
Nishibe, S. et al. Increase of the catalytic activity of phospholipase C-γ 1 by tyrosine phosphorylation. Science 250, 1253–1256 (1990).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20, https://doi.org/10.1016/j.cmet.2007.10.002 (2008).
Schicho, R. et al. The atypical cannabinoid O-1602 protects against experimental colitis and inhibits neutrophil recruitment. Inflamm. Bowel Dis. 17, 1651–1664, https://doi.org/10.1002/ibd.21538 (2011).
Clark, J. D. et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65, 1043–1051 (1991).
Lin, L. L. et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72, 269–278 (1993).
Aikawa, S., Hashimoto, T., Kano, K. & Aoki, J. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem. 157, 81–89, https://doi.org/10.1093/jb/mvu077 (2015).
Yang, M. et al. G protein-coupled lysophosphatidic acid receptors stimulate proliferation of colon cancer cells through the β-catenin pathway. Proc. Natl. Acad. Sci. U. S. A. 102, 6027–6032, https://doi.org/10.1073/pnas.0501535102 (2005).
Zhang, H. et al. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Krüppel-like factor 5. J. Biol. Chem. 282, 15541–15549, https://doi.org/10.1074/jbc.M700702200 (2007).
Prestwich, G. D. et al. Phosphatase-resistant analogues of lysophosphatidic acid: agonists promote healing, antagonists and autotaxin inhibitors treat cancer. Biochim. Biophys. Acta 1781, 588–594, https://doi.org/10.1016/j.bbalip.2008.03.008 (2008).
Khurana, S. et al. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp. Cell Res. 314, 530–542, https://doi.org/10.1016/j.yexcr.2007.10.028 (2008).
Lin, S., Lee, S. J., Shim, H., Chun, J. & Yun, C. C. The absence of LPA receptor 2 reduces the tumorigenesis by Apc Min mutation in the intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1128–1138, https://doi.org/10.1152/ajpgi.00321.2010 (2010).
Dudakov, J. A., Hanash, A. M. & van den Brink, M. R. Interleukin-22: immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785, https://doi.org/10.1146/annurev-immunol-032414-112123 (2015).
Duffin, R. et al. Prostaglandin E2 constrains systemic inflammation through an innate lymphoid cell-IL-22 axis. Science 351, 1333–1338, https://doi.org/10.1126/science.aad9903 (2016).
Serhan, C. N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 510, 92–101, https://doi.org/10.1038/nature13479 (2014).
Levy, M. et al. Microbiota-modulated metabolites shape the intestinal microenvironment by regulating NLRP6 inflammasome signaling. Cell 163, 1428–1443, https://doi.org/10.1016/j.cell.2015.10.048 (2015).
Munoz, M. et al. Interleukin-22 induces interleukin-18 expression from epithelial cells during intestinal infection. Immunity 42, 321–331, https://doi.org/10.1016/j.immuni.2015.01.011 (2015).
Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature 535, 65–74, https://doi.org/10.1038/nature18847 (2016).
Kurashima, Y. et al. Extracellular ATP mediates mast cell-dependent intestinal inflammation through P2X7 purinoceptors. Nature communications 3, 1034, https://doi.org/10.1038/ncomms2023 (2012).
Rigoni, A. et al. Mast cells infiltrating inflamed or transformed gut alternatively sustain mucosal healing or tumor growth. Cancer Res. 75, 3760–3770, https://doi.org/10.1158/0008-5472.CAN-14-3767 (2015).
Giannou, A. D. et al. Mast cells mediate malignant pleural effusion formation. J. Clin. Invest. 125, 2317–2334, https://doi.org/10.1172/JCI79840 (2015).
Gulati, A. S. et al. Mouse background strain profoundly influences Paneth cell function and intestinal microbial composition. PLoS One 7, e32403, https://doi.org/10.1371/journal.pone.0032403 (2012).
Esworthy, R. S. et al. Analysis of candidate colitis genes in the Gdac1 locus of mice deficient in glutathione peroxidase-1 and -2. PLoS One 7, e44262, https://doi.org/10.1371/journal.pone.0044262 (2012).
Pena, J. A. et al. Alterations in myeloid dendritic cell innate immune responses in the Gαi2-deficient mouse model of colitis. Inflamm. Bowel Dis. 15, 248–260, https://doi.org/10.1002/ibd.20744 (2009).
Berg, D. J. et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J. Clin. Invest. 98, 1010–1020, https://doi.org/10.1172/jci118861 (1996).
Sato, H. et al. Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis. J. Biol. Chem. 283, 33483–33497, https://doi.org/10.1074/jbc.M804628200 (2008).
Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917, https://doi.org/10.1139/o59-099 (1959).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Numbers JP15H05905, JP16H02613 (to M.M.), JP25460087 (to Y.T.), JP16H01372 (to K.Y.) and JP16K18882 (to Y.M.), and AMED-CREST (to M.M.) and PRIME (to K.Y.) from Japan Agency for Medical Research and Development.
Author information
Authors and Affiliations
Contributions
R.M., Y.T., and M.S. designed and performed the experiments. Y.N. performed microarray analysis. Y.M. and K.Y. performed lipidomics analysis. K.F. assisted M.S. M.M. wrote the manuscript with input from all the other authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murase, R., Taketomi, Y., Miki, Y. et al. Group III phospholipase A2 promotes colitis and colorectal cancer. Sci Rep 7, 12261 (2017). https://doi.org/10.1038/s41598-017-12434-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12434-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.