Abstract
Sharp eyespot is a major fungal disease of wheat caused by Rhizoctonia cerealis in cool and humid environments worldwide. In this study, 224 single seed descent derived F13, F14 and F15 recombinant inbred lines (RILs) from the cross between CI12633 (a resistant cultivar) and Yangmai 9 (a susceptible cultivar) were assessed for sharp eyespot resistance (R.cerealis isolate R0301) in field and greenhouse conditions in three growing seasons. Different agronomic characteristics were also evaluated in the field with no disease infection. All the lines were genotyped with the Illumina iSelect 90 K SNP wheat chip and 101 SSR markers. Sharp eyespot resistance was significantly negatively correlated with heading date and tiller angle, and significantly positively correlated with the diameter of the basal first internode and second internode. Five QTL with a likelihood of odds ratio score of higher than 3.0 were detected on chromosomes 2BS, 4BS, 5AL and 5BS, respectively. These identified QTL may be used in future wheat breeding programs through marker assisted selection for developing sharp eyespot resistant cultivars.
Similar content being viewed by others
Introduction
Wheat sharp eyespot, caused by R. cerealis, is a serious fungal disease of wheat worldwide, leading to yield losses and threatening food security1. Although fungicides can be effective in reducing yield losses from the disease at the seedling stage, the occurrence of fungicide-resistant isolates of the pathogen has increased the need for host resistance to limit sharp eyespot epidemics2,3. Furthermore, the fungicides are not effective after the jointing stage due to the difficulty of spraying to the bottom half of the plants. Breeding resistant cultivars is, therefore, an ideal method of controlling wheat sharp eyespot disease.
While many studies have been carried out over the last decade to identify sharp eyespot resistance genes, no wheat germplasm resources with confirmed immunity or complete resistance to sharp eyespot have been found. Partial resistance was found in some wheat cultivars or Chinese landraces such as ARz, CI12633, Shanhongmai and AQ4,5. QTL for sharp eyespot resistance were identified on chromosomes 1A, 1D, 2A, 2B, 2D, 3A, 3B, 3D, 4A, 5D, 6B, 7B and 7D, respectively4,6,7,8,9. Among these QTL, a QTL from chromosome 7D was consistently detected in different populations with the highest phenotypic variation.
High-density linkage maps are very effective for the detection of QTL for important agronomic traits, fine mapping and identification of candidate genes, map-based cloning, and marker-assisted selection10,11,12. For sharp eyespot resistance, however, only a small number of DNA markers (<600) were used for mapping thus some minor QTL could not be detected. The development of high-density wheat single nucleotide polymorphism (SNP) iSelect array which comprises approximately 90000 gene-associated SNPs13 makes it possible for mining useful QTL for sharp eyespot resistance through high-resolution maps.
Agronomic traits, such as plant height, heading date, tiller angle, and the diameter of the internode (the first and second), may aggravate or alleviate disease development. For example, one resistant QTL was found to be significantly associated with plant heading time4. The close relation was reported between agronomic traits and other resistance genes. In rice, tiller angle showed a significant effect on plant’s resistance to sheath blight which is similar to wheat sharp eyespot14. These factors must, therefore, be considered when studying sharp eyespot resistance in wheat.
CI12633 is an R. cerealis-resistant winter wheat line. Our study aimed to clarify the relationship between sharp eyespot resistance and the five independent agronomic factors and to identify sharp eyespot resistance QTL and tightly linked SNP markers for use in future marker-assisted selection programs or positional cloning.
Results
Performance of the measured traits in three years
The distribution pattern of the disease index for sharp eyespot in the 224 RILs indicated that resistance to sharp eyespot is controlled by multiple genes and not by a single gene in the CI12633/Yangmai 9 population (Fig. 1). The skewness and kurtosis were all near zero, which indicated that resistance trait in CI12633 exhibited a normal distribution at all the environments. Sharp eyespot symptoms were examined in five environments: field in 2013–2014 (F2014), field in 2014–2015 (F2015), field in 2015–2016 (F2016), greenhouse in 2014–2015 (GH2015), and greenhouse in 2015–2016 (GH2016). CI12633 was rated as resistant with a mean sharp eyespot disease index of 27.79% (11.32–42.77%) while Yangmai 9 was rated as susceptible with a mean score of 83.04% (78.76–86.08%). The disease index for the 224 RILs showed continuous distribution, with ranges of 31.59–92.53%, 30.36–87.65%, 18.35–64.96%, 24.21–88.98%, and 30.02–87.61% in individual environments (Table 1).
Histograms showing frequency distribution pattern of sharp eyespot disease index (%) values in five environments. F2014, evaluated in field during the 2013–2014 cropping season; F2015, evaluated in field during the 2014–2015 cropping season; F2016, evaluated in field during the 2015–2016 cropping season; GH2015, evaluated in greenhouse during the 2014–2015 cropping season; GH2016, evaluated in greenhouse during the 2015–2016 cropping season; Mean of all environments, averaged data across five environments.
Some disease index detected from RILs which influenced by others pathogen must be abandoned in the data analysis. In the investigation, we found that disease index of sharp eyespot can be infected by wheat powdery mildew and yellow mosaic virus. Usually, disease index of RILs infected by these pathogen was very high.
The analysis of variance (ANOVA) for disease index showed a significant variation for the genotypes and five environments (Table 2). The disease was serious in all environments except F2016 for the small amount of rain in this year which led to air humidity was insufficient for sharp eyespot being hardly occurred even though sprinkled with water every day. Additionally, we found that the correlations for disease index between different trials were all positively significant at the P = 0.01 level (Table 3), indicating that the expression of the disease resistance was consistent across the environments thus the data were further used for QTL analysis.
Correlations between disease index with other agronomic traits
The disease index were negatively correlated with heading data (HD) (Table 3). Early heading lines tended to have greater disease index (more susceptible to sharp eyespot) in comparison to lines with late heading dates. The disease index also showed significant positive correlation with first internode diameter (FID) and second internode diameter (SID), indicating that plants with more slender stem are less prone to disease infection. Plant height (PH) is an important trait of wheat, which had no significantly negatively correlated with disease index in each environment (Table 3). A significant negative correlation between disease index and tiller angle (TA) was found in both F2014 and F2015 trials. Lines with greater TA showed less sharp eyespot symptom.
SNP polymorphism and genetic mapping
The CI12633/Yangmai 9 RIL population was genotyped with the wheat 90 K SNP array which resulted in 2829 polymorphic markers. In addition to the SNP markers, 205 SSR markers were also used to genotype the population. After removing unlinked markers, the resulting map consisted of 2012 markers (1911 SNP markers and 101 SSR markers) spanning 12434.23 cM in length with an average locus density of 6.18 cM/locus.
QTL mapping analysis in five experiments
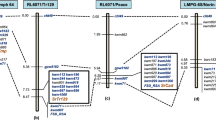
A total of five QTL were identified for sharp eyespot resistance using composite interval mapping, these QTL were identified on chromosomes 2BS, 4BS, 5AL (2) and 5BS, and were designated QSe.jaas-2BS, QSe.jaas-4BS, QSe.jaas-5AL.1, QSe.jaas-5AL.2 and QSe.jaas-5BS respectively. They explained 5.2–37.7% of the phenotypic variation (Table 4; Fig. 2). All resistance alleles were derived from CI12633 except for QSe.jaas-2BS which was from Yangmai 9.
Three QTL, QSe.jaas-2BS, QSe.jaas-5AL.1 and QSe.jaas-5AL.2, were detected in three or more environments and explained 20.7–27.9%, 8.3–14.7% and 5.8–11.3% of the phenotypic variation, respectively (Table 4; Fig. 2). QSe.jaas-2BS was flanked by RAC875_c730_234 and RAC875_c16697_1502. QSe.jaas-5AL.1 was located in the interval GENE-3601_145~Ku_c21002_908. QSe.jaas-5AL.2 was flanked by IAAV3043 and wsnp_Ex_c55777_58153636. The QSe.jaas-4BS, and QSe.jaas-5BS with larger effects for sharp eyespot resistance were both identified in only two environments and explained 32.2–37.7% and 28.7–36.2% of the phenotypic variance, and located in the interval RAC875_c49792_228~ Kukri_c34353_821 and wsnp_Ku_c11721_19085513~ BS00068710_51 respectively.
QTL for HD, PH, TA, FID and SID were also analysed. As shown in Table 5, all the QTL for different traits were located in different positions to those for sharp eyespot resistance except for the QTL controlling FID (QFid-jaas-2BS) and SID (QSid-jaas-2BS). This indicated that the diameter of the wheat basal internode is an important factor in determining sharp eyespot resistance.
Discussion
Phenotyping is a critical step for gene mapping
Limited research has been conducted to date on this disease because of the difficulties associated in accurately phenotyping the plant’s response to sharp eyespot3. One of the methods is the use of wheat kernels inoculated with the pathogen and subsequent quantitative categorization using a scale of 0 to 5 based on disease severity15. This method is dependent on the placement of the wheat kernel, i.e. the infected kernels should be placed close to but not touching the roots of the plants. The direct contact of plant roots with the inoculated pathogen will allow the pathogen to attack the plant persistently, leading to a higher disease index even in resistant plants, particularly when the environmental conditions are suitable for pathogen growth16. In this study, the inoculation was carefully conducted to prevent the direct contact between plant roots and the pathogen and all inoculated plants were dug up for the evaluation of disease index. This is a time-consuming and labor-intensive phenotyping method but the reliability of the results was improved. Two other methods of inoculation, toothpick and seedbed inoculation, are generally used by researchers, with many studies finding that toothpick inoculation was more effective2,6,7,8. In this research, however, the toothpick inoculation method could not be used because the sheath of CI12633 and its descendants was too thin and narrow and would break with the insertion of the toothpick.
QTL for sharp eyespot resistance
A previous study by Chen et al.4 identified several QTL for sharp eyespot resistance. These QTL located on chromosomes 1A, 2B, 3B, 4A, 5D, 6B, and 7B, respectively. Among these, the QTL detected on chromosome 2BS was also identified in our study, though their precise location was unclear. BLAST analysis of the GrainGenes and WHEAT URGI websites found that the markers RAC875_c730_234 and RAC875_c16697_1502 were similar to the SSR markers Xbarc101–2 and barc200, respectively, suggesting that QSe.jaas-2BS and QSe.cau-2BS may be the same5,7. The QTL on Chromosomes 4BS, 5AL (2) and 5BS identified in the current study were nor reported before. Interestingly, the resistance QTL on 2B (QSe.jaas-2BS) with larger effect came from the susceptible wheat Yangmai 9. Some resistance gene have been identified previously in susceptible parents. For instance, a rice sheath blight (SB) resistance QTL allele came from the susceptible cultivar Lemont has been successfully fine mapped by Zuo et al.16. It is more likely that the wheat Yangmai 9 is not highly susceptible to sharp eyespot disease. In Yangmai 9, the resistance QTL QSe.jaas-2BS may play a decisive role.
It’s not uncommon that a lot of disease resistance QTL mapped so far do not behave consistently in difference environments. In rice, a similar phenomenon was observed by Channamallikarjuna et al.17 and Zuo et al.16 in maping rice SB resistance QTLs. In this study, none of the QTL could be detected in all environments, most likely due to that the outbreak of this disease is easily influenced by environments. Thus, the identification of QTL for sharp eyespot resistance should be based on multi-experiments/environments.
The association between sharp eyespot resistance and agronomic traits
The association between QTL for sheath blight resistance and agronomic traits in rice has been reported18. Little research is conducted on the association between agronomic traits and wheat sharp eyespot19. Wheat sharp eyespot pathogens favor cool, humid and shady environments thus is generally more predominant in the lower part of the plant than the upper part. Small internode diameter is conducive to field ventilation and light crossing, thereby changing the growth condition of R. cerealis. Wheat varieties such as Shanhongmai, Xifeng, and Limai 16 with small internode diameter are generally more resistance to sharp eyespot5. Our experiment confirmed that disease index showed significant positive correlation with FID and SID and the resistant QTL on 2B (QSe.jaas-2BS) was associated with the internode diameter. Although significant correlations were observed between sharp eyespot resistance and HD, FID, SID, or TA, more detailed analysis are required to establish any definitive link between these phenological traits and plant’s response to this disease.
In summary, the markers closely linked to sharp eyespot resistance genes identified in present investigation can be used for marker assisted selection in wheat improvement.
Materials and Methods
Plant materials
The 224 F13–15 RILs used for QTL mapping were developed from a cross between CI12633 and Yangmai 9. Yangmai 9, a high-yielding wheat cultivar grown in the wheat region along the middle and lower reaches of the Yangtze from 2000 to 2007, is susceptible to sharp eyespot; CI12633 is a taller winter wheat line that exhibits a high level of sharp eyespot resistance5.
Sharp eyespot inoculum preparation
The R.cerealis isolate R0301 is the dominant strain in Jiangsu province, China, and was provided by Professors Huaigu Chen and Shibin Cai of the Jiangsu Academy of Agricultural Sciences, China. R0301 was activated on freshly prepared potato-dextrose agar (PDA) just before use. Inoculum was produced on sterilized wheat kernels using a method similar to that of Lipps and Herr15 and Chen et al.4.
Evaluation of disease severity in the field
The reactions of the 224 RILs and their parents to sharp eyespot were evaluated at Yangzhou in Jiangsu province during the 2013–2014, 2014–2015, and 2015–2016 cropping seasons. As humid and wet weather conditions prevail in Yangzhou during spring this area is very conducive for sharp eyespot outbreak. All research materials were sown on October 20th for each of the three years, in a randomized complete block design with three replicates. CI12633 and Yangmai 9 were included every 50 lines in each replicate. Each plot was 0.8 m long and rows were spaced 0.4 m apart; in each row, approximately 50 seeds were sown. To achieve a plant density of 40 plants per row some seedlings were removed. High plant densities provided favorable conditions for the spread of sharp eyespot. In early March, when the weather became warm and wet, the plants were inoculated by placing R0301-colonized wheat kernels on the soil surface close to the plants, but the kernels should not be touched with the plants. The kernels were then covered with earth and water was sprinkled three times a day for the first month in non-raining days. Overall, the weather was very suitable for sharp eyespot outbreak two of three seasons with consistent rainfall occurring throughout the experimental period until the final disease recording.
In early May, when the susceptible parent Yangmai 9 began to succumb to R. cerealis infection, all sample plants except the edge plants were dug up and individually assessed for disease (approximately 200–250 wheat stems of each line). The infection types were categorized qualitatively from 0 to 5: 0, no lesion; 1, the lesion appeared on the sheaths rather than stems; 2, the width of the lesion was <50% of the infected stem perimeter; 3, the width of the lesion is >50 and <75% of the infected stem perimeter; 4, the width of the disease lesion is >75% of the infected stem perimeter; 5, white spike or dead plant. Disease index = ((0 × X0 + 1 × X1 + 2 × X2 + 3 × X3 + 4 × X4 + 5 × X5)/[(X0 + X1 + X2 + X3 + X4 + X5) × 5]) × 100, where X 0 − X5 indicated plants with infection types 0–5, respectively15.
Evaluation of disease severity in a greenhouse
To better facilitate R. cerealis infection and development, the 224 RILs and their parents were sown in a greenhouse during the 2014–2015 and 2015–2016 seasons. All materials were sown in early November in a randomized complete block design with three replicates. The planting and management patterns were similar to those in the field. A water sprinkler system was constructed over the plants that was used to keep the experimental environment moist. In mid-February, when wheat plants were at the tillering growth stage, R0301-colonized wheat kernels were added to the soil surface and covered with soil. The water sprinkler system and temperature control system were then used to facilitate R. cerealis infection and development. When Yangmai 9 began to succumb to sharp eyespot, all the plants were dug up for disease assessment. The disease score was calculated as described above.
Agronomic trait data
All the RILs and both parents were planted in field conditions in the same randomized block design with three replicates without R0301 inoculation in the 2013–2014 and 2014–2015 seasons. Four traits, heading date (HD), plant height (PH), tiller angle (TA), the first internode diameter (FID), and second internode diameter (SID), were measured for each wheat line. HD was recorded when 50% of the plants in any plot were at heading. PH was measured in centimeters from the soil surface to the tip of the ear excluding the awns at the milk-ripening stage. TA was judged as loose or compact. FID and SID were measured with Vernier calipers once the sheath was removed.
Molecular marker analyses and map construction
Genomic DNA was extracted from young leaves using a DNeasy Plant DNA extraction kit (QIAGEN). Genotyping was done with the Illumina iSelect 90 K SNP wheat chip containing 81,587 SNP markers. This part work was completed by China Golden Marker (Beijing) Biotech Co.Ltd.(CGMB). Linkage groups were generated with the software Joinmap V4.0 and genetic distances between markers were estimated based on the Kasambi mapping function.
QTL analyses
QTL analyses were conducted by composite interval mapping (CIM) with the software WinQTLCart 2.5. To declare QTL significant, the threshold logarithm of the odds score was arbitrarily set at 3.0. Adjusted means for percent disease index values each year were calculated before pooling the results from all the years for statistical analyses. The QTL were named according to the International rules of Genetic Nomenclature (http://wheat.pw.usda.gov/ggpages/wgc/98/Intro.htm). If one QTL only detected one time, it will be discarded.
Statistical analysis
Analysis of variance (ANOVA) analyses within the five seasons of field and greenhouse sharp eyespot data were conducted using PROC ANOVA in SAS 9.1.2 (SAS Institute Inc., Cary, NC, USA). Correlations between experiments were calculated using Excel.
References
Rong, W. et al. A Wheat cinnamyl alcohol dehydrogenase TaCAD12 contributes to host resistance to the sharp eyespot disease. Front. Plant. Sci. 7, 17–23 (2016).
Li, W., Guo, Y., Zhang, A. & Chen, H. Genetic structure of populations of the wheat sharp eyespot pathogen rhizoctonia cerealis anastomosis group D subgroup I in China. Phytopathology. 107, 224–230 (2017).
Hamada, M. S. et al. The escalating threat of Rhizoctonia cerealis, the causal agent of sharp eyespot in wheat. Pest. Manag. Sci. 67, 1411–1419 (2011).
Chen, J. et al. Mapping of QTL conferring resistance to sharp eyespot (Rhizoctonia cerealis) in bread wheat at the adult plant growth stage. Theor. Appl. Genet. 126, 2865–2878 (2013).
Ren, L. et al. Screening of resistance to sharp eyespot in wheat. Journal of Plant Genetic Resources. 11, 108–111 (2010).
Ren, L. et al. SSR markers linked resistance QTLs to sharp eyespot (Rhizoctoniacerealis) in wheat. Journal of YangZhou UniverSity (Agricultural and Life Science Edition). 25, 16–19 (2004).
Cai, S. et al. Germplasm development and mapping of resistance to sharp eyespot (Rhizoctonia cerealis) in wheat. Scientia Agricultura Sinica. 39, 928–934 (2006).
Tang, T. et al. Study on QTL mapping of sharp eyespot resistance (Rhizoctonia cerealis) in wheat ARz. Journal of Triticeae Crops. 24, 11–16 (2004).
Zhang, X. et al. QTL and molecular marker for resistance gene of wheat sharp eyespot. Journal of Plant Genetic Resources. 6, 276–279 (2005).
Avni, R. et al. Ultra-dense genetic map of durum wheat × wild emmer wheat developed using the 90K iSelect SNP genotyping assay. Mol. Breeding. 34, 1549–1562 (2014).
Gupta, P., Rustgi, S. & Mir, R. Array-based high-throughput DNA markers for crop improvement. Heredity. 101, 5–18 (2008).
Gao, L. et al. Candidate loci involved in domestication and improvement detected by a published 90K wheat SNP array. Sci. Rep. 7, 44530 (2017).
Wang, S. et al. Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant biotechnology journal. 12, 787–796 (2014).
Zuo, S. et al. Fine-mapping of qSB-9 TQ, a gene conferring major quantitative resistance to rice sheath blight. Mol. Breeding. 34, 2191–2203 (2014).
Lipps, P. & Herr, L. Etioligy of Rhizoctonia cerealis in sharp eyespot of wheat. Phytopathology 72, 1574–1577 (1982).
Zuo, S. et al. Fine mapping of qSB-11 LE, the QTL that confers partial resistance to rice sheath blight. Theor. Appl. Genet. 126, 1257–1272 (2013).
Channamallikarjuna, V. et al. Identification of major quantitative trait loci Qsbr11-1 for sheath blight resistance in rice. Mol. Breeding. 25, 155–166 (2010).
Hossain, M., Jena, K., Bhuiyan, M. & Wickneswari, R. Association between QTLs and morphological traits toward sheath blight resistance in rice (Oryza sativa L.). Breeding Science. 66, 613–626 (2016).
Wei, L., Muranty, H. & Zhang, H. Advances and prospects in wheat eyespot research: contributions from genetics and molecular tools. Journal of Phytopathology. 159, 457–470 (2011).
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangsu Province [Grant No. BK20130457 and BK20140499], the National Natural Science Foundation of China (31471497) and Jiangsu agricultural science and technology innovation fund (cx(14)2002).
Author information
Authors and Affiliations
Contributions
W.X.J. performed phenotype, analysis, and wrote the manuscript; C.K., Z.R.H., Z.S.J. and B.T.D. performed phenotype; J.Z.N., W.R.L. and G.D.R. constructed and performed phenotype; Z.B.Q. designed the experiment and assisted with manuscript writing.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, X., Cheng, K., Zhao, R. et al. Quantitative trait loci responsible for sharp eyespot resistance in common wheat CI12633. Sci Rep 7, 11799 (2017). https://doi.org/10.1038/s41598-017-12197-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12197-7
This article is cited by
-
Pseudomonas fluorescens HC1-07 transformed with phenazine-1-carboxylic acid biosynthesis genes has improved biocontrol activity against Rhizoctonia root rot and Fusarium crown rot of wheat
BioControl (2023)
-
Analysis of gene expression changes in wheat in response to Rhizoctonia cerealis infection using RNA-Seq
Journal of Plant Pathology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.