Abstract
Boar taint (BT) is an offensive odour or taste observed in pork from a proportion of non-castrated male pigs. Surgical castration is effective in avoiding BT, but animal welfare issues have created an incentive for alternatives such as genomic selection. In order to find candidate biomarkers, gene expression profiles were analysed from tissues of non-castrated pigs grouped by their genetic merit of BT. Differential expression analysis revealed substantial changes with log-transformed fold changes of liver and testis from −3.39 to 2.96 and −7.51 to 3.53, respectively. Co-expression network analysis revealed one module with a correlation of −0.27 in liver and three modules with correlations of 0.31, −0.44 and −0.49 in testis. Differential expression and co-expression analysis revealed candidate biomarkers with varying biological functions: phase I (COQ3, COX6C, CYP2J2, CYP2B6, ACOX2) and phase II metabolism (GSTO1, GSR, FMO3) of skatole and androstenone in liver to steroidgenesis (HSD17B7, HSD17B8, CYP27A1), regulation of steroidgenesis (STARD10, CYB5R3) and GnRH signalling (MAPK3, MAP2K2, MAP3K2) in testis. Overrepresented pathways included “Ribosome”, “Protein export” and “Oxidative phosphorylation” in liver and “Steroid hormone biosynthesis” and “Gap junction” in testis. Future work should evaluate the biomarkers in large populations to ensure their usefulness in genomic selection programs.
Similar content being viewed by others
Introduction
Boar taint (BT) is an offensive odour that occurs in cooked or heated pork from a proportion of non-castrated male pigs. Two compounds are mainly responsible for BT: skatole (3-methyl-indole) and androstenone (5α-androst-16-ene-3-one)1,2. Skatole is a by-product of enzymatic breakdown of L-tryptophan by bacterial action in the large intestine and subsequently absorbed into the blood3. Androstenone is a steroid produced in the testis where it is transported by blood to the salivary gland and functions as a mating pheromone4. It is synthesised from pregnenolone in association with sexual maturity together with other testicular steroids, such as testosterone and oestrogens5,6. Due to their lipophilic properties, the compounds accumulate in the adipose tissue and concentrations are correlated with the level of sexual maturity at the time of slaughter7,8 and/or the intensity of liver degradation9,10. A distinct relationship between androstenone and skatole exists: high concentration of androstenone prevents breakdown of skatole by inhibiting enzymes responsible for skatole metabolism and causes accumulation in adipose tissues11. Furthermore, androstenone itself is an active contributor to BT, and it has been proposed that elevated levels of androstenone are primary to elevated levels of skatole in the hierarchy of BT development12. Threshold levels for skatole and androstenone concentrations in fat considered to cause BT are 0.5 to 1 µg/ g for androstenone and 0.20 to 0.25 µg/g for skatole2.
Boar taint is the main factor preventing the production of non-castrated male pigs in many countries13 and often leads to depreciation of the carcass, even though their production is associated with a number of significant economic advantages including increased efficiency, leaner carcasses and lower faecal and urinary nitrogen losses14. Furthermore, production of non-castrated males is often viewed as advantageous in the context of animal welfare due to the absence of surgical castration. Indeed, this practice is often performed without anaesthesia or analgesia which results in pain and stress for the animal15, but the practice is justified by its alleviation of aggression and sexual activity which can result in leg fractures and skin damage16. Surgical castration is also an effective technique for avoiding BT, but increased attention to the animal welfare issues has led to a voluntary initiative to end the practice in the European Community from January 1, 201817.
In order to avoid BT in the production of non-castrated males without surgical castration, two overall solutions have been proposed: (i) chemicals and/or chemotherapeutics such as immunocastration and (ii) optimised animal breeding programs. For the first solution, immunocastration by Improvac® (Pfizer Ltd, NY, USA) has been found to be an effective strategy against BT with no detrimental effects on carcass and meat quality18,19,20,21,22,23 and with environmental benefits such as increased or at least similar growth rates and feed conversion efficiencies between immunised and surgically castrated pigs24. However, the European Food Safety Authority (EFSA) has anticipated poor public acceptance of immunocastration25,26 due to the possible perception of a link with hormone treatment and possible harmful residues24,27.
For the latter solution, genomic-based breeding programs have been proposed. As skatole and androstenone are moderate to highly heritable traits9,11,28,29,30, reduction of BT would be possible through breeding programs. By this strategy, breeding organisations would apply genomic selection on individuals with desirable genotypes that are predisposed to low genetic merit of BT compounds without the need for trait recording31. Previous work has indeed established low and mostly favourable genetic correlations between BT compounds and production traits such as the quality of meat without negatively affecting important male fertility trait32. Thus, selection of low genetic merit of BT can be deemed an effective and sustainable solution. By employing molecular genetic methods, identification of male pigs with low genetic merit of BT is possible, before they actually develop the condition or have their traits recorded33. However, this requires knowledge on highly predictive key genes associated with the trait of interest. In turn, these genes can be applied as biomarkers, e.g. Mazzoni, et al.34 and Salleh, et al.35. Finding biomarkers with strong predictive powers for a trait can be greatly enhanced with omics technologies, such as transcriptomics36. By measuring changes in gene expression profiles, analysis of differentially expressed (DE) genes associated with BT has been performed in a variety of breeds and experimental designs (as reviewed by Zadinová, et al.37). While many studies employed microarrays to obtain gene expression profiles, a few employed RNA sequencing (RNA-Seq) which provides far more precise measurements of transcript expression levels and their isoforms than traditional technologies38. Another powerful analysis within transcriptomics is construction of co-expression networks which reveal co-expressed (CE) genes with a high connectivity (“hub genes”). Hub genes are often regulatory genes, with major impact on genetic networks and may affect the trait of interest39,40,41. To the authors knowledge, no studies have applied a systems biology approach36 to genetic merit of BT. By this approach, findings from DE and CE analyses from RNA-Seq data are integrated in order to enhance discriminatory power of highly associated genes, useful as candidate biomarkers within animal breeding.
The aims of this study were: (i) to detect important DE genes in gene expression profiles of liver and testis in male pigs associated with divergent genetic merit of BT, (ii) to build co-expression networks by application of the Weighted Gene Co-expression Network Analysis (WGCNA) method42 and extract important hub genes, (iii) to integrate results and extract candidate biomarkers associated with genetic merit of BT and evaluate their potential within animal breeding (Fig. 1).
Illustration of the study design. From a total of 114 Landrace male pigs, 48 pigs were grouped as having low, medium and high boar taint (BT) according to estimated breeding values (EBVs) of skatole and human nose score of their respective sires. Each group consisted of 16 pigs. At 100 kg weight, they were slaughtered and two tissues were sampled. Of these, liver and testis tissues were subjected to paired-end RNA sequencing. These were analysed for differentially expressed (DE) genes and co-expressed (CE) genes which were extracted from co-expression networks by a range of statistical methods. In turn, this led to the discovery of candidate biomarkers for BT and BT related pathways.
Results
Genetic merit of boar taint
The animal model in this study comprised of commercial Danish Landrace male pigs (n = 114). The pigs were produced from sires with known genetic merit of BT assessed from estimated breeding values (EBVs) of skatole concentrations in fat and human nose scores (HNS). The genetic merit of BT was defined as the summarised EBVs calculated as the sum of EBVs of skatole and HNS obtained from the respective sire of each pig. In brief, EBVs are a measure of the relative genetic performance compared with a contemporary group of a trait and used as an industry standard in animal breeding. The full concept, definition and methods used to calculate EBVs are given in Bourdon and Bourbon43. Based on the distribution of summarised EBVs, a total of 48 pigs (16 low, 16 medium and 16 high) were selected and used in the analysis, representing a low, medium and high genetic merit of BT. The means of the summarised EBVs (±standard deviation) for each group were 0.71 (±0.19), −0.01 (±0.09) and −0.38 (±0.17) for high, medium and low genetic merit of BT, respectively. A list of selected animals and their EBVs are available in Supplementary file 1.
RNA sequencing of liver and testis tissue
RNA-Seq was performed on liver and testis tissue of the selected pigs. Post-mapping quality control revealed that a mean of 33.25 million reads throughout the 96 samples had been successfully mapped to the reference genome. The number of reads (±SD) aligned to genes in liver and testis tissue were 7.76 (±1.61) million and 7.04 million (±2.44), respectively. Quality control revealed a distribution of 50.14% exonic reads, 41.29% intergenic reads and 8.57% intronic reads in liver and 47.42% exonic reads, 37.89% intergenic reads and 14.79% intronic reads in testis. After filtering of genes with expression levels equal to or fewer than five counts, the number of genes included in the differential expression and co-expression analyses were 10,545 for liver and 12,731 for testis, respectively.
Identification of differentially expressed genes
Differential expression analysis of liver and testis by contrast of high vs low genetic merit of BT from the three-group study design revealed 507 and 5,943 differentially expressed (DE) genes (FDR < 0.05), respectively. An additional filtering step which compared expression profiles for each DE gene throughout the three groups revealed that 241 and 2,315 genes were significantly (P < 0.05) different across groups in liver and testis, respectively. The log-transformed fold change of the DE genes in liver and testis ranged from −3.39 to 2.96 and −7.51 to 3.53, showing substantial gene expression differences. The top significant DE genes were MORN2, AASDHPPT, SPCS3, SEC. 11 C and NDUFC1 in liver and LAMP1, TNPO1, TOPORS, GFPT1 and COX4I1 in testis. The top 25 most significant DE genes found in liver and testis are presented in Table 1 and Table 2, respectively. Full result tables are included in Supplementary file 2.
Gene functional enrichment analysis
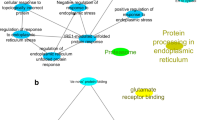
Gene functional enrichment analysis revealed a total of 39 and 421 significantly enriched (Adj. P-val. < 0.05) gene ontology (GO) terms in liver and testis, respectively (Supplementary file 3). Subsequent filtering of redundant GO terms by semantic similarity further reduced the amount in order to visually represent the most enriched terms in each tissue (Fig. 2). In liver, “Peptide metabolic processes” (Adj. P-val. = 1.1 × 10−4) were the most significantly enriched GO terms of Biological Process (BP), “Mitochondrial proton-transporting ATP synthase complex, coupling factor F(o)” (Adj. P-val. = 0.9 × 10−7) were the most significantly enriched Cellular Component (CC) terms and”Transferase activity, transferring one-carbon groups” (Adj. P-val. = 1.8 × 10−2) were the most significantly enriched Molecular Function (MF) terms (Fig. 2a). In testis, the GO terms “Organelle organization” (Adj. P-val. = 1.8 × 10−50) were the most significantly enriched BP, “Intracellular part” (Adj. P-val. = 4.4 × 10−115) were the most significantly enriched CC and finally, “Transmembrane signalling receptor activity” (Adj. P-val. = 1.0 × 10−61) were the most significantly enriched MF (Fig. 2b). A total of 11 GO terms were found to be significantly (Adj. P-val. < 0.05) enriched in both tissues which comprised 5 BP and 6 CC terms. According to results obtained from each individual tissue, the most significantly enriched common GO terms were “Mitochondrion” (Adj. P-val. = 3.2 × 10−5), “Peptide metabolic process” (Adj. P-val. = 1.1 × 10−4) and “Cellular amide metabolic process” (Adj. P-val. = 1.9 × 10−4) in liver and “Catalytic complex” (Adj. P-val. = 1.0 × 10−25), “Transferase complex” (Adj. P-val. = 5.0 × 10−20) and “Ribonucleoprotein complex biogenesis” (Adj. P-val. = 1.9 × 10−5) in testis.
Gene functional enrichment analysis of a fixed number (n = 507) of the top DE genes from each tissue revealed the MF GO terms “Transferase activity, transferring one-carbon groups”, “S-adenosylmethionine-dependent methyltransferase activity”, “Cytochrome-C oxidase activity” with child GO term “Hydrogen ion transmembrane transporter activity” and “Peptidase activator activity” with child GO term “Cysteine-type endopeptidase regulator activity involved in apoptotic process” significantly enriched (Adj. P-val. < 0.05) in liver. Furthermore, “Translation factor activity, RNA binding”, “Helicase activity”, “Ubiquitin-protein transferase activity”, “ Lysine-acetylated histone binding”, “Peptide N-acetyltransferase activity”, “P-P-bond-hydrolysis-driven transmembrane transporter activity” and “NAD + binding” were significantly enriched (Adj. P-val. < 0.05) in testis. The MF GO terms from both tissues were visualised with the DE genes mapped to their corresponding GO terms and the expression levels in the “low BT” and “high BT” groups for liver (Fig. 3a) and testis (Fig. 3b).
Network visualisation of molecular function gene ontology terms found in (a) liver and (b) testis obtained by from a fixed amount of the top 507 differentially expressed genes. Each term is represented by a circle whose diameter is negatively correlated with the adjusted P value (pV). Each node represents a gene where the expression levels are indicated in the circle where the green group (left side) represents the expression level in low BT group and the red group (right side) represents the expression level in high BT group.
Pathway enrichment analysis
Pathway analysis revealed six and 20 significantly enriched (Adj. P-val. < 0.05) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways in liver and testis, respectively (Supplementary file 4). The top three significantly enriched pathways found were “Ribosome” (Adj. P-val. = 5.0 × 10−4), “Protein export” (Adj. P-val. = 6.4 × 10−4) and “Alzheimer’s disease” (Adj. P-val. = 4.3 × 10−3) in liver and “Neuroactive ligand-receptor interaction” (Adj. P-val. = 1.1 × 10−9), “Cytokine-cytokine receptor interaction” (Adj. P-val. = 1.1 × 10−6) and “Thyroid hormone signalling pathway” (Adj. P-val. = 7.8 × 10−5) in testis. A selection of pathways deemed biologically interesting were visualised with their DE genes mapped to their corresponding pathways and the expression levels in the “low BT” and “high BT” in liver (Fig. 4a) and testis (Fig. 4b). These pathways were “Ribosome”, “Oxidative phosphorylation”, “Gluthathione metabolism”, “Protein export”, “Folate biosynthesis” and “Drug metabolism” in liver and “Taste transduction”, “GnRH signalling pathway”, “Drug metabolism”, “Steroid hormone biosynthesis” and “Gap junction” in testis.
Network visualisation of a selection of the most biologically interesting and overrepresented pathways in (a) liver and (b) testis. Each pathway is represented by a circle whose diameter is negatively correlated with the adjusted P-value (pV) of the given term. Each node represents a gene where the expression levels are indicated in the circle where the green group (left side) represents the expression level in low BT group and the red group (right side) represents the expression level in high BT group.
Co-expression network analysis
In liver, no modules were found to be significantly (P < 0.05) associated with genetic merit of BT, but one module (lightgreen) containing 593 genes was found to have a strong tendency of association with genetic merit of BT (P = 0.06) with a negative correlation (module-trait relationship (r) = −0.27). Hence, the module was selected for additional analysis. SERPINC1, PRDX3, HSD17B8, GSTZ1 and NIT1 were found to be hub genes (Fig. 5a). The intramodular connectivity defined as the node degree (k) (links per node) of the individual hub gene ranged from 23 to 36 and module membership measure ranged from 0.77 to 0.84. Enrichment analysis of the genes from the lightgreen module revealed “Small molecule catabolic process”, “Lipid metabolic process” and “Cofactor metabolic process” as the top three significantly (adj. P-val. < 0.05) enriched BP GO terms (Fig. 5b). Due to its biological relevance, GO term “Organic hydroxyl compound metabolic process” was selected for further analysis which revealed child GO terms of “Cellular hormone metabolic process”, “Hormone metabolic process”, “Steroid metabolic process”, “Cholesterol metabolic process” and “Lipid homeostasis” (Fig. 5c).
Composite visualisation of the lightgreen module in liver. (a) Network visualisation of hub genes in the module. Hub genes are indicated by large font and circle size; (b) Bar plot of gene ontology (GO) terms related to the module. Y-axis indicate the GO term, X-axis indicate the adjusted P-value and (c) analysis of genes related to the GO term “Organic hydroxy compound metabolic process”. Abbreviations: BP Biological process, CC cellular component, MF molecular function.
In testis, two modules were found to be significantly (P < 0.05) associated with genetic merit of BT: darkgreen and tan containing 180 and 519 genes, respectively. Furthermore, the darkturquoise module was found to have a tendency of association with genetic merit of BT (P = 0.05). The module darkgreen containing 180 genes was positively correlated with the summarised EBVs (r = 0.31), whereas tan had a negative correlation (r = −0.49). COPZ2, RAMP2, VDAC1 and SLC25A6 were found to be hub genes in the darkgreen module (Fig. 6a) with intramodular connectivity ranging from 24 to 35 and module membership measure ranging from 0.34 to 0.53. The top three most significantly (adj. P-val. < 0.05) enriched BP GO terms attributed to darkgreen module were “Carboxylic acid catabolic process”, “Small molecule catabolic process” and “Sterol molecule metabolic process” (Fig. 6b). The GO term “Sterol metabolic process” was selected for further analysis which revealed further GO annotations of “Cholesterol biosynthetic process”, “Coenzyme binding”, “Flavin adenine dinucleotide binding”, “Oxidoreductase activity, acting on the CH-CH group of donors”, and “Oxidoreductase activity, acting on the CH-CH group of donors, NAD or NADP as acceptor” (Fig. 6c). Full result tables from co-expression analysis are included in Supplementary file 5.
Composite visualisation of the darkgreen module in testis. (a) Network visualisation of hub genes in the module. Hub genes are indicated by large font and circle size; (b) Bar plot of gene ontology (GO) terms related to the module. Y-axis indicates the GO term, X-axis indicates the adjusted P-value and (c) analysis of genes related to the GO term “Steroid metabolic process”. Abbreviations: BP Biological process, CC cellular component, MF molecular function.
Candidate biomarker selection
By combining results from differential expression analysis with co-expression analysis and applying a reductionist strategy, a total of 16 candidate biomarkers were detected as potential biomarkers: COQ3, COX6 C, GSTO1, GSR, FMO3, CYP2J2, CYP2B6 and ACOX2 in liver (Fig. 7a) and HSD17B7, HSD17B8, MAPK3, MAP2K2, MAP3K2, STARD10, CYB5R3 and CYP27A1 in testis (Fig. 7b). Briefly, the reductionist strategy comprised selection of candidate biomarkers from genes that were found as significant (FDR < 0.05) in the differential expression analysis and significant (P < 0.05) in additional filtering by expression profile comparison. Furthermore, the genes were either: (i) annotated to relevant GO terms and/or pathways and/or (ii) part of a co-expression module associated with genetic merit of BT.
Selected candidate biomarkers with their associated box plot from (a) liver and (b) testis. Y-axis indicates normalised expression levels. X-axis indicates low, medium and high boar taint (BT) groups. Finally, the P-value (p) indicates the result from a separate Kruskal-Wallis test on expression levels of the candidate biomarker throughout the three BT groups.
Discussion
In this study, candidate biomarkers associated with divergent genetic merit of boar taint (BT) was identified from RNA sequencing (RNA-Seq) of liver and testis of Danish Landrace pigs. The genetic merit of BT was defined from the estimated breeding values (EBVs) of skatole concentrations and human nose score (HNS) obtained from the sires of each pig. The HNS method has been shown to be a rapid, sensitive and cost-effective system with good correlations with androstenone and skatole levels and contains the ability to capture subjective variation not obtained by laboratory assays44. However, the HNS method is prone to some limitations that are intrinsically linked to using human beings as trained panellists: (i) regional differences in panels perception of BT compounds and training of panellists45; (ii) moderate reproducibility due to differences in human sensitivity and perception of BT even in experienced panellists46 and (iii) accuracy of panels is related to the number of panellists and their individual olfactory acuity47. The EBVs (the relative genetic performance compared with a contemporary group43) were calculated by Genomic BLUP Animal Models48,49 and is used by breeding organisations as a measure of genetic merit. This is related to the advantage of EBVs which can be calculated for a large number of animals without phenotypic data via their pedigree or genomic relationship with animals having phenotypic data by a Best Linear Unbiased Prediction (BLUP) framework. Collecting phenotypic data of BT traits on large number of animals is also very prohibitive. As this study was concerned with animal breeding under industry settings, we integrated EBVs from the breeding sires with gene expression profiles of their progeny to find candidate biomarkers associated with genetic merit of BT. Hence, biomarkers will be able to identify pigs with low genetic merit of BT for selection, before they develop BT and have the trait recorded which is of high relevance in practical and cost-effective breeding programs. The EBVs were adjusted for age (in days) and carcass weight (in kg) at slaughter. Thus, systematic differences in sexual maturation between pigs were accounted for in order to ensure consistent steroidogenic potential in the three groups, as warranted in previous research33. Gene expression profiles were not subjected to further correction by any covariates other than technical normalisation. Previous work found EBVs as a very reliable phenotype indicator50 and they have been successfully applied in gene co-expression analysis51.
A shortcoming of the current study is the lack of phenotypic data. Due to the commercial nature of the pigs, we did not have access to phenotypic measurements. In a previous study by Lervik, et al.50, the correlation between EBVs of androstenone and the androstenone phenotype was high. On the other hand, skatole has a slightly lower heritability than androstenone30 and we expect some disparity due to lack of correlation between EBVs and phenotype in the individual pig at slaughter weight (~100 kg). Thus, the lack of phenotypic data means that any disparity between genetic merit and BT phenotype cannot be identified or quantified. However, we emphasise that the current study identifies candidate biomarkers of genetic merit of BT and the candidate biomarkers found in this study cannot be used directly for phenotype identification without prior validation.
Liver metabolism of xenobiotic substances is divided into two phases: phase I metabolism which comprises oxidation, reduction and hydroxylation steps to make the substrate more soluble in water by adding or exposing a functional group and phase II sulfoconjugation which adds polar groups for increased hydrophilicity and excretion52,53. Related to the phase II sulfoconjougation, transferase activity was found to be highly enriched GO term in liver, but not in testis (Fig. 2a). Transferases are a large group of enzymes involved in transfer of specific functional groups from one molecule to another and previous studies have indicated their importance in catalysis of phase II sulfoconjugation of 16-androstene steroids in liver and testis52,54. All eleven DE genes annotated to “Single-carbon transferase activity” in liver (Fig. 3a) were downregulated in high BT pigs, indicating a strong decrease in phase II metabolism of BT compounds. Furthermore, transferase activity was found enriched in both tissues which emphasises the importance of transferase-related genes of BT. Interestingly, COQ3 encodes an O-methyltransferase that is required for two steps in the biosynthetic pathway of coenzyme Q 10 (CoQ10). CoQ10 is involved in the respiratory chain and participates in aerobic cellular respiration which generates energy in the form of ATP55. In mammals, tissues with high energy demands, such as heart, liver and kidney, have the highest CoQ10 concentrations56,57. As the BT condition is dependent on the level of liver degradation, it is likely that the downregulated expression of COQ3 reflects insufficient metabolism. Hence, COQ3 is considered a candidate biomarker for BT in liver (Fig. 7a).
Important enriched MF terms in liver included “cytochrome-c oxidase activity” which comprised isoforms of COX6 and COX7. These genes encode subunits of cytochrome-c oxidase, the terminal enzyme of the mitochondrial respiratory chain catalysing the electron transfer from reduced cytochrome-c to oxygen58. Previous studies reported genes encoding cytochrome oxidases of the mitochondrial respiratory chain to be associated with skatole levels12,59,60,61,62. Specifically, the cytochrome subunit family COX4I1 and COX8 C encoding cytochrome-c oxidases were involved in regulation of skatole metabolism63,64, which was attributed to their involvement in the phase I oxidation reactions in liver. In this study, the cytochrome-c oxidases COX6 C, COX7AL, COX7B2 and COX7A1 were all downregulated in the high BT pigs which may reflect the situation of deficient skatole metabolism in high BT pigs. Another strong indicator of the importance of cytochrome-c oxidases in the context of BT is the high enrichment of mitochondrial CC GO terms where the enzymes are located. Hence, COX6 C is considered a candidate biomarker for BT in liver (Fig. 7a). Another important MF term found enriched in liver is the oxidoreductases, specifically the “CH-CH oxidoreductases” which participate in the phase I metabolism of BT compounds and play important enzymatic roles, some which have not yet been precisely clarified65. In this study, the “CH-CH oxidoreductases” comprised ACAD10, ACADSB, ACOX2, CPOX and DUS4L (Supplementary file 3) which are commonly found in gene expression studies for BT in liver65 and testis66,67. Interestingly, all five genes were downregulated in the high BT pigs (Supplementary file 2), which may be attributed to the insufficient liver degradation of BT compounds. ACOX2 encodes a protein of the acyl-Coenzyme A oxidase family which involves degradation of long branched fatty acids and bile acid intermediates in peroxisomes68. The similar ACOX1 was previously found to be slightly upregulated in high BT pigs65. The current finding suggests a contrary expression pattern for ACOX2 as the gene is indeed downregulated in high BT pigs. ACOX2 is also found in the lightgreen CE module in liver where the gene is functionally annotated to “Steroid metabolic process” (Fig. 5c). Hence, ACOX2 is considered a candidate biomarker for BT (Fig. 7a).
Analysis of overrepresented pathways in liver revealed a strong correlation towards active transcription (“Ribosome”), export of proteins (“Protein export”) and oxidation (“Oxidative phosphorylation”) (Fig. 4a). Analysis of the specific DE genes annotated to “Drug metabolism” revealed FMO3 and CYP2B6. Flavin-containing monooxygenases (FMOs) are a family of enzymes that converts lipophilic compounds into polar metabolites, thus decreasing their activity69. The FMOs are involved in metabolic activation or detoxification of numerous xenobiotics such as pesticides, drugs and carcinogens70. FMOs are regulated by sex steroid hormones and FMO3 was found to be repressed by testosterone and upregulated by castration71. Furthermore, FMO3 was previously speculated to be associated with off flavour in pork72. Consistently, we found FMO3 downregulated in high BT as androstenone and testosterone levels are positively correlated7. Hence, FMO3 is considered a candidate biomarker for BT in liver (Fig. 7a). The cytochrome P450 (CYP) proteins are also monooxygenases and similar in function to FMOs; they are involved in drug metabolism and synthesis of cholesterol, steroids and other lipids73. In human and pig, 18 families of CYPs have been identified with 57 and 54 genes, respectively73. The CYPs have previously been reviewed as candidate genes for BT37. In the current study, ten CYPs were identified as DE genes: CYP2J2, CYP2B6 in liver and CYP4V2, CYP1B1, CYP2D7, CYP2R1, CYP51A1, CYP20A1, CYP26B1 and CYP27A1 in testis (Supplementary file 2). In liver, CYP2B6 was upregulated in the high BT group which clearly reflects its implication in phase I metabolism. Another member of the CYP2 family was the CYP2J2 which was found to be upregulated in high BT. Interestingly, previous studies showed that the CYP2B was found only to metabolise skatole to a low degree and to our knowledge, no data is available for CYP2J (see a review by Rasmussen and Zamaratskaia74). In testis, CYP27A1 was found to be strongly upregulated in high BT pigs. In porcine hepatocytes, CYP27A1 has been found to be active in 25-hydroxylation of the prohormone vitamin D3 and to carry out the 27-hydroxylation of cholesterol-derived intermediates in bile acid biosynthesis75. In this context, it is likely that CYP27A1 is affected by the increased steroid production characterised in high BT pigs and to our knowledge, we are the first to report association of the gene with BT. Hence, CYP2J2, CYP2B6 and CYP27A1 are considered candidate biomarkers for BT in liver and testis, respectively (Fig. 7).
GSTO1 and GSR are part of “Glutathione metabolism” and “Drug metabolism” pathways and participate in the phase II metabolism. GSTO1 encodes a member of the glutathione S-transferases (GSTs) that are known for their role in catalysis of conjugation reactions in endogenous substances, haeme, fatty acids, xenobiotics and products of oxidative processes76. In context of BT, GSTO2 has been speculated to be involved in excretion of skatole from the porcine body77. We found GSTO1 to be clearly downregulated in high BT pigs (Fig. 4a), which could explain the BT condition observed by decreased excretion of skatole. The finding is consistent with previous gene expression studies in liver65 and testis66. Due to its implication in relevant pathways and clear expression pattern, GSTO1 could be considered a candidate biomarker (Fig. 7a). GSR encodes a glutathione reductase enzyme that catalyses the reduction of glutathione disulphide to glutathione, an important antioxidant and critical for the cell to resist oxidative stress and to maintain its reducing environment78. The expression of GSR is probably related to liver degradation capacity as GSR exhibited a clear pattern of downregulation in high BT pigs. Hence, GSR is considered a candidate biomarker in liver (Fig. 7a). However, the precise relationship between GSR and BT compounds should be subject to further research.
Analysis of DE genes from testis revealed “Transmembrane signalling receptor activity” to be a highly enriched MF GO term. This may reflect the fact that steroid hormone biosynthesis is a highly active function in the testis of high BT pigs, as previous research has found a high genetic correlation between androstenone (plasma and fat) and sex steroids in Duroc and Landrace pigs79. Activation and regulation of steroid hormone biosynthesis requires a high level of cellular communication observed by enrichment of the MF GO terms “Poly(A) RNA binding” and “Enzyme binding” which reflects the high pathway activity. Furthermore, the CC GO terms such as “Intracellular part” and “Nuclear part” revealed that most processes are located in the intracellular part or nuclear part where transcription and translation are undertaken in the cell (Fig. 2b). The BP term “Organelle organization” was highly enriched which probably reflects high activity of transcription and protein metabolic processes. When a fixed number of top DE genes between tissues were subjected to MF GO term enrichment analysis, “Translation factor activity, RNA binding” had four upregulated genes in high BT pigs which probably is attributed to the high level of steroidgenesis. Interestingly, genes associated with the term “Ubiquitin-protein transferase activity” were almost exclusively downregulated in the high BT pigs. This family of transferases is responsible for the transfer of ubiquitin from one protein to another which can signal the degradation of the protein by the proteasome. The downregulation of the associated genes implies a markedly less regulated steroidgenesis which probably results in overproduction of steroid hormones in testis. This hypothesis is supported by the significant overrepresentation of the pathway “Steroid hormone biosynthesis” which comprises the 17 β-hydroxysteroid dehydrogenase (HSD) genes HSD17B7 and HSD17B8 (Fig. 4b). The 17β-HSD encode enzymes that are part of the last catalysis step in the formation of androgens and estrogens80 and the various types provide specific cell types with the mechanisms to regulate intracellular androgens and/or estrogens81. The importance of the HSD family in relation to BT compounds has been well documented in both RNA-Seq and microarray studies and both HSD17B2 and HSD17B4 have previously been suggested as biomarkers for reduced BT37,66,77,82. We also found the HSD17B7 and HSD17B8 to be highly upregulated in the high BT pigs. HSD17B7 is involved in biosynthesis of cholesterol83 and sex steroids84 and regulates bioavailability of both androgens and estrogens, yet interestingly, previous work has confirmed that the HSD17B7 is not the key enzyme responsible for androstenone and testosterone metabolism in porcine hepatocyte85. However, we found the HSD17B7 upregulated in testis and not liver, which consequently justifies the proposition of it being a candidate biomarker in testis (Fig. 7b). The HSD17B8 is involved in regulation of concentrations of biologically active estrogens and androgens86. Recently, the protein encoded by the HSD17B8 was identified as a major interaction partner in gene networks of pigs with divergent androstenone levels. The major function of the protein was hypothesised to be testosterone inactivation87. The testicular testosterone concentrations were positively correlated with androstenone concentrations due to active testosterone synthesis which could also imply active androstenone synthesis87. However, we found that HSD17B8 was upregulated in testis from high BT pigs which is contrary to the hypothesis that HSD17B8 inactivates testosterone synthesis. We suggest that the gene plays an important role in BT and is considered a candidate biomarker, but further research should clarify its exact role in regulation of BT compounds (Fig. 7b).
The selection of pathways of biological interest included the “Drug metabolism”, “Estrogen signalling pathway” and “GnRH signalling pathway” (Fig. 4b). In “GnRH signalling pathway”, genes had both up- or downregulation patterns, but mostly downregulated. MAPK3 and MAP2K2 were upregulated whereas MAP3K2 were downregulated in high BT pigs, respectively (Fig. 4b). The MAPK pathway encodes protein kinases which have been shown to modulate numerous male reproductive functions such as spermatogenesis, sperm maturation and activation and other functions of the germ cells88. Interestingly, MAPK3, MAP2K2 and MAP3K2 were also a part of the “Estrogen signalling pathway” (Supplementary file 4). The “Estrogen signalling pathway” was significantly overrepresented, which is probably due to secretion of large amounts of estrogen from the testis89. Estrogen concentrations have been found to be correlated with androstenone in adipose tissue90,91. The roles of the MAPK pathway in variation of BT has recently been shown in a gene expression study on Duroc boars92. The authors found that upregulation of MAPK coincided with pubertal increase in androstenone and testosterone production92 as the upregulation was observed several weeks earlier than complex morphological restructuring of testis into reproductive tissue. Furthermore, proteins encoded by MAPK may regulate the Steroidogenic Acute Regulatory protein (STAR) (a cholesterol traffic regulator) and have a complex interaction with steroidgenesis (see review by Manna and Stocco93). Interestingly, STARD10, which may play a metabolic role in sperm maturation and lipid transport93, was found upregulated in testis (Supplementary file 2) and may have other localised functions such as enrichment of lipids in milk in the mammary glands94. Previous works have documented the role of a subfamily of STAR in BT in testis, especially STARD6 and STAR 66,92. To our knowledge this is the first time a relationship between STARD10 and BT has been reported and the association warrants further investigation of the role of STARD10 in BT. Due to the complexity of MAPK in BT and the warrant for further research of these genes, MAPK3, MAP2K2, MAP3K2 and STARD10 are considered candidate biomarkers (Fig. 7b).
Co-expression analysis in liver revealed the lightgreen module to be associated and slightly downregulated with the summarised EBVs. Among the five hubs genes (HSD17B8, GSTZ1, SERPINC1, PRDX3 and NIT1), HSD17B8 was also DE in testis and selected as a candidate biomarker. Furthermore, HSD17B8 was co-expressed with CYB5A. Expression levels of CYB5A has previously been linked to androstenone levels94. Hence, the finding of HSD17B8 as a hub gene strengthens the hypothesis of a regulatory role in androstenone synthesis. GSTZ1 was found as a hub gene in the lightgreen module and as a DE gene in liver which encodes a member of the GSTs95. Other hub genes included SERPINC1 (Fig. 5a) which encode a gene that inhibits thrombin as well as other activated serine proteases of the coagulation system and regulates the blood coagulation cascade96. Members of the SERPIN family have previously been associated with skatole metabolism in liver77 and it is very likely that the hub genes are all related through various mechanisms in the clearing of skatole and/or androstenone from the liver either directly or indirectly through regulation or activation of other genes and/or pathways. Interestingly, SERPINC1 was co-expressed with HSD17B13 which is not exactly clear as 17β-HSD catalyses biosynthesis of androgens and estrogens but the finding warrants further research into the association of SERPINC1 and BT. The darkgreen module was significantly enriched for the MF GO term “Steroid metabolic process” which comprised SULT1B1 and CYP7A1. Downregulation of CYP7A1 has been speculated to increase androstenone levels through reduced fat catabolism82 which is consistent with the current study where the gene was downregulated in high BT pigs. Considering the finding of CYP7A1 as co-expressed in liver, additional research should be focused on its role in BT. SULT1B1 encodes a member of the sulfotransferases which catalyse the sulphate conjugation of steroid hormones, neurotransmitters, drugs and xenobiotic compounds97 and previous work established them as key enzymes involved in the testicular and hepatic metabolism of androstenone66. The closely related SULT1A1 were found to be involved in phase II metabolism of skatole98 and a mutation in the gene is thought to be responsible for skatole accumulation99 which has prompted researchers to consider it a candidate biomarker for BT37. Indeed, the sulfotransferases seem important in the metabolism of BT compounds as a negative correlation between accumulation of androstenone and SULT2A1 in which low activity of SULT2A1 was found in animals with high concentrations of androstenone100. Similar results were found in a study performed on Duroc, Norwegian Landrace and Yorkshire67 which is also consistent with the findings in the current study where SULT1B1 was downregulated in high BT pigs. The finding of SULT1B1 as a co-expressed gene reflects a possible regulatory association and warrants further research on its precise role in BT. In the current study, only SULT4A1 was found as a DE gene in testis and upregulated in high BT pigs but it did not pass additional filtering (Supplementary file 2).
In testis, the significantly associated darkgreen module contained genes significantly enriched for “Carboxylic acid catabolic process”, “Small molecule catabolic process” and “Sterol molecule metabolic process” (Fig. 6b). Further analysis of the “Sterol metabolic process” term revealed that several genes overlapped with “Cholesterol biosynthetic process” (Fig. 6c). Interestingly, the previously selected candidate biomarker HSD17B7 was part of the darkgreen module and the finding of HSD17B7 as a co-expressed gene may point to some kind of regulatory function in BT. However, this is in contrast to previous research where HSD17B7 was excluded as a key enzyme responsible for androstenone and testosterone metabolism in porcine liver cells85. Another important enriched gene in the module was CYB5R3 which encodes a cytochrome b5 reductase101. The subfamily CYB5 has been found particular important in androstenone biosynthesis and expression levels and a mutation in CYB5A was speculated to decrease androstenone levels94,102. From its role as a reductase, CYB5R3 is a modulator of the activity of CYB5A 103. Interestingly, work on nuclear receptor transactivation found that CYB5R1 probably increased levels of 16-androstene steroids but ambiguous results were found for CYB5R3 104. In the current study, CYB5R1 was also found as a DE gene in testis (Supplementary file 3) but did not pass additional filtering. Due to strong upregulation and co-expression of CYB5R3 in high BT pigs, we consider the gene as a candidate biomarker in testis (Fig. 7b).
In this study, the candidate biomarkers were genes with functions within phase I (COQ3, COX6 C, CYP2J2, CYP2B6, ACOX2) and phase II metabolism (GSTO1, GSR, FMO3) of skatole and androstenone in liver to steroidgenesis (HSD17B7, HSD17B8, CYP27A1), regulation of steroidgenesis (STARD10, CYB5R3) and GnRH signalling (MAPK3, MAP2K2, MAP3K2) in testis. For comparison, a recently published review by Zadinová, et al.37 summarised genes currently believed to be controlling levels of androstenone (CYP11A1, CYP17A, CYP21A2, CYB5A, LHB, HSD17B7), androstenone metabolism (HSD3B, SULT2A1, SULT2B1, TEAD3), phase I skatole metabolism (CYP2A6, CYP2E1, CYB5A) and phase II skatole metabolism (SULT1A1). Other important genes were FMO3 72, GSTO2 77, COX4I1 64, MAPK14 79, STARD6 92 and ACOX1 77. Thus, a high level of consistency is evident between known gene families involved in BT and the selection of candidate biomarkers in this study. Importantly, our study presents two new genes (COQ3, GSR) and novel gene members and isoforms of BT which is probably attributed to the use of deep sequencing of ~33 mio reads per sample to register even small fluctuations in gene expression. Some candidate biomarkers probably share similar functions with known genes of BT such as the case of HSD3B and HSD17B 91 or may be involved in regulation of known candidate biomarkers such as CYB5R3 that regulates CYB5A 103. Furthermore, co-expression network analysis provided valuable insight into the complexity of both DE and non-DE genes and their functions in BT such as CYP7A1 and SULT1B1 and enabled discovery of hub genes with a possible regulatory association such as HSD17B8 and SERPINC1 co-expressed with CYB5A and HSD17B13¸ respectively.
Future work should focus on the validation of the detected biomarkers at the population-wise level as well as in other breeds to develop a sensitive and specific industry-targeted test for optimised genomic-based animal breeding to reduce BT. Gene expression data should be combined with genotypes to find expression quantitative trait loci (eQTLs) of BT and/or further utilise the power of the RNA-Seq data with additional analysis of splicing events, polymorphisms and different isoforms of transcripts105 of the candidate biomarkers proposed in this study.
Methods
A complete overview of the experimental design is presented in Fig. 1.
Animal model
Male Landrace pigs (n = 114) were housed at the commercial testing station Bøgildgård in Denmark (N 56°27′52.60′′ W 9°38′62.97′′) operated by the Pig Research Centre, SEGES (Copenhagen, Denmark) and raised with ad libitum feed and water supply. The pigs were produced from sires with known genetic merit of boar taint (BT) assessed from estimated breeding values (EBVs) of skatole concentrations and human nose score (HNS). To account for both traits, the genetic merit of BT was defined as the sum of the EBVs of skatole concentration and HNS obtained from the respective sire of each pig and denoted as the summarised EBV following equation (1):
where the summarised EBV i is the sum of the EBV for skatole concentration in fat and the EBV for HNS for the individual pig i obtained from its respective sire j. All EBVs were collected from the Danish pig breeding database (Pig Research Centre, SEGES, Copenhagen, Denmark). The raw values for skatole concentrations were obtained from measurements on carcass fat samples by a calorimetric method106. HNS were collected by a standardised method107. The EBVs (the relative genetic performance compared with a contemporary group) were calculated by Genomic BLUP Animal Models48,49. The EBVs were corrected for age (days) and weight (kg) in order to account for systematic differences in sexual maturation and to obtain full steroidogenic potential33 in the three groups.
Subsequently, the animals were grouped as low, medium or high genetic merit of BT, according to the summarised EBVs. A total of 48 pigs out of the 114 pigs were selected for analysis from each of the three groups as high, medium and low genetic merit of BT (Supplementary file 1). The selection of pigs was based on the most extreme values within each of the low and high groups, and the closest values to the mean in the medium group. To account for any pedigree effects, the maximum amount of relatedness allowed between pigs but within sire groups were only half-siblings. However, the sires themselves were not related as far as we could find in the dataset.
Tissue collection and preparation
Pigs were slaughtered at a weight of ~100 kg at a commercial slaughterhouse (Danish Crown, Herning, Denmark). Slaughter was performed by submersion into CO2 until unconsciousness ensued followed by exsanguination. Following slaughter, the liver was extracted from the carcass and 150 mg of tissue were retrieved by punch biopsy and immediately immersed into 1.5 ml RNAlater (QIAGEN, Hilden, Germany) in 2 ml Eppendorf tubes (Eppendorf, Hamburg, Germany). The carcasses were kept in a cold room at 4 °C for approximately 1.5 h before 150 mg testis tissue were retrieved by punch biopsy and immersed into 1.5 ml RNAlater (QIAGEN). All samples (n = 96) were stored at −20 °C for 14 days until RNA extraction and sequencing at a commercial facility (AROS A/S, Aarhus, Denmark).
RNA extraction and sequencing
Total RNA was extracted from the 96 samples by RNeasy Mini Kit (QIAGEN) following instructions of the manufacturer. Concentration of RNA was measured by Nanodrop® 2000 (ThermoScientific, Massachusetts, USA). The quality of the RNA was measured with a Bioanalyzer (Agilent, California, USA) to ensure an RNA integrity number (RIN) of at least 6; otherwise, the samples were discarded. For samples with an RIN values of 7 or above, the sequencing libraries were prepared using the TruSeq stranded mRNA (Illumina, San Diego, USA) kit following instructions of the manufacturer. Samples with RIN values from 6 to 7 were therefore prepared using the TruSeq total stranded RNA (Illumina) kit following instructions of the manufacturer. In both protocols, 400 ng of total RNA were used as input and fragmentation was performed following instructions of the manufacturer, but with an incubation step of 1 min at 94 °C protocol to produce libraries that are compatible with sequencing on a paired-end 100 base pair flow cell (PE-100bp FC, Illumina, San Diego, USA). Libraries were subjected to quality control with respect to size profile by test on an Agilent DNA 1000 (Agilent, California, USA) and library concentration by KAPA quantitative PCR (qPCR) kit and three independent 106-fold dilutions of libraries following instructions of the manufacturer (Kapa Biosystems, Massachusetts, USA). The samples were sequenced with an Illumina HiSeq. 2500 (Illumina, San Diego, USA) which amounted to a theoretical 40 million reads per sample.
Quality control and gene counting
Data from sequencing machine were converted from base calls into FastQ-files by CASAVA®-software (Illumina, San Diego, USA). Quality control (QC) of RNA-Seq reads was conducted with FastQC108. Reads were trimmed for known Illumina TruSeq adapter sequences using the programme CutAdapt109. Poor reads were trimmed by Trimmomatic110 using default parameters. The trimmed reads were then mapped to the Sus scrofa reference genome (10.2, version 79) obtained from Ensembl111 by the STAR aligner using default parameters112. Post-mapping QC was performed with Qualimap113. The mapped reads were counted to each gene by HTSeq count using default parameters114. All subsequent statistical analysis were performed in R version 3.1.0115. Only genes with a mean count of more than five were included in the gene count matrices. Due to relatively poor annotation of the Sus scrofa genome, Ensembl identifiers were translated into their human orthologous gene symbols by the R package biomaRt116,117, using the Homo sapiens reference genome (h38, version 84, Ensembl).
Differential expression analysis
Normalisation of gene counts was performed by voom variance-stabilization function with sample quality weights118 implemented in the R package limma. Differentially expressed (DE) genes were detected using the R package limma119. Briefly, limma fits a linear model to each gene using the function lmFit following equation (2):
where \({\gamma }_{ij}\) is the measured expression level of gene j for individual pig i, \({\beta }_{j,BT}\) is the estimated regression coefficient calculated by regression of the gene expression value of the i th individual pig categorised to its genetic merit of BT group (“low”, “medium” or “high”) for the j th gene and ε ij is the error component. By using the function eBayes in limma, the moderated t-test statistics for differences in the variance of gene expression of a gene across replicates were calculated and subjected to multiple testing correction of the P values by the Benjamini-Hochberg procedure120 which resulted in a false discovery rate (FDR) and the logarithm-transformed fold change for each gene. Genes were categorised as differentially expressed genes when FDR was below 0.05 and were found by contrasting “high BT vs low BT” groups from a design matrix that included all three groups. Additional filtering was performed by comparison of expression profiles of the individual DE gene throughout the three groups by a Kruskal-Wallis test. Gene functional enrichment analysis was performed with the Cytoscape121 plug-in ClueGO version 2.2.5122. By providing DE genes and genes attributed to co-expression modules with significant association to the summarised EBVs, ClueGO performed overrepresentation test and visualisation for gene ontology (GO) terms and KEGG pathways using default parameters. Test results were subjected to multiple testing correction of the P values by Bonferroni correction. For semantic filtering of redundant GO terms, the online tool REVIGO123 was used. As the BT condition involves numerous enzymatic functions and for the sake of simplicity, the most interesting molecular function (MF) GO terms from a fixed number (n = 507) of top DE genes from each tissue to make analysis consistent. Similar DE analyses based on 3 or more categories and treatment groups with similar sample sizes / biological replicates and RNA-Seq data in animals have been applied for various conditions including Mazzoni, et al.34 for cattle reproduction and Salleh, et al.35 for cattle feed efficiency.
Co-expression analysis
In order to identify strongly co-expressed (CE) genes (“hub genes”) which may have regulatory functions and/or major impact on the trait, we employed a co-expression analysis approach for each tissue which has previously been successfully employed by our group40. Scale-free undirected co-expression networks were built using the R package WGCNA42,124 and modules with significant association (P < 0.05) to genetic merit of BT were detected and analysed. The genetic merit of BT was inputted as the high, medium and low groups defined from the summarised EBVs as described in the animal model section. The co-expression analyses was performed independently for liver and testis by the following procedure: An adjacency matrix comprising Pearson’s correlations between expression levels of genes was created from the 7,500 most highly expressed genes from the tissue in question which satisfied the requirements of both acceptable computational time and biological meaningfulness. Subsequently, the matrix was raised by a power (β) of 10 in each tissue which was found to be an appropriate value by the function pickSoftThreshold() to reach a scale-free topology index (R 2) of at least 0.90. The topological overlap measure (TOM), which assesses the degree of shared neighbours between pairs of genes, was calculated from the adjacency matrix and used to build a gene dendrogram which detects modules and assigns colour by the DynamicTreeCut algorithm125, using a minimum of 30 genes per module. The module eigengene, the first principal component of each module, represents the expression value of each module and was used to detect biologically relevant modules. The module-trait relationship was calculated as the Pearson’s correlation between the module eigengene and the traits of interest. Modules significantly (P < 0.05) associated with genetic merit of BT were exported for topological analysis and visualisation in the software VisANT126. An arbitrarily chosen subset of 150 gene pairs with the highest TOM within the selected modules was selected and hub genes were defined as genes with highest intramodular connectivity. The intramodular connectivity was assessed by a chosen topological analysis of the module: genes with a node degree distribution (k) (links per node) of ≥12 were assigned as hub genes for the specific tissue.
Candidate biomarkers
Selection of candidate biomarkers for each tissue was made by applying a reductionist strategy of genes that had significant (FDR < 0.05) differential expression and significant (P < 0.05) difference in expression profiles by a Kruskal-Wallis test throughout the three groups and were either: (i) annotated to relevant GO terms and/or pathways and/or (ii) part of a co-expression module related to a summarised EBVs of skatole and HNS. Each chosen candidate biomarker was visualised on a box plot with the P value of the Kruskal-Wallis test.
Declarations
Ethics approval and consent to participate
Animal Care and Use Committee approval was not obtained for this study, because tissue samples were obtained from a commercial slaughter facility.
Availability of data and material
The datasets generated during and/or analysed during the current study are available in NCBI’s Gene Expression Omnibus127 and are accessible through GEO Series accession number GSE93734 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE93734).
All data analysed during this study are included in this published article [and its supplementary information files].
References
Bonneau, M. et al. An international study on the importance of androstenone and skatole for boar taint: IV. Simulation studies on consumer dissatisfaction with entire male pork and the effect of sorting carcasses on the slaughter line, main conclusions and recommendations. Meat Sci 54, 285–295 (2000).
Lundström, K., Matthews, K. & Haugen, J.-E. Pig meat quality from entire males. Animal 3, 1497–1507 (2009).
Claus, R., Weiler, U. & Herzog, A. Physiological aspects of androstenone and skatole formation in the boar—a review with experimental data. Meat Sci 38, 289–305 (1994).
Brooks, R. & Pearson, A. Steroid hormone pathways in the pig, with special emphasis on boar odor: a review. J Anim Sci 62, 632–645 (1986).
Bonneau, M. Compounds responsible for boar taint, with special emphasis on androstenone: a review. Livest Prod Sci 9, 687–705 (1982).
Andresen, Ø. Boar taint related compounds: Androstenone/skatole/other substances. Acta Vet Scand 48, S5 (2006).
Zamaratskaia, G., Babol, J., Andersson, H. & Lundström, K. Plasma skatole and androstenone levels in entire male pigs and relationship between boar taint compounds, sex steroids and thyroxine at various ages. Livest Prod Sci 87, 91–98 (2004).
Babol, J., Zamaratskaia, G., Juneja, R. & Lundström, K. The effect of age on distribution of skatole and indole levels in entire male pigs in four breeds: Yorkshire, Landrace, Hampshire and Duroc. Meat Sci 67, 351–358 (2004).
Robic, A., Larzul, C. & Bonneau, M. Genetic and metabolic aspects of androstenone and skatole deposition in pig adipose tissue: A review. Genet Sel Evol 40, 1 (2008).
Wesoly, R. & Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2, 221–242 (2012).
Tajet, H. & Andresen, Ø. Estimation of genetic parameters of boar taint; skatole and androstenone and their correlations with sexual maturation. Acta Vet Scand 48, 1 (2006).
Squires, E. J. & Lundström, K. Relationship between cytochrome P450IIE1 in liver and levels of skatole and its metabolites in intact male pigs. J Anim Sci 75, 2506–2511 (1997).
Wäckers, F., Olson, D., Rains, G., Lundby, F. & Haugen, J. E. Boar taint detection using parasitoid biosensors. J Food Sci 76, S41–S47 (2011).
Bonneau, M. Use of entire males for pig meat in the European Union. Meat Sci 49, S257–S272 (1998).
Prunier, A. et al. A review of the welfare consequences of surgical castration in piglets and the evaluation of non-surgical methods. Anim Welf 15, 277 (2006).
Rydhmer, L. et al. Aggressive and sexual behaviour of growing and finishing pigs reared in groups, without castration. Acta Agric Scand A Anim Sci 56, 109–119 (2006).
European Declaration on alternatives to surgical castration of pigs http://ec.europa.eu/food/animals/welfare/practice/farm/pigs/castration_alternatives_en (2010).
Baumgartner, J. et al. Aktuelle Aspekte der Kastration mannlicher Ferkel-2. Mitteilung: Alternativmethoden zur chirurgischen Kastration und zusammenfassende Bewertung Castration of male piglets (II): alternatives and conclusion. Wien Tierarztl Monatsschr, 198–209 (2004).
Jaros, P. et al. Effect of active immunization against GnRH on androstenone concentration, growth performance and carcass quality in intact male pigs. Livest Prod Sci 92, 31–38 (2005).
Kim, Y. et al. Effects of immunocastration on physiological changes, the characteristics of carcass and meat quality in boars. J Anim Sci Technol 49, 753–760 (2007).
Zamaratskaia, G. et al. Effect of a Gonadotropin‐releasing Hormone Vaccine (ImprovacTM) on Steroid Hormones, Boar Taint Compounds and Performance in Entire Male Pigs. Reprod Domest Anim 43, 351–359 (2008).
Morales, J. et al. Evaluation of production performance and carcass quality characteristics of boars immunised against gonadotropinreleasing hormone (GnRH) compared with physically castrated male, entire male and female pigs. Span J Agric Res, 599–606 (2010).
Škrlep, M. et al. Effect of immunocastration (Improvac®) in fattening pigs I: Growth performance, reproductive organs and malodorous compounds. Slov Vet Zb 47, 57–64 (2010).
De Roest, K., Montanari, C., Fowler, T. & Baltussen, W. Resource efficiency and economic implications of alternatives to surgical castration without anaesthesia. Animal 3, 1522–1531 (2009).
Fredriksen, B., Johnsen, A. M. S. & Skuterud, E. Consumer attitudes towards castration of piglets and alternatives to surgical castration. Res Vet Sci 90, 352–357 (2011).
Spoolder, H., Bracke, M., Mueller‐Graf, C. & Edwards, S. Preparatory work for the future development of animal based measures for assessing the welfare of pigs‐Report 1: Preparatory work for the future development of animal based measures for assessing the welfare of sow, boar and piglet including aspects related to pig castration. EFSA Supporting Publications 8 (2011).
Heinritzi, K., Ritzmann, M. & Otten, W. Alternatives for castration of suckling piglets, determination of catecholamines and wound healing after castration of suckling piglets at different points of time. Dtsch Tierarztl Wochenschr 113, 94–97 (2006).
Grindflek, E. et al. Large scale genome-wide association and LDLA mapping study identifies QTLs for boar taint and related sex steroids. BMC Genomics 12, 1 (2011).
Sellier, P., Le Roy, P., Fouilloux, M., Gruand, J. & Bonneau, M. Responses to restricted index selection and genetic parameters for fat androstenone level and sexual maturity status of young boars. Livest Prod Sci 63, 265–274 (2000).
Strathe, A. B., Velander, I., Mark, T. & Kadarmideen, H. Genetic parameters for androstenone and skatole as indicators of boar taint and their relationship to production and litter size traits in Danish Landrace. J Anim Sci 91, 2587–2595 (2013a).
Frieden, L., Looft, C. & Tholen, E. Breeding for reduced boar taint. Lohmann Inf 46, 21–27 (2011).
Strathe, A. B. et al. Genetic parameters for male fertility and its relationship to skatole and androstenone in Danish Landrace boars. J Anim Sci 91, 4659–4668 (2013b).
Zamaratskaia, G. & Squires, E. Biochemical, nutritional and genetic effects on boar taint in entire male pigs. Animal 3, 1508–1521 (2009).
Mazzoni, G. et al. Identification of potential biomarkers in donor cows for in vitro embryo production by granulosa cell transcriptomics. PLoS One 12, e0175464 (2017).
Salleh, M. et al. RNA-Seq transcriptomics and pathway analyses reveal potential regulatory genes and molecular mechanisms in high-and low-residual feed intake in Nordic dairy cattle. BMC Genomics 18, 258 (2017).
Kadarmideen, H. N. Genomics to systems biology in animal and veterinary sciences: progress, lessons and opportunities. Livest Sci 166, 232–248 (2014).
Zadinová, K., Stupka, A. S., Čítek, J. & Vehovský, D. U. Boar taint–the effects of selected candidate genes associated with androstenone and skatole levels–a review. Anim Sci Pap Rep 34, 107–128 (2016).
Wang, Z., Gerstein, M. & Snyder, M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10, 57–63 (2009).
Kadarmideen, H. N. & Reverter, A. Combined genetic, genomic and transcriptomic methods in the analysis of animal traits. Anim Health Res Rev 2, 16 (2007).
Kogelman, L. J. et al. Identification of co-expression gene networks, regulatory genes and pathways for obesity based on adipose tissue RNA Sequencing in a porcine model. BMC Med Genomics 7, 1 (2014).
Kadarmideen, H. N., Watson-Haigh, N. S. & Andronicos, N. M. Systems biology of ovine intestinal parasite resistance: disease gene modules and biomarkers. Mol Biosyst 7, 235–246 (2011).
Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 1 (2008).
Bourdon, R. M. & Bourbon, R. M. Understanding animal breeding. Vol. 2 (Prentice Hall Englewood Cliffs, NJ, 1997).
Windig, J. et al. Genetic parameters for androstenone, skatole, indole, and human nose scores as measures of boar taint and their relationship with finishing traits. J Anim Sci 90, 2120–2129 (2012).
Dijksterhuis, G. et al. An international study on the importance of androstenone and skatole for boar taint: II. Sensory evaluation by trained panels in seven European countries. Meat Sci 54, 261–269 (2000).
Whittington, F. et al. Comparison of heating methods and the use of different tissues for sensory assessment of abnormal odours (boar taint) in pig meat. Meat Sci 88, 249–255 (2011).
Meier-Dinkel, L., Gertheiss, J., Müller, S., Wesoly, R. & Mörlein, D. Evaluating the performance of sensory quality control: the case of boar taint. Meat Sci 100, 73–84 (2015).
Strathe, A. B., Mark, T., Velander, I. & Kadarmideen, H. in 65th Annual Meeting of the European Federation of Animal Sciences.
Christensen, O. F., Madsen, P., Nielsen, B., Ostersen, T. & Su, G. Single-step methods for genomic evaluation in pigs. Animal 6, 1565–1571 (2012).
Lervik, S. et al. Androstenone and testosterone levels and testicular morphology of Duroc boars related to estimated breeding value for androstenone. Theriogenology 79, 986–994 (2013).
Kogelman, L. J. et al. Genetic architecture of gene expression in ovine skeletal muscle. BMC Genomics 12, 607 (2011).
Goldstein, J. A. & Faletto, M. B. Advances in mechanisms of activation and deactivation of environmental chemicals. Environ Health Perspect 100, 169 (1993).
Parkinson, A. & Ogilvie, B. W. Biotransformation of xenobiotics. (McGraw-Hill New York, 2001).
Sinclair, P., Squires, E., Raeside, J. & Renaud, R. Synthesis of free and sulphoconjugated 16-androstene steroids by the Leydig cells of the mature domestic boar. J Steroid Biochem Mol Biol 96, 217–228 (2005).
Crane, F. L. Biochemical functions of coenzyme Q10. J Am Coll Nutr 20, 591–598 (2001).
Åberg, F., Appelkvist, E.-L., Dallner, G. & Ernster, L. Distribution and redox state of ubiquinones in rat and human tissues. Arch Biochem Biophys 295, 230–234 (1992).
Battino, M. et al. Natural distribution and occurrence of coenzyme Q homologues. Membr Biochem (2009).
Fontanesi, F., Soto, I. C., Horn, D. & Barrientos, A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am J Physiol Cell Physiol 291, C1129–C1147 (2006).
Lundström, K. et al. Skatole levels in pigs selected for high lean tissue growth rate on different dietary protein levels. Livest Prod Sci 38, 125–132 (1994).
Skinner, T., Doran, E., McGivan, J., Haley, C. & Archibald, A. Cloning and mapping of the porcine cytochrome‐p450 2E1 gene and its association with skatole levels in the domestic pig. Anim Genet 36, 417–422 (2005).
Moe, M. et al. Association between SNPs within candidate genes and compounds related to boar taint and reproduction. BMC Genet 10, 1 (2009).
Lin, Z., Lou, Y. & Squires, E. Molecular cloning, expression and functional characterization of the cytochrome P450 2A6 gene in pig liver. Anim Genet 35, 314–316 (2004).
Lobjois, V. et al. A muscle transcriptome analysis identifies positional candidate genes for a complex trait in pig. Anim Genet 39, 147–162 (2008).
Ramos, A. M. et al. The distal end of porcine chromosome 6p is involved in the regulation of skatole levels in boars. BMC Genet 12, 1 (2011).
Moe, M. et al. Gene expression profiles in liver of pigs with extreme high and low levels of androstenone. BMC Vet Res 4, 1 (2008).
Moe, M. et al. Gene expression profiles in testis of pigs with extreme high and low levels of androstenone. BMC Genomics 8, 1 (2007).
Leung, M. C., Bowley, K.-L. & Squires, E. J. Examination of testicular gene expression patterns in Yorkshire pigs with high and low levels of boar taint. Anim Biotechnol 21, 77–87 (2010).
Baumgart, E., Vanhooren, J. C., Fransen, M., Mannaerts, G. P. & Veldhoven, P. P. Mammalian Peroxisomal Acyl‐CoA Oxidases. Ann N Y Acad Sci 804, 678–679 (1996).
Cashman, J. R. Some distinctions between flavin-containing and cytochrome P450 monooxygenases. Biochem Biophys Res Commun 338, 599–604 (2005).
Ziegler, D. M. Recent studies on the structure and function of multisubstrate flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol 33, 179–199 (1993).
Falls, J. G., Ryu, D.-Y., Cao, Y., Levi, P. E. & Hodgson, E. Regulation of mouse liver flavin-containing monooxygenases 1 and 3 by sex steroids. Arch Biochem Biophys 342, 212–223 (1997).
Glenn, K., Ramos, A. & Rothschild, M. Analysis of FMO genes and off flavour in pork. J Anim Breed Genet 124, 35–38 (2007).
Burkina, V., Rasmussen, M. K., Pilipenko, N. & Zamaratskaia, G. Comparison of xenobiotic-metabolising human, porcine, rodent, and piscine cytochrome P450. Toxicology (2016).
Rasmussen, M. K. & Zamaratskaia, G. Regulation of porcine hepatic cytochrome p450—implication for boar taint. Comput Struct Biotechnol J 11, 106–112 (2014).
Hosseinpour, F., Ibranovic, I., Tang, W. & Wikvall, K. 25-Hydroxylation of vitamin D 3 in primary cultures of pig hepatocytes: evidence for a role of both CYP2D25 and CYP27A1. Biochem Biophys Res Commun 303, 877–883 (2003).
Listowsky, I., Abramovitz, M., Homma, H. & Niitsu, Y. Intracellular binding and transport of hormones and xenobiotics by glutathiones-transferases. Drug Metab Rev 19, 305–318 (1988).
Gunawan, A. et al. Identification of the novel candidate genes and variants in boar liver tissues with divergent skatole levels using RNA deep sequencing. PLoS One 8, e72298 (2013a).
Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta 1830, 3217–3266 (2013).
Robic, A. et al. Expression levels of 25 genes in liver and testis located in a QTL region for androstenone on SSC7q1. 2. Anim Genet 42, 662–665 (2011).
Adamski, J. et al. Molecular cloning of a novel widely expressed human 80 kDa 17β-hydroxysteroid dehydrogenase IV. Biochem J 311, 437–443 (1995).
Labrie, F. et al. The key role of 17β-hydroxysteroid dehydrogenases in sex steroid biology. Steroids 62, 148–158 (1997).
Gunawan, A. et al. RNA deep sequencing reveals novel candidate genes and polymorphisms in boar testis and liver tissues with divergent androstenone levels. PLoS One 8, e63259 (2013b).
Marijanovic, Z. et al. Closing the gap: identification of human 3-ketosteroid reductase, the last unknown enzyme of mammalian cholesterol biosynthesis. Mol Endocrinol 17, 1715–1725 (2003).
Peltoketo, H., Nokelainen, P., Piao, Y.-s., Vihko, R. & Vihko, P. Two 17β-hydroxysteroid dehydrogenases (17HSDs) of estradiol biosynthesis: 17HSD type 1 and type 7. J Steroid Biochem Mol Biol 69, 431–439 (1999).
Chen, G. et al. Investigation of testosterone, androstenone, and estradiol metabolism in HepG2 cells and primary culture pig hepatocytes and their effects on 17βHSD7 gene expression. PLoS One 7, e52255 (2012).
Fomitcheva, J., Baker, M. E., Anderson, E., Lee, G. Y. & Aziz, N. Characterization of Ke 6, a new 17β-hydroxysteroid dehydrogenase, and its expression in gonadal tissues. J Biol Chem 273, 22664–22671 (1998).
Sahadevan, S. et al. Pathway based analysis of genes and interactions influencing porcine testis samples from boars with divergent androstenone content in back fat. PLoS One 9, e91077 (2014).
Li, M. W., Mruk, D. D. & Cheng, C. Y. Mitogen-activated protein kinases in male reproductive function. Trends Mol Med 15, 159–168 (2009).
Claus, R. & Hoffmann, B. Oestrogens, compared to other steroids of testicular origin, in bloodplasma of boars. Acta Endocrinol 94, 404–411 (1980).
Zamaratskaia, G. et al. Boar taint is related to endocrine and anatomical changes at puberty but not to aggressive behaviour in entire male pigs. Reprod Domest Anim 40, 500–506 (2005).
Chen, G. et al. Gene expression of 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase in relation to androstenone, testosterone, and estrone sulphate in gonadally intact male and castrated pigs. J Anim Sci 85, 2457–2463 (2007).
Lervik, S. et al. Gene expression during testis development in Duroc boars. Animal 9, 1832–1842 (2015).
Manna, P. R. & Stocco, D. M. The role of specific mitogen-activated protein kinase signaling cascades in the regulation of steroidogenesis. J Signal Transduct 2011 (2011).
Olayioye, M. A. et al. StarD10, a START domain protein overexpressed in breast cancer, functions as a phospholipid transfer protein. J Biol Chem 280, 27436–27442 (2005).
Di Cianni, F. et al. MAP3K7 and GSTZ1 are associated with human longevity: a two-stage case–control study using a multilocus genotyping. Age 35, 1357–1366 (2013).
de la Morena-Barrio, M. E. et al. Regulatory regions of SERPINC1 gene: Identification of the first mutation associated with antithrombin deficiency. Thromb Haemost 107, 430–437, https://doi.org/10.1160/TH11-10-0701 (2012).
Weinshilboum, R. & Otterness, D. in Conjugation—Deconjugation Reactions in Drug Metabolism and Toxicity 45–78 (Springer, 1994).
Babol, J., Squires, E. J. & Lundström, K. Hepatic metabolism of skatole in pigs by cytochrome P4502E1. J Anim Sci 76, 822–828 (1998).
Lin, Z., Lou, Y. & Squires, J. Molecular cloning and functional analysis of porcine SULT1A1 gene and its variant: a single mutation SULT1A1 causes a significant decrease in sulfation activity. Mamm Genome 15, 218–226 (2004).
Sinclair, P., Gilmore, W., Lin, Z., Lou, Y. & Squires, E. Molecular cloning and regulation of porcine SULT2A1: relationship between SULT2A1 expression and sulfoconjugation of androstenone. J Mol Endocrinol 36, 301–311 (2006).
Billen, M. & Squires, E. The role of porcine cytochrome b5A and cytochrome b5B in the regulation of cytochrome P45017A1 activities. J Steroid Biochem Mol Biol 113, 98–104 (2009).
Davis, S. & Squires, E. Association of cytochrome b5 with 16-androstene steroid synthesis in the testis and accumulation in the fat of male pigs. J Anim Sci 77, 1230-1235 (1999).
Gray, M. A. & Squires, E. J. Effects of nuclear receptor transactivation on boar taint metabolism and gene expression in porcine hepatocytes. J Steroid Biochem Mol Biol 133, 110–119 (2013).
Gray, M. A. & Squires, E. J. Effects of nuclear receptor transactivation on steroid hormone synthesis and gene expression in porcine Leydig cells. J Steroid Biochem Mol Biol 133, 93–100 (2013).
Marguerat, S. & Bähler, J. RNA-seq: from technology to biology. Cell Mol Life Sci 67, 569–579 (2010).
Mortensen, A. & Sørensen, S. Relationship between boar taint and skatole determined with a new analysis method. Danish Meat Research Institute, Man (1984).
Mathur, P. et al. A human nose scoring system for boar taint and its relationship with androstenone and skatole. Meat Sci 91, 414–422 (2012).
Andrews, S. FastQC: a quality control tool for high throughput sequence data, http://www.bioinformatics.babraham.ac.uk/projects/fastqc (2010).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. journal 17, 10–12, https://doi.org/10.14806/ej.17.1.200 pp (2011).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics, btu170 (2014).
Andrew Yates et al. Ensembl 2016. Nucleic Acids Res D710–D716 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, (15–21 (2013).
Okonechnikov, K., Conesa, A. & García-Alcalde, F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics 32, 292–294 (2016).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics, btu638 (2014).
R: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria, 2014).
Durinck, S. et al. BioMart and Bioconductor: a powerful link between biological databases and microarray data analysis. Bioinformatics 21, 3439–3440 (2005).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat Protoc 4, 1184–1191 (2009).
Law, C. W., Chen, Y., Shi, W. & Smyth, G. K. Voom: precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol 15, 1 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res, gkv007 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol, 289–300 (1995).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504 (2003).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Supek, F., Bošnjak, M., Škunca, N. & Šmuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS One 6, e21800 (2011).
Langfelder, P. & Horvath, S. Fast R functions for robust correlations and hierarchical clustering. J Stat Softw 46 (2012).
Langfelder, P., Zhang, B. & Horvath, S. Defining clusters from a hierarchical cluster tree: the Dynamic Tree Cut package for R. Bioinformatics 24, 719–720 (2008).
Hu, Z. et al. VisANT: data-integrating visual framework for biological networks and modules. Nucleic Acids Res 33, W352–W357 (2005).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30, 207–210 (2002).
Acknowledgements
Anders B Strathe is acknowledged for skilled technical assistance with sample collection in the slaughterhouse. We thank the Danish Pig Research Centre (VSP-SEGES) for animals and breeding data used in this study and the staff of Danish Crown Herning for assistance during sample collection at the slaughterhouse. This “AGES” project was funded by the Green Development and Demonstration Programme (GUDP, grant number VETENH/ANHJ NR. 14029) and Veterinary Department of the Ministry of Food, Agriculture and Fisheries, Denmark and University of Copenhagen, Faculty of Health and Medical Sciences, Denmark.
Author information
Authors and Affiliations
Contributions
H.N.K. conceived the study design and supervised the research project, acronymed “AGES”. R.S.J., H.N.K., L.J.A.K. and D.N.D. completed the tissue sampling experiments at the Danish Crown slaughterhouse in Herning. R.S.J. processed tissue samples for sequencing. M.D. analysed sequence data by differential expression and co-expression network analysis, wrote the first draft of the manuscript and interpreted the results. R.S.J. participated in interpretation of co-expression network analysis. D.N.D. helped with graphical presentation and construction of the final conclusion. L.J.A.K. validated and interpreted co-expression networks and final conclusion. H.N.K. participated in writing, revising and interpretation of the results. All authors wrote, read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Drag, M., Skinkyté-Juskiené, R., Do, D.N. et al. Differential expression and co-expression gene networks reveal candidate biomarkers of boar taint in non-castrated pigs. Sci Rep 7, 12205 (2017). https://doi.org/10.1038/s41598-017-11928-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11928-0
This article is cited by
-
Novel role of COX6c in the regulation of oxidative phosphorylation and diseases
Cell Death Discovery (2022)
-
Screening of fungi from the phylum Basidiomycota for degradation of boar taint aroma compounds
European Food Research and Technology (2022)
-
Recent genetic advances on boar taint reduction as an alternative to castration: a review
Journal of Applied Genetics (2021)
-
Metabolomic networks and pathways associated with feed efficiency and related-traits in Duroc and Landrace pigs
Scientific Reports (2020)
-
Whole blood transcriptome analysis in ewes fed with hemp seed supplemented diet
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.