Abstract
The present study aimed to systematically evaluate the effectiveness and safety of the intrafascial and interfascial nerve sparing (ITR-NS and ITE-NS) radical prostatectomy. PubMed, Embase, and Cochrane Library databases were searched for eligible studies. Meta-analysis with random-effects model was performed. Six comparative trials were selected and embraced in this research, including one randomized controlled trial, three prospective comparative trials, and two retrospective comparative trials. With regard to perioperative parameters, no significant association of operative time, blood loss, transfusion rates, duration of catheterization, and hospital stay existed between ITR-NS and ITE-NS. With respect to the functional results, ITR-NS had advantages in terms of both continence and potency recovery compared with ITE-NS. In reference to the oncologic results, the ITR-NS showed lower overall positive surgical margin (PSM) compared with ITE-NS but pT2 PSM and biochemical recurrence free rates were similar to the two surgical types. This study demonstrates that ITR-NS has better continence at 6 mo and 36 mo and better potency recovery at 6 mo and 12 mo postoperatively, regardless of the surgical technique. The cancer control of ITR-NS was also better than that of ITE-NS. This may be explained by the fact that patients in ITE-NS group present higher risk cancer than patients in ITR-NS group.
Similar content being viewed by others
Introduction
Prostate cancer is the most common nonskin malignancy in western men and the second leading cause of cancer-related death among men in United States1, 2. Radical prostatectomy (RP) is the standard surgical treatment for localized prostate cancer. However, the postoperative impotence and especially the urinary incontinence following RP are still a matter of trouble for patients3,4,5.
Many approaches, such as open retropubic RP, laparoscopy RP, and robot-assisted RP, have been applied in RP. Recently, Reeves et al.6 performed a systematic review and meta-analysis that summarized the existing evidence on the influence of the preservation of the NVBs on continence after RP. Their study suggested that early urinary continence rate (at 6 mo time point) was improved for patients undergoing nerve-sparing RP compared with patients undergoing non-nerve-sparing RP. In recent years, certain urologists have compared intrafascial nerve-sparing (ITR-NS) with interfascial nerve-sparing (ITE-NS) RP, and the results are inconclusive7,8,9,10,11,12. The ITR-NS technique is considered a dissection that follows a plane on the prostate capsule and it allows a whole-thickness preservation of the NVBs13. Reeves and colleagues did not assess the specific nerve-sparing technique6.
To answer this important question, we carried out the present systematic review and meta-analysis to summarize the current existing evidence for clinical practice. In this study, we comprehensively evaluated the functional outcomes, oncologic outcomes, and perioperative parameters of ITR-NS and ITE-NS.
Methods
This systematic review and meta-analysis was performed according to the Preferred Reporting Items of Systematic Reviews and Meta-analysis (PRISMA) statement14. The study protocol of this systematic review and meta-analysis was published in the PROSPERO register (registration number: CRD42016038687).
Search strategy
A literature search of the PubMed, Embase, and Cochrane Library was conducted up to March 2016 (updated to July 2017) to identify potentially relevant trials. The following terms were searched: (“prostatic neoplasms” OR “prostate” OR “prostate cancer”) AND (“prostatectomy” OR “radical prostatectomy”) AND (“interfascial” OR “intrafascial”). The study language was restricted to English. In addition, reference lists in the recent reviews, meta-analysis, and included articles were checked for identifying any potentially relevant studies. The detailed search strategy for each database is presented in Supplementary Table 1.
Eligibility criteria
The inclusion criteria were established according to PICOS (patients, intervention, comparison, outcomes, and study design) principle as presented in Table 1. The exclusion criteria were as following: duplicated studies, single cohort studies (i.e. studies without comparison groups), case-control studies and cross-sectional studies were excluded.
Study selection and data extraction
Two authors independently screened titles and abstracts of all search results. Studies were selected based on the pre-specified inclusion and exclusion criteria. Any discrepancy was resolved by discussion. Two authors independently extracted the following data from each identified study: study details (name of first author, country, year, contact details, conflicts of interest), methods (study design, duration, clinical setting), patients (sample size, baseline characteristics), intervention (surgical approach, comparison group), and outcome (postoperative urinary continence rate, potency recovery rate, PSM, pT2 PSM, BCR free rates, operative time, blood loss, transfusion rates, duration of catheterization, and hospital stay).
Risk of bias and quality of evidence assessment
We used the Cochrane collaboration’s tool for risk of bias assessment of RCTs and the Newcastle-Ottawa Scale for risk of bias assessment of observational studies15,16,17. The Cochrane collaboration’s tool assesses risk of bias in six domains: (1) selection bias; (2) performance bias; (3) detection bias; (4) attribution bias; (5) reporting bias; and (6) other bias15. The Newcastle-Ottawa Scale assesses risk of bias in three domains: (1) selection of the study population; (2) comparability of groups; and (3) ascertainment of outcome16. We evaluated that follow-up was adequate if the maximum follow-up was more than 2 yr (i.e. 24 mo). The quality of evidence was assessed according to the GRADE system using GRADEpro GDT software.
Statistical analysis
Meta-analysis was performed to aggregate the results if studies were sufficiently similar. Due to the clinical heterogeneity implicated in the included studies, random-effects model was applied to estimate summary risk ratios (RRs) and corresponding 95% confidence intervals (CIs). Sensitivity analysis was conducted through sequentially excluding retrospective studies. Subgroup analysis according to timing of outcome measurement was performed if sufficient data was available. Heterogeneity was tested using chi-square (p ≤ 0.1) test and I2 metric. All statistical analysis was performed using RevMan 5.3 (Cochrane Collaboration, Oxford, UK). A two-sided p value less than 0.05 represented a statistically significant difference, except for heterogeneity test. Publication bias was detected using funnel plot if the included studies were more than five for each outcome.
Results
Literature search and study characteristics
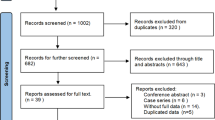
Figure 1 shows the PRISMA flowchart of the systematic review and meta-analysis. Our search initially yielded a total of 216 records. After exclusion of duplicate articles, 131 records were screened through titles and abstracts. Finally, 6 studies involving 1663 patients (ITR-NS: 916 patients, ITE-NS: 747 patients) were included in this systematic review and meta-analysis7,8,9,10,11,12.
The characteristics of included studies are presented in Table 2. These six comparative trials included one RCT7, three prospective comparative trials8, 9, 12, and two retrospective comparative trials10, 11. There were two studies7, 9 using laparoscopic RP, two studies8, 11 involving robot-assisted RP, and two studies10, 12 applying open retropubic RP. The definition of the ITR-NS and ITE-NS in the included studies is presented in Supplementary Table 2. Study sample sizes ranged from 418 and 42012. Studies were published from 2010 to 2015 in Europe7, 10,11,12 and Asia8, 9. All the studies used bilateral nerve-sparing radical prostatectomy technique.
Risk of bias assessment
Tables 3 and 4 showed the risk of bias assessment of included studies. The random sequence generation, allocation concealment, and blinding of the RCT were all unclear (Table 3). Therefore, the risk of bias of the RCT was unclear. The majority of the longitudinal controlled studies were considered to have low to moderate risk of bias (Table 4). Three studies7, 10, 11 had the proportion of high Gleason score (8–10 score). Patients in one study8 who underwent RP were relatively younger than those in other studies included in this meta-analysis. Furthermore, the surgical techniques used in the included studies were different. These factors would introduce some selection bias and clinical heterogeneity.
Primary outcomes
Urinary continence
Four studies7, 9, 10, 12 reported the postoperative urinary continence recovery rate at 3 mo; two studies7, 9 reported it at 6 mo; five studies7, 9,10,11,12 reported it at 12 mo; one study12 reported it at 36 mo (Table 5). Heterogeneity was detected in the 3 mo time point (p = 0.006, I2 = 76%). The results of meta-analysis with random-effects model showed that patients undergoing ITR-NS had significantly better continence outcomes reported on 6 mo (RR = 1.18, 95% CI 1.08–1.30, p = 0.0002) and 36 mo (RR = 1.13, 95% CI 1.02–1.25, p = 0.02) compared with those undergoing ITE-NS (Fig. 2a). No significant difference was found at 3 mo (RR = 1.08, 95% CI 0.91–1.28; p = 0.37) and 12 mo (RR = 1.03, 95% CI 0.99–1.08; p = 0.14) (Fig. 2a). Sensitivity analysis showed similar results to overall analysis (Fig. 2b). The quality of evidence was very low for continence recovery at different timing (Supplementary Table 3).
Erectile function
One study10 reported the postoperative potency recovery rate at 3 mo; two studies7, 9 reported it at 6 mo; four studies7,8,9,10 reported it at 12 mo (Table 6). Heterogeneity was detected in the 6 mo time point (p = 0.04, I2 = 59%). The results of meta-analysis with random-effects model showed that patients undergoing ITR-NS had significantly better potency outcomes reported on 6 mo (RR = 1.49, 95% CI 1.01–2.18, p = 0.04) and 12 mo (RR = 1.40, 95% CI 1.24–1.57, p < 0.00001) compared with those suffering from ITE-NS (Fig. 3a). No significant difference was found at 3 mo (RR = 1.35, 95% CI 0.98–1.85, p = 0.06) (Fig. 3a). Sensitivity analysis showed similar results to overall analysis (Fig. 3b). The quality of evidence was very low for potency at different timing (Supplementary Table 3).
PSM
Four studies7, 9,10,11 reported the PSM rate after the RP (Table 7). No evidence of heterogeneity was found between the studies (p = 0.42, I2 = 0%). The results of meta-analysis with random-effects model showed that patients undergoing ITR-NS had significantly lower PSM rate compared with those experiencing ITE-NS (RR = 0.64, 95% CI 0.48–0.86, p = 0.003; Fig. 4a). Sensitivity analysis showed that the significant difference was disappeared when retrospective studies were excluded (Fig. 4b). In ITR-NS compared with ITE-NS, RR was 0.87 (0.54–1.40, p = 0.56; Fig. 4b) for PSM rate. The quality of evidence was very low for PSM (Supplementary Table 3).
pT2 PSM
Three7, 10, 11 studies reported the pT2 PSM rate after the RP (Table 7). Low to moderate between-study heterogeneity was detected (p = 0.27, I2 = 23%). The results of meta-analysis with random-effects model showed that patients receiving ITR-NS had similar pT2 PSM rate compared with those undergoing ITE-NS (RR = 0.67, 95% CI 0.37–1.19, p = 0.17; Fig. 4a). Sensitivity analysis showed similar results to overall analysis (Fig. 4b). The quality of evidence was very low for pT2 PSM (Supplementary Table 3).
BCR free rates
One study7 reported the BCR free rates at 6 mo and four studies7, 9,10,11 reported them at 12 mo (Table 8). Moderate between-study heterogeneity was detected in the 12 mo point (p = 0.17, I2 = 40%). The results of meta-analysis with random-effects model showed that patients undergoing ITR-NS had similar BCR free rate compared with those experiencing ITE-NS (6 mo: RR = 0.98, 95% CI 0.94–1.02, p = 0.31; 12 mo: RR = 0.99, 95% CI 0.95–1.03, p = 0.53; Fig. 5a). Sensitivity analysis showed similar results to overall analysis (Fig. 5b). The quality of evidence was very low for BCR free rates at different timing (Supplementary Table 3).
Secondary outcomes (perioperative parameters)
The perioperative parameters are presented in Table 9. Two studies7, 9 reported the transfusion rate. The results of meta-analysis with random-effects model showed that patients suffering from ITR-NS had similar transfusion rates compared with those undergoing ITE-NS (RR = 0.50, 95% CI 0.05–5.47, p = 0.57; Fig. 6). The mean operation time ranged from 6010 to 169.41 min8. The mean blood loss ranged from 879 to 200 ml7. The mean duration of catheterization ranged from 57 to 11.09 d8. The mean hospital stay was 8 d9.
Publication bias
Publication bias was detected only for continence recovery. The result of funnel plot provided certain evidence that publication bias existed (Fig. 7).
Discussion
In this systematic review and meta-analysis of six studies, we compared the effectiveness and safety of ITR-NS and ITE-NS on prostate cancer treatment. Irrespective of the surgical technique, we found that ITR-NS had better functional results (urinary continence and erectile function) and oncologic outcome (PSM, pT2 PSM, and BCR) compared with ITE-NS. These findings were supported by sensitivity analyses which took the prospective studies into consideration alone. The results suggested that there was a difference in continence between techniques at 6 months and 36 months but not at 12 months. This might be caused by the various procedures of different techniques or it was a spurious result.
To our knowledge, this systematic review and meta-analysis is the first study to comprehensively evaluate this topic. The previous reviews or systematic reviews or meta-analysis evaluated the techniques of RP5, 18,19,20 (such as RARP, laparoscopic, and retropubic open), the PSM and perioperative complication rates of primary surgical treatments21, the primary surgical treatments for prostate cancer22,23,24,25,26, transperitoneal and extraperitoneal robot-assisted RP27, and the efficacy and safety of conventional laparoscopic RP with a transperitoneal approach versus that of an extraperitoneal approach28. Therefore, none of these studies focused on the surgical technique of RP. In 2015, Reeves et al.6 systematically reviewed the association of NVBs sparing in RP with postoperative urinary continence outcomes. They found that avoiding damage to the nerve activity surrounding the prostate promotes urinary control in the first 6 mo after nerve sparing RP. In addition, from theoretically, the ITR-NS had better function than ITE-NS in functional outcomes as we mentioned in introduction. The result of our systematic review and meta-analysis was supported by the Reeves’s study6.
Tewari et al.29 proposed a grading system based on four grades of dissection according to veins surrounding the prostate. Schatloff et al.30 proposed a grading system based on five grades of dissection according to arterial periprostatic vasculature. The grade 1 of Tewari’s approach and grade 5 of Schatloff’s approach was equal to ITR-NS. As we acknowledged that cancer control is the most important goal of RP. The different dissection planes concept aims for an incremental security margin of prostate, instead of true incremental nerves sparing13. In this review, we found that ITR-NS was not significantly presented with risk of PSM, pT2 PSM and BCR free rate compared with ITR-NS. This might be due to restricted patient selection of the included studies. Therefore, the choice of surgical technique or dissection plane should be made based on the specific situation of patients in clinical practice, such as clinical examination, biopsy results, and imaging results6.
Although we used a systematic method to perform the meta-analysis, certain limitations also should be taken into consideration. First, our systematic review only identified one RCT, and the absence of high quality RCT might weaken the reliability of the meta-analysis. Second, low to moderate between-study heterogeneity was detected, which might be attributed to different surgical techniques, study design, selection bias, and surgeon experience. Selection bias between the two techniques was a major bias in the present meta-analysis, which implied that higher risk patients tended to undergo interfascial technique and lower risk patients tended to an intrafascial technique. However, patients with Gleason score more than 8 were only in two trials and the PSA levels were all similar as presented in Table 2. In addition, the sensitivity analysis also showed similar results to the overall analysis. Therefore, the selection bias was not obvious in this systematic review. Two studies7, 9 used laparoscopic RP; two studies8, 11 used robot-assisted RP; and two studies10, 12 used open retropubic RP. These six comparative trials included one RCT7, three prospective comparative trials8, 9, 12, and two retrospective comparative trials10, 11. We performed sensitivity analysis through excluding retrospective studies. The summary result of PSM rate was changed while excluding the retrospective studies. Therefore, the robustness of the result is weak. Third, we only included studies published in English. In addition, grey literature was not included. Hence, language bias might occur in this study. Fourth, due to limited number of included studies, we did not fully detect the publication bias. Of course, the publication bias is inevitable because we included studies published in English and excluded grey literature. The publication bias might decrease the reliability and credibility of this meta-analysis and systematic review. Moreover, the sample size and statistical power were relatively insufficient to identify the true difference of the two surgical techniques. Ultimately, the meta-analysis is a secondary analysis and its quality is based on the included studies. Our meta-analysis included studies with a RCT with high risk of bias and five longitudinal studies with moderate risk of bias. Therefore, the quality of the evidence was consequentially degraded.
With respect to further researches, multi-center clinical trials, if possible, RCTs should be performed to evaluate the effectiveness and safety of ITR-NS and ITE-NS. In addition, further studies also should elucidate the functional anatomy of urinary continence and erectile function.
In conclusion, this systematic review and meta-analysis demonstrates that ITR-NS has better continence at 6 mo and 36 mo and better potency recovery at 6 mo and 12 mo postoperatively, regardless of the surgical technique. This finding might be due to more nerves were saved and less damage of the periprostatic tissue in ITR-NS compared with ITE-NS. The cancer control of ITR-NS was also better than that of ITE-NS. This may be explained by the fact that patients in ITE-NS group present higher risk cancer than patients in ITR-NS group. Further studies are needed to verify the conclusion in future.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J Clin 65, 5–29 (2015).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015).
Mandel, P., Graefen, M., Michl, U., Huland, H. & Tilki, D. The effect of age on functional outcomes after radical prostatectomy. Urol Oncol 33(203), e211–208 (2015).
Murphy, D. G. & Costello, A. J. How can the autonomic nervous system contribute to urinary continence following radical prostatectomy? A “boson-like” conundrum. Eur Urol 63, 445–447 (2013).
Ficarra, V. et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol 62, 405–417 (2012).
Reeves, F. et al. Preservation of the neurovascular bundles is associated with improved time to continence after radical prostatectomy but not long-term continence rates: results of a systematic review and meta-analysis. Eur Urol 68, 692–704 (2015).
Stolzenburg, J. U. et al. A comparison of outcomes for interfascial and intrafascial nerve-sparing radical prostatectomy. Urology 76, 743–748 (2010).
Ko, W. J., Hruby, G. W., Turk, A. T., Landman, J. & Badani, K. K. Pathological confirmation of nerve-sparing types performed during robot-assisted radical prostatectomy (RARP). BJU Int 111, 451–458 (2013).
Zheng, T. et al. A matched-pair comparison between bilateral intrafascial and interfascial nerve-sparing techniques in extraperitoneal laparoscopic radical prostatectomy. Asian J Androl 15, 513–517 (2013).
Khoder, W. Y., Waidelich, R., Buchner, A., Becker, A. J. & Stief, C. G. Prospective comparison of one year follow-up outcomes for the open complete intrafascial retropubic versus interfascial nerve-sparing radical prostatectomy. Springerplus 3, 335 (2014).
Ihsan-Tasci, A. et al. Oncologic results, functional outcomes, and complication rates of transperitoneal robotic assisted radical prostatectomy: single centre’s experience. Actas Urol Esp 39, 70–77 (2015).
Khoder, W. Y. et al. Do we need the nerve sparing radical prostatectomy techniques (intrafascial vs. interfascial) in men with erectile dysfunction? Results of a single-centre study. World J Urol 33, 301–307 (2015).
Walz, J. et al. A Critical Analysis of the Current Knowledge of Surgical Anatomy of the Prostate Related to Optimisation of Cancer Control and Preservation of Continence and Erection in Candidates for Radical Prostatectomy: An Update. Eur Urol (2016).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339, b2535 (2009).
Higgins, J. P. T. & Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, Available from www.cochrane-handbook.org (2011).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605 (2010).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8, 2–10 (2015).
Ficarra, V. et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 62, 418–430 (2012).
Novara, G. et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur Urol 62, 382–404 (2012).
Novara, G. et al. Systematic review and meta-analysis of perioperative outcomes and complications after robot-assisted radical prostatectomy. Eur Urol 62, 431–452 (2012).
Tewari, A. et al. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: a systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur Urol 62, 1–15 (2012).
Coelho, R. F. et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a critical review of outcomes reported by high-volume centers. J Endourol 24, 2003–2015 (2010).
Moran, P. S. et al. Robot-assisted radical prostatectomy compared with open and laparoscopic approaches: a systematic review and meta-analysis. Int J Urol 20, 312–321 (2013).
Robertson, C. et al. Relative effectiveness of robot-assisted and standard laparoscopic prostatectomy as alternatives to open radical prostatectomy for treatment of localised prostate cancer: a systematic review and mixed treatment comparison meta-analysis. BJU Int 112, 798–812 (2013).
Pan, X. W. et al. Robot-Assisted Radical Prostatectomy vs. Open Retropubic Radical Prostatectomy for Prostate Cancer: A Systematic Review and Meta-analysis. Indian J Surg 77, 1326–1333 (2015).
Allan, C. & Ilic, D. Laparoscopic versus Robotic-Assisted Radical Prostatectomy for the Treatment of Localised Prostate Cancer: A Systematic Review. Urol Int 96, 373–378 (2016).
Lee, J. Y., Diaz, R. R., Cho, K. S. & Choi, Y. D. Meta-analysis of transperitoneal versus extraperitoneal robot-assisted radical prostatectomy for prostate cancer. J Laparoendosc Adv Surg Tech A 23, 919–925 (2013).
De Hong, C. et al. Comparison of efficacy and safety of conventional laparoscopic radical prostatectomy by the transperitoneal versus extraperitoneal procedure. Sci Rep 5, 14442 (2015).
Tewari, A. K. et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int 108, 984–992 (2011).
Schatloff, O. et al. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol 61, 796–802 (2012).
Acknowledgements
This work was supported by The National Key Research and Development Program of China (Grant No. 2016YFC0106300).
Author information
Authors and Affiliations
Contributions
H.W., X.T.Z., and X.H.W. designed this study; H.W. and S.L. searched databases and collected full-text papers; X.Y.M., M.J.S., and D.L.H. extracted and analyzed data; H.W. and X.T.Z. wrote the manuscript, X.H.W. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Weng, H., Zeng, XT., Li, S. et al. Intrafascial versus interfascial nerve sparing in radical prostatectomy for localized prostate cancer: a systematic review and meta-analysis. Sci Rep 7, 11454 (2017). https://doi.org/10.1038/s41598-017-11878-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11878-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.