Abstract
1α,20S-Dihydroxyvitamin D3 [1,20S(OH)2D3], a natural and bioactive vitamin D3 metabolite, was chemically synthesized for the first time. X-ray crystallography analysis of intermediate 15 confirmed its 1α-OH configuration. 1,20S(OH)2D3 interacts with the vitamin D receptor (VDR), with similar potency to its native ligand, 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] as illustrated by its ability to stimulate translocation of the VDR to the nucleus, stimulate VDRE-reporter activity, regulate VDR downstream genes (VDR, CYP24A1, TRPV6 and CYP27B1), and inhibit the production of inflammatory markers (IFNγ and IL1β). However, their co-crystal structures revealed differential molecular interactions of the 20S-OH moiety and the 25-OH moiety to the VDR, which may explain some differences in their biological activities. Furthermore, this study provides a synthetic route for the synthesis of 1,20S(OH)2D3 using the intermediate 1α,3β-diacetoxypregn-5-en-20-one (3), and provides a molecular and biological basis for the development of 1,20S(OH)2D3 and its analogs as potential therapeutic agents.
Similar content being viewed by others
Introduction
The classical pathway of vitamin D3 (D3) activation involves two key steps: 25-hydroxylation to produce 25-hydroxyvitamin D3 [25(OH)D3], and 1α-hydroxylation by cytochrome CYP27B1 to produce the active 1α,25-dihydroxyvitamin D3 [1,25(OH)2D3] (Fig. 1)1. This natural ligand of the vitamin D receptor (VDR) regulates expressions of various genes including that encoding catabolic CYP24A1 through the VDR. Other activities mediated via the VDR include anti-inflammation, anti-proliferation, pro-differentiation, pro-apoptosis, immunomodulation, mineral homeostasis and anti-angiogenesis2,3,4. In addition, D3 can also be activated by a novel metabolic pathway initiated by CYP11A1 (P450scc) producing 20S-hydroxyvitamin D3 [20S(OH)D3] as the major product5. As an activation enzyme, CYP27B1 is able to hydroxylate 20S(OH)D3 producing the natural metabolite 1α,20S-dihydroxyvitamin D3 [1,20S(OH)2D3] and has been used to produce µg amounts of this product in vitro 6, 7. Alternative biosynthesis using CYP11A1 to 20S-hydroxylate commercially available 1α-hydroxyvitamin D3 [1(OH)D3] increased the production of 1,20S(OH)2D3 to 0.5–1 mg7.
1,20S(OH)2D3 has been found to upregulate the expression of CYP24A1 mRNA, suggesting that it can modulate the expression of genes downstream of the VDR7. It also inhibits cell growth and shows potent anti-leukemic and anti-melanoma activities, while displaying less calcemic (toxic) effect than 1,25(OH)2D3 7,8,9,10. In addition, 1,20S(OH)2D3 was found in human epidermis suggesting an endogenous role in the skin11. However, the lack of detailed information on the interactions between 1,20S(OH)2D3 and VDR makes it difficult to understand its mechanism of action, and some of the differential effects of 1,20S(OH)2D3 and 1,25(OH)2D3. Here we present the high resolution X-ray crystal structure of 1,20S(OH)2D3 in complex with the VDR, as well as further characterization of its biological activities. Importantly, while 1,20S(OH)2D3 has great potential as a therapeutic agent, the production of 1,20S(OH)2D3 6, 7 and its analogs12, 13 has been limited to date by the need for purified enzymes, CYP27B1 or CYP11A1, for their biosynthesis. Now we report the first chemical synthesis of 1,20S(OH)2D3 facilitating its production for further testing of its biological activities.
Results and Discussion
Retrosynthesis of 1,20S(OH)2D3
A retrosynthetic strategy including a common 1α-OH intermediate 3 was proposed (Fig. 2). The D3-like structure could be obtained from UVB transformation of 2 12,13,14, of which the 20S-OH and side chain could be achieved by Grignard reaction of 3 12,13,14,15. Introduction of 1α-OH to 4 could be carried out by a multi-step conversion following the synthesis of androstenolone16.
Synthesis of 1,20S(OH)2D3
The synthesis (Fig. 3) started with deacetylation and TBS protection of pregnenolone acetate (4) to give intermediate 6. NaBH4 treatment of 6 selectively afforded the 20 R epimer as a major product which was then protected with an acetyl group to go through the DDQ oxidation safely (75% yield) to produce intermediate 10. After replacing 20-OAc with 20-OTBS, a 1α,2α-epoxide group was introduced by adding KOH and H2O2 solution to afford intermediate 13 (73%), followed by Birch reduction to give 1α,3β-diol 14 (61%) as a major product16,17,18. To confirm the 1α-OH formation, 14 was protected with an acetyl group to produce 15, which was characterized by 1D and 2D NMR spectrometry, and crystalized from hexane for X-ray structure analysis (see Supplementary Fig. S2). After removal of 20-OTBS, intermediate 16 was oxidized by DMP to 1α,3β-diacetoxypregn-5-en-20-one (3, 95%), which was then transformed into the 5,7-diene 7DHC intermediate (17, 52%) following a well-established procedure12,13,14. To avoid potential separation problems caused by acetyl protection after Grignard reaction, ester hydrolysis was carried out prior to Grignard reaction (87%) to afford 1α,20S-7DHC (2) where the formation of 20S confirmation was discussed in previous reports12,13,14. UVB irradiation of 2 in ethyl ether followed by pre-vitamin D3 isomerization afforded the desired product 1 (13%), which was compared with its enzymatic counterpart after HPLC separation.
Synthesis of 1α,20S-dihydroxyvitamin D3. Reagents and conditions: (a) K2CO3, MeOH, r.t., overnight. (b) TBSCl, imidazole, DMF, r.t., overnight. (c) NaBH4, DCM: MeOH (1:1), 0 °C - r.t., overnight. (d) Ac2O, Et3N, DMAP, DCM, r.t., overnight. (e) TBAF, THF, r.t., 12 h. (f) DDQ, 1,4-dioxane, reflux, 4 h. (g) KOH, MeOH, r.t., 3 h. (h) TBSCl, imidazole, DMF, r.t., overnight. (i) KOH in MeOH, 30% H2O2, MeOH, −40 °C – 0 °C, 12 h. (j) Li, NH3 (liquid), −80 °C, 30 min; addition of starting material in THF, −80 °C, 2 h; −40 °C, 1 h; NH4Cl, −80 °C, 2 h. (k) Ac2O, Et3N, DMAP, DCM, r.t., overnight. (l) TBAF, THF, r.t., 48 h. (m) DMP, DCM, r.t., 12 h. (n) Dibromantin, AIBN, benzene: hexane (1:1), reflux 20 min; TBAB, THF, r.t., 75 min; TBAF, r.t., 50 min. (o) K2CO3, MeOH, r.t., overnight. (p) i) Mg, I2, 1-bromo-4-methylpentane, THF, reflux, 1 h; ii) 18, THF, 0 °C – r.t., overnight. (q) UVB irradiation, Et2O, 50 °C, 15 min; r.t., 10 d; HPLC, MeCN:H2O. AIBN, azobisisobutyronitrile; DDQ, 2,3-dichloro-5,6-dicyanobenzoquinone; DMP, Dess–Martin periodinane; DMAP, 4-dimethylaminopyridine; HPLC, high-performance liquid chromatography; TBAB, tetra-n-butylammonium bromide; TBAF, tetra-n-butylammonium fluoride; TBSCl, tert-butyldimethylsilyl chloride.
Pregnenolone acetate (4) has often been used as the starting material for 20S(OH)D3 analogs12,13,14, in which 1α-hydroxylation was necessary to display potent stimulation of the VDR12, 13. Owing to the lack of appropriate 1α-OH intermediates, the production of 1α-OH derivatives of 20S(OH)D3 analogs was dependent on the purification of recombinant CYP27B1. The limited amount of 1α-OH derivatives that could be made was thus a hurdle for extensive biological testing. The production of 1α,3β-diacetoxypregn-5-en-20-one (3) in this report enables production of various analogs of 1,20S(OH)2D3 for future studies.
We experienced inconsistent yields during the Birch reduction of epoxide 13 in the initial trials. In fact, the addition of NH4Cl (a quenching step) is the key to the success of this reaction. Quick addition (<10 min) of NH4Cl gave predominantly intermediate 14a, whereas slow addition (>2 h) afforded mainly the desired product 14. To our knowledge, 14a as a semi-reduced intermediate was obtained and characterized for the first time.
HPLC showed matched retention times of chemical and enzymatic 1,20S(OH)2D3
In addition to the UV spectra and NMR identification (Supplementary Information), the chemically synthesized 1,20S(OH)2D3 was analysed by HPLC under two different solvent systems, either an acetonitrile in water gradient or a methanol in water gradient. We conclude that the chemically synthesized 1,20S(OH)2D3 and the enzymatically produced counterpart are identical on the basis of their UV and NMR spectra, as well as their HPLC retention times (Fig. 4). Co-migration of the chemically and enzymatically synthesized 1,20S(OH)2D3 was further confirmed by chromatography of a mixture of the two (see Supplementary Information).
Identification of 15 as having a 1α-OH by NMR analysis
To identify the formation of the 1α-hydroxyl, the structure of intermediate 15 was characterized from its NMR spectra (Supplementary Information). The NOESY spectrum of 15 gave a strong NOE integral (0.42, see Supplementary Fig. S1) of 1Hβ to 19-CH3, using the NOE integral of 1Hβ to 2Hβ as an internal reference. In contrast, the NOE signal of 1Hβ to 2Hα was not observed, suggesting the presence of 1α-OAc group in 15.
Confirmation of 15 by X-ray crystallographic analysis
To confirm the structure of 15, crystals were produced in hexane for X-ray crystallographic analysis (Supplementary Information). The X-ray structure of 15 (CCDC code: 1527430, Fig. 3) confirmed its absolute structure as the desired product reported in the Fig. 3.
Transcriptional activity
The ability of 1,20S(OH)2D3 to activate the VDR was analysed in three cell lines (HaCaT, Caco-2 and Jurkat) transduced with a lentiviral vitamin D response element (VDRE) reporter (luciferase)12,13,14,15. Compared with 1,25(OH)2D3 and 22-oxa-1,25(OH)2D3 (22-Oxa), two known VDR agonists, 1,20S(OH)2D3 showed potent transcriptional activity with EC50s of 450 nM in HaCaT cells, 285 nM in Caco-2 cells and 19.1 nM in Jurkat cells (Table 1). Although less potent than 22-Oxa in all three cell lines, 1,20S(OH)2D3 is equally potent to (HaCaT and Caco-2 cells) or less potent than (Jurkat cells) 1,25(OH)2D3, the native ligand of the VDR.
X-ray crystallographic analysis of the zVDR ligand binding domain in complex with 1,20S(OH)2D3
To characterize molecular interactions in order to understand the mechanisms underlying the differential VDRE stimulatory effects, the Danio rerio VDR (zVDR) ligand binding domain (LBD) was crystallized in the presence of 1,20S(OH)2D3 or 1,25(OH)2D3. The overall structure of VDR-1,20S(OH)2D3 (PDB code: 5MX7) is highly homologous to the VDR-1,25(OH)2D3 structure, adopting the canonical active conformation. When compared to the zVDR LBD-1,25(OH)2D3 structure19, 20, the Cα atoms of the zVDR LBD–1,20S(OH)2D3 complex have a root mean square deviation of 0.25 Å over 238 residues. The ligand binds similarly to 1,25(OH)2D3 with the notable difference being that the 20S-OH forms a weak H-bond with His305 (3.42 Å) and does not interact with His397 (note that the residues numbers correspond to hVDR). The H-bond with His305 causes a ligand-induced conformational change in the receptor where His305 (loop6-7) is shifted by 0.63 Å to enable this H-bonding interaction. (Fig. 5). The 1α-OH and 3β-OH form similar hydrogen bonds to the zVDR to those seen with 1,25(OH)2D3.
X-ray crystal structures of 1,20S(OH)2D3 and 1,25(OH)2D3 in complex with zVDR LBD. (A) Binding pose and interactions of 1,20S(OH)2D3 in in complex with zVDR LBD. (B) Binding pose and interactions of 1,25(OH)2D3 in in complex with zVDR LBD. (C) Details of the interactions mediated by the side chain of 1,20S(OH)2D3 with residues of the zVDR LBD at a 4.0 Å distance cutoff. The residues numbers correspond to hVDR. (D) Overlay of 1,25(OH)2D3 (carbon atoms in orange and oxygen atoms in red) with 1,20S(OH)2D3 (grey) within zVDR LBD complexes with the indication of the hydrogen bonds formed by the ligands. The Hydrogen bonds are shown by red or yellow dashed lines and Van Der Waals interactions are shown by grey dashed lines. Hydrogen bonds are shown by dashed lines, and hydrogen-bonding residues are labelled.
An additional difference in the structures is that the 20S-OH forms a Van der Waals interaction with Val300. While most of the Van der Waals interactions are maintained, the side chain and terminal methyl groups that are differently positioned to interact differently with some of the residues (Fig. 5). Weaker interactions are formed with Leu227 (4.1 Å instead of 3.8 Å with C26) and Tyr399 (4.1 Å instead of 3.8 Å with C27), interactions compensated by stronger interactions with Val234 (3.9 Å instead of 4.2 Å with C22), and Leu412 (3.9 Å instead of 4.2 Å with C27). Overall, the hydrogen bonding interaction of 20S-OH with His305 and hydrophobic contacts formed by the ligand explains its agonist activity, however, with less potency than that of 1,25(OH)2D3.
VDR translocation activity
1,25(OH)2D3 binds to cytosolic or membrane-assocoated VDR3, then translocation of 1,25(OH)2D3-bound VDR from the cytoplasm to the nucleus is a key step to exert its gene-regulatory effects1, 3. In SKMEL-188 melanoma cells transduced with pLenti-CMVVDR-EGFP-pgk-puro21, both 1,20S(OH)2D3 and 1,25(OH)2D3 showed stimulatory effects on this translocation with EC50 values of 2.14 × 10−9 and 7.87 × 10−9 M (Fig. 6A), respectively. The results indicate that 1,20S(OH)2D3 induces VDR translocation in a similar fashion to 1,25(OH)2D3.
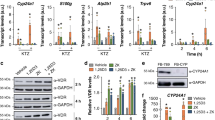
VDR translocation and gene regulation activities of 1,20S(OH)2D3. (A) The effect on vitamin D receptor (VDR) translocation from the cytoplasm to the nucleus. Data are mean ± SEM (n ≥ 3). The dose-dependent stimulation of VDR translocation was analysed by one-way ANOVA with # p < 0.05 and ## p < 0.01. The differences between control and treatment were analysed with Student’s t-test, where *p < 0.05 and **p < 0.01. (B) 1,20S(OH)2D3 regulates mRNA expression of genes VDR, CYP24A1, TRPV6 and CYP27B1 in HaCaT cells at 100 nM after 24 h treatment (n = 3). *p < 0.05, **p < 0.01 and ***p < 0.001.
Regulatory activity of 1,20S(OH)2D3 on VDR downstream genes
To investigate how 1,20S(OH)2D3 affects VDR target genes through VDR activation, expression of VDR, CYP24A1, TRPV6 and CYP27B1 genes at the mRNA level was determined in HaCaT cells (Fig. 6B). 1,20S(OH)2D3 was capable of mildly upregulating the expression (1.6-fold) of the gene encoding its own receptor, the VDR, while being moderately stronger than 1,25(OH)2D3 (1.3-fold) and comparable to 22-Oxa (1.7-fold). 1,25(OH)2D3 is known to induce expression of the vitamin D catabolic enzyme, CYP24A114, 22. Similarly, 1,20S(OH)2D3 strongly stimulates CYP24A1 mRNA levels 34-fold, as compared to 10-fold for 1,25(OH)2D3 and 78-fold for 22-Oxa. In addition, TRPV6 encoding an intestinal calcium channel is also a well-known target of VDR for mineral homeostasis14, 23. The mRNA levels of TRPV6 were increased by 1.4-, 1.4- and 2.6-fold for 1,20S(OH)2D3, 1,25(OH)2D3 and 22-Oxa, respectively. Moreover, VDR activation induced by its agonists inhibits the expression of the vitamin D activation enzyme, CYP27B124, 25. Although less than 1,25(OH)2D3 and 22-Oxa, 1,20S(OH)2D3 slightly but significantly inhibited the expression of CYP27B1. These results indicate that 1,20S(OH)2D3 is able to activate the VDR, and exert its effects through regulating VDR target genes in a similar manner to 1,25(OH)2D3. Since 1,20S(OH)2D3 affected the expression of VDR, TRPV6 and CYP27B1 weakly (~2-fold), further investigation on their protein levels will be beneficial to confirm the actions of 1,20S(OH)2D3.
Anti-inflammatory activity
The anti-inflammatory effect of 1,20S(OH)2D3 was determined in mouse splenocytes stimulated by lipopolysaccharide prior to treatments with the secosteroids. The concentrations of IFNγ and IL1β in the culture media were significantly reduced by 1,20S(OH)2D3, compared with the control (Table 1). The effect of 1,20S(OH)2D3 (1.0 nM) was comparable with or slightly weaker than that of 1,25(OH)2D3 but weaker than 22-Oxa for reduction of IFNγ production. In contrast, 1,20S(OH)2D3 (100 nM) showed equal efficacy to 22-Oxa, and higher than that of 1,25(OH)2D3 for reduction of IL1β production. These studies suggested that 1,20S(OH)2D3, acting similarly to 1,25(OH)2D3 and 22-Oxa, is a potent anti-inflammatory agent.
Conclusions
Similar to 1,25(OH)2D3, 1,20S(OH)2D3 can interact with the VDR with high potency, as evidenced by its ability to stimulate its translocation to the nucleus, regulate VDR downstream genes (including but not limited to VDR, CYP24A1, TRPV6 and CYP27B1), and exert strong anti-inflammatory activity. The crystal structure of 1,20S(OH)2D3 bound to the VDR reveals differences from the 1,25(OH)2D3-bound form with respect to their interactions, including the important role of the H-bond between the 20S-OH and His305 that shifts the position of this residue compared to the 1,25(OH)2D3-bound form. This difference may contribute to their differential activities of these secosteroids such as the lower calcemic activity of 1,20S(OH)2D3 compared to 1,25(OH)2D3 8. This study provides a molecular basis for the rational design and practical synthesis of novel 1,20S(OH)2D3 analogs that interact with VDR for future drug development. 1,20S(OH)2D3 was successfully chemically synthesized for the first time, providing ample material for further characterization of its biological activities, including animal studies in the future. The 1α,3β-diacetoxypregn-5-en-20-one (3) intermediate can serve as a common precursor for production of other 1,20S(OH)2D3 analogs which will facilitate the synthesis of similar secosteroids containing a 1α-OH group.
Methods
General procedures
Reagents and solvents for the synthesis were anhydrous (purchased or self-dried) to ensure good product yield. Solvents used for separations were ACS chemical grade, purchased from commercial sources and used upon arrival. NH4Cl was sublimed in our lab for Birch reduction. Reactions for light sensitive compounds (7DHC or D3 structures) were protected from light by wrapping flasks with aluminum foil, and were monitored under UV lights. Moisture-sensitive reactions were carried out under argon gas in flame-dried flasks. Reactions for non-UV active compounds were visualized on TLC by 5% phosphomolybdic acid in ethanol. All NMR data were collected on a Bruker Avance III 400 MHz NMR or a Varian Inova 500 MHz NMR. Samples were dissolved in 0.5 mL CDCl3, methanol-d 4, DMSO-d 6 or actone-d 6, and NMR data were collected at r.t. Mass spectra of compounds were acquired using a Bruker LC-IT-MS system with an ESI source. High-resolution MS spectra and extracted ion chromatogram (EIC) were obtained using a Waters ACQUITY UPLC I-Class System equipped with a Xevo G2-S QTof mass spectrometer based on our previously reported conditions26, 27. Reaction mixtures were extracted with ethyl acetate, DCM or hexanes, washed with aqueous Na2CO3, brine, and water, and then dried over anhydrous Na2SO4. The solution was transferred to a round-bottom flask and dried by rotary evaporator. The purities of final products were determined by HPLC as >98% (Fig. 4).
Crystallization of intermediate 15
To a clean test tube (13 × 100 mm), 18 mg of compound 15 powder and 3 mL anhydrous n-hexane were added. The tube was shaken until the solid was completely dissolved, then sealed with 5 layers of sealing film (Para film) membrane. The resulting solution was allowed to stand in a quiet environment for 10 days, by which time the hexane had evaporated, leaving crystals of 15 which were collected for crystallographic analysis (Supplementary Information).
Crystallization and structural analysis of 1,20S(OH)2D3–VDR complex
cDNA encoding zVDR LBD (156–453 AA) was subcloned into pET28b vector to generate an N-terminal His-tag fusion protein. Purification was carried out as previously described, including metal affinity chromatography and gel filtration28. The protein was concentrated using Amicon ultra-30 (Millipore) to 3–7 mg/mL and incubated with a two-fold excess of ligand and a three-fold excess of the coactivator SRC-1 peptide (686-RHKILHRLLQEGSPS-698). Crystals were obtained in 50 mM Bis–Tris pH 6.5, 1.6 M lithium sulfate and 50 mM magnesium sulfate. Protein crystals were mounted in a fiber loop and flash-cooled under a nitrogen flux after cryo-protection with 20% glycerol. Data collection from a single frozen crystal was performed at 100 K on the ID23-1 beamline at ESRF (France). The raw data were processed and scaled with the HKL2000 program suite29. The crystals belong to the space group P6522, with one LBD complex per asymmetric unit. The structure was solved and refined using BUSTER30, Phenix31 and iterative model building using COOT32. Crystallographic refinement statistics are presented in Supplementary Table S9. All structural figures were prepared using PyMOL (www.pymol.org/).
Biosynthesis of 1,20S(OH)2D3
Enzymatic synthesis of 1,20S(OH)2D3 involved the 20S-hydroxylation of 1α(OH)D3 by recombinant bovine CYP11A1 and was carried out as described in detail before7. HPLC comparison was determined by using an Agilent HPLC 1100 system and a Phenomenex Luna-PFP C18 column (5 µm, 250 mm × 4.6 mm, Torrance, CA) at 25 °C and a flow rate of 1.0 mL/min. MeCN: H2O and MeOH: H2O were used as mobile phases with a gradient comprising 50–100% organic solvent for 30 min. 263 nm was used to display chromatograms.
VDRE-reporter assay
HaCat, Caco-2 and Jurkat cells were transduced by lentiviral VDRE-reporter (luciferase) vector12,13,14,15. Caco-2 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin/amphotericin antibiotic solution (Ab) (Sigma-Aldrich, St. Louis, MO). HaCaT cells were grown in DMEM supplemented with 5% FBS and 1% Ab. Jurkat cells were grown in RPMI 1640 medium containing 10% FBS and 1% Ab. All cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2. All cell lines were selected for at least one week by medium containing additional 1.0 µg/mL puromycin before treatment with secosteroid. Each cell line was then plated in a 96-well plate (10,000 cells/100 µL medium/well) using FBS-free media and incubated for 24 h. 1,20S(OH)2D3, 1,25(OH)2D3 and 22-Oxa at a series of concentrations in 10% DMSO were added separately to 96-well plates (1.0 µL/well), while 10% DMSO was used as control. After 24 h incubation, 100 µL of ONE-GloTM Luciferase Assay System (Promega, Madison, WI) was added to each well. After 5 min at r.t., the signal was recorded by a BioTek Synergy HT microplate reader (BioTek Instruments, Inc., Winooski, VT, US). All concentrations of secosteroids were tested in triplicate.
VDR translocation assay
The effects of 1,20 S(OH)2D3 on VDR translocation from the cytoplasm to the nucleus were tested on the previously described SKMEL-188 cell model21, 33, using cells stably transduced with pLenti-CMV-VDREGFP-pgk-puro (VDR and EGFP expressed as fusion protein)34. Cells were treated with secosteroids (up to 100 nM) for 90 min followed by analysis with Cytation 5 (BioTek, Winooski, VT, US). Translocation to the nucleus was determined by counting cells with a fluorescent nucleus and the results are presented as the percentage of the total cells that displayed nuclear staining, as described previously21. The data were obtained from at least two separate experiments, with images taken in the central area from at least three different wells and counted as described21, 34.
Real-time PCR assay
HaCaT cells were seeded in 60 mm dishes (1 million/dish) in 10 mL DMEM supplemented with 5% FBS and 1% Ab. After overnight incubation they were cultured in FBS-free medium for another 12 h to synchronize the cells. The media were then removed and secosteroids in DMEM (5% FBS and 1% Ab) with a concentration of 100 nM were added to the dishes. After 24 h incubation, media were removed, and 10 mL PBS was used to wash the dish. Cells were then detached by trypsin, centrifuged in Eppendorf tubes, washed with PBS (5 mL), and stored at −80 °C. Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA, USA) was used to isolate the RNA, and Transcriptor First Strand cDNA Synthesis Kit (Roche Inc., Mannheim, Germany) was used for reverse transcription (100 ng RNA/reaction). Real-time PCR was carried out using cDNA which was diluted 10-fold in sterile water and a SYBR Green PCR Master Mix. The forward reverse primers for VDR, CYP24A1, TRPV6 and CYP27B1 genes were designed based on the rat and mouse sequences using Primer Quest software (Integrated Device Technology, San Jose, CA, USA). Reactions (n = 3) were performed at 50 °C for 2 min, 95 °C for 10 min and then 40 cycles of 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s. Data were collected and analyzed on a Roche Light Cycler 480. Using a comparative Ct method25, the amount of the final amplified product was normalized to the amount of β-actin as a housekeeping gene.
IFNγ production assay
All animal experiments in this study were performed in accordance with the NIH animal use guidelines and protocol (protocol No.: 15–043.0) approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee Health Science Center (UTHSC, Memphis, TN). Splenocytes were isolated from 7-week old C57BL/6 female mice and cultured at 2 × 106/mL 500 μL/well for 72 h at 37 °C in a humidified atmosphere. Harvested supernatants were analyze for levels of murine IFNγ by ELISA (R & D Systems Minneapolis, MN) according to the manufacturer’s instructions. Results are expressed as mean [IFNγ] ± SEM of triplicate determinations (pg/mL) of culture supernatant. The difference in [IFNγ] between EtOH vehicle + anti-CD3e MOAB (0.08 μg/mL) and [IFNγ] in each of the cultures containing D3 analog was analyzed by one way ANOVA Sigma. The amount of EtOH in the EtOH + PBS and EtOH + anti-CD3e MOAB culture was equal to that in the cultures of the D3 analogs at a level of 10−9 M. Culture medium was RPMI 1640 supplemented with 9% charcoal stripped fetal bovine serum, non-essential amino acids, HEPES buffer Glutamax, penicillin 100 μg/mL, streptomycin 100 μg/mL, fungizone 1 μg/mL (GIBCO, Grand Island, NY) and 50 μM β-mercaptoethanol (Sigma, St. Louis, MO)35.
IL1β production assay
Splenocytes were isolated from 7-week old C57BL/6 female mice and cultured at 2 × 106/mL, 500 μL/well, for 24 h at 37 °C in a humidified atmosphere. The vitamin D analogs or EtOH vehicle were added to the splenocyte cultures 2 h prior to addition of Lipopolysaccharide W E. coli 055:B5 (LPS) (Difco Lab. Defrost MI) 100 ng/mL or PBS vehicle. Harvested supernatants were analyzed for levels of murine IL1β by ELISA (R & D Systems Minneapolis, MN) according to the manufacturer’s instructions. Results are expressed as mean IL1β concentration ± SEM of triplicate determinations (pg/mL) of culture supernatant. The amount of EtOH in the EtOH + PBS and EtOH + LPS culture was equal to that in the cultures of the vitamin D analogs at a level of 10-7 M. Culture medium was RPMI 1640 supplemented with 9% charcoal stripped fetal bovine serum, non-essential amino acids, HEPES buffer Glutamax, penicillin 100 μg/mL, streptomycin 100 μg/mL, fungizone 1 μg/mL (GIBCO, Grand Island, NY) and 50 μM β-mercaptoethanol (Sigma, St. Louis, MO). The difference in [IL1β] between control and D3 analog treatment was analyzed by one way ANOVA (Sigma Plot 13.0).
References
Lin, Z. & Li, W. The roles of vitamin D and its analogs in inflammatory diseases. Curr. Top. Med. Chem. 16, 1242–1261 (2016).
Wierzbicka, J., Piotrowska, A. & Zmijewski, M. A. The renaissance of vitamin D. Acta Biochim. Pol. 61, 679–686 (2014).
Haussler, M. R. et al. Vitamin D receptor: molecular signaling and actions of nutritional ligands in disease prevention. Nutr. Rev. 66, S98–112, doi:10.1111/j.1753-4887.2008.00093.x (2008).
Christakos, S. et al. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 96, 365–408, doi:10.1152/physrev.00014.2015 (2016).
Slominski, A. T. et al. In vivo evidence for a novel pathway of vitamin D(3) metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 26, 3901–3915, doi:10.1096/fj.12-208975 (2012).
Li, W. et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids 75, 926–935, doi:10.1016/j.steroids.2010.05.021 (2010).
Tuckey, R. C. et al. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J. Steroid Biochem. Mol. Biol. 112, 213–219, doi:10.1016/j.jsbmb.2008.10.005 (2008).
Slominski, A. T. et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. PLoS One 5, e9907, doi:10.1371/journal.pone.0009907 (2010).
Slominski, A. T. et al. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J. Steroid Biochem. Mol. Biol. 144(Pt A), 28–39, doi:10.1016/j.jsbmb.2013.10.012 (2014).
Slominski, A. T. et al. Novel vitamin D hydroxyderivatives inhibit melanoma growth and show differential effects on normal melanocytes. Anticancer Res. 32, 3733–3742 (2012).
Slominski, A. T. et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 5, 14875, doi:10.1038/srep14875 (2015).
Lin, Z. et al. Chemical synthesis and biological activities of 20S,24S/R-dihydroxyvitamin D3 epimers and their 1alpha-hydroxyl derivatives. J. Med. Chem. 58, 7881–7887, doi:10.1021/acs.jmedchem.5b00881 (2015).
Lin, Z. et al. Synthesis and Biological Evaluation of Vitamin D3 Metabolite 20S,23S-Dihydroxyvitamin D3 and Its 23R Epimer. J. Med. Chem. 59, 5102–5108, doi:10.1021/acs.jmedchem.6b00182 (2016).
Lin, Z. et al. Design, Synthesis and Biological Activities of Novel Gemini 20S-Hydroxyvitamin D3 Analogs. Anticancer Res. 36, 877–886 (2016).
Wang, Q. et al. Total synthesis of biologically active 20S-hydroxyvitamin D3. Steroids 104, 153–162, doi:10.1016/j.steroids.2015.09.009 (2015).
Yin, Y. Z., Liu, C., Tang, L. Q. & Liu, Z. P. Recoverable Pd/C catalyst mediated dehydrogenation of sterols and an improved synthesis of 1alpha-hydroxydehydroepiandrosterone. Steroids 77, 1419–1422, doi:10.1016/j.steroids.2012.08.018 (2012).
Poza, J. et al. Synthesis and evaluation of new 6-hydroximinosteroid analogs as cytotoxic agents. Bioorg. Med. Chem. 15, 4722–4740, doi:10.1016/j.bmc.2007.05.003 (2007).
Mouriño, A. An improved synthesis of 1α, 3β-dihydroxycholesta-5, 7-diene. Synth. Commun. 8, 117–125 (1978).
Otero, R. et al. Carborane-based design of a potent vitamin D receptor agonist. Chem. Sci. 7, 1033–1037 (2016).
Huet, T. et al. A vitamin D receptor selectively activated by gemini analogs reveals ligand dependent and independent effects. Cell Rep. 10, 516–526, doi:10.1016/j.celrep.2014.12.045 (2015).
Slominski, A. T. et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am. J. Physiol. Cell Physiol. 300, C526–541, doi:10.1152/ajpcell.00203.2010 (2011).
St-Arnaud, R. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J. Steroid Biochem. Mol. Biol. 121, 254–256, doi:10.1016/j.jsbmb.2010.02.002 (2010).
Clapham, D. E., Julius, D., Montell, C. & Schultz, G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 57, 427–450, doi:10.1124/pr.57.4.6 (2005).
Holick, M. F. Resurrection of vitamin D deficiency and rickets. J. Clin. Invest. 116, 2062–2072, doi:10.1172/JCI29449 (2006).
Zbytek, B. et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J. Invest. Dermatol. 128, 2271–2280, doi:10.1038/jid.2008.62 (2008).
Pingili, A. K. et al. 6beta-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension 65, 1279–1287, doi:10.1161/HYPERTENSIONAHA.115.05396 (2015).
Lin, Z., Yang, R., Guan, Z., Chen, A. & Li, W. Ultra-performance LC separation and quadrupole time-of-flight MS identification of major alkaloids in Plumula Nelumbinis. Phytochem. Anal. 25, 485–494, doi:10.1002/pca.2517 (2014).
Huet, T. et al. Structure–function study of gemini derivatives with two different side chains at C-20, Gemini-0072 and Gemini-0097. MedChemComm 2, 424–429 (2011).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Bricogne, G. et al. BUSTER version 2.11. 2. Cambridge, United Kingdom: Global Phasing Ltd (2011).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, (213–221 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Slominski, A., Zbytek, B. & Slominski, R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int. J. Cancer 124, 1470–1477 (2009).
Kim, T.-K. et al. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol. Cell. Endocrinol. 361, 143–152 (2012).
Slominski, A. T. et al. RORalpha and ROR gamma are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J. 28, 2775–2789, doi:10.1096/fj.13-242040 (2014).
Acknowledgements
This work was supported by NIH grants 1R21AR063242 (W.L., D.D.M.), 1S10OD010678 (W.L.), 1S10RR026377 (W.L.), R21AR066505 (A.S.) and 1R01AR056666 (A.S.), ANR grant ANR-13-BSV8-0024-01 (N.R.), and institutional funds (N.R.) from Instruct which is part of the European Strategy Forum on Research Infrastructures (ESFRI). We thank Dr. Dejian Ma in UTHSC, Dr. Stephen W. White in St. Jude Children’s Research Hospital, and the staff of the ID23-1 beamline at ESRF synchrotron for the experimental assistance. Z.L. thanks the support from the pre-doctoral Alma and Hal Reagan Fellowship from UTHSC, and a national scholarship award from China Scholarship Council. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Contributions
W.L. and Z.L. designed and coordinated the project. W.L., D.M., N.R. and Z.L. planned and designed the experiments. Z.L., H.C., E.T., Z.J., T.K., Z.W. and A.P. performed the experiments. A.B. and N.R. resolved VDR-compound co-crystal, J.B. resolved the crystal of the small molecule intermediate. D.M., A.S., A.P., R.T., N.R. and W.L. analyzed data. Z.L. drafted the manuscript together with N.R., all authors revised and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Accession codes: PDB ID code 5MX7 for co-crystal of 1,20S(OH)2D3 and VDR. CCDC ID Code 1527430 for intermediate 15.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, Z., Chen, H., Belorusova, A.Y. et al. 1α,20S-Dihydroxyvitamin D3 Interacts with Vitamin D Receptor: Crystal Structure and Route of Chemical Synthesis. Sci Rep 7, 10193 (2017). https://doi.org/10.1038/s41598-017-10917-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10917-7
This article is cited by
-
Photoprotective Properties of Vitamin D and Lumisterol Hydroxyderivatives
Cell Biochemistry and Biophysics (2020)
-
Investigation of 20S-hydroxyvitamin D3 analogs and their 1α-OH derivatives as potent vitamin D receptor agonists with anti-inflammatory activities
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.