Abstract
Monoecious species provide an excellent system to study the specific determinants that underlie male and female flower development. Quercus suber is a monoecious species with unisexual flowers at inception. Despite the overall importance of this and other tree species with a similar reproductive habit, little is known regarding the mechanisms involved in the development of their male and female flowers. Here, we have characterised members of the ABCDE MADS-box gene family of Q. suber. The temporal expression of these genes was found to be sex-biased. The B-class genes, in particular, are predominantly, or exclusively (in the case of QsPISTILLATA), expressed in the male flowers. Functional analysis in Arabidopsis suggests that the B-class genes have their function conserved. The identification of sex-biased gene expression plus the identification of unusual protein-protein interactions suggest that the floral organ identity of Q. suber may be under control of specific changes in the dynamics of the ABCDE model. This study constitutes a major step towards the characterisation of the mechanisms involved in reproductive organ identity in a monoecious tree with a potential contribution towards the knowledge of conserved developmental mechanisms in other species with a similar sex habit.
Similar content being viewed by others
Introduction
Development of separate male and female flowers in the same individual (monoecy) or in different individuals (dioecy) is a highly adaptive trait that enhances cross-pollination and gene fluidity1. Flowers can become unisexual after floral organ specification due to carpel or stamen abortion or arrest. Other unisexual flowers are derived from floral meristems that fail to initiate female or male organ primordia, being unisexual at inception2,3,4,5,6,7. In these flowers, the likely sex-determinant genes should control the mechanisms between floral meristem initiation and floral organ identity8.
Development of floral organs has been extensively studied in hermaphrodite species where floral organ identity is controlled by well-described gene hubs, which in the majority of the cases, code for MADS-box transcription factors9. Detailed analysis of the genetic mechanisms controlling flower organ identity led to the proposal of the ABCDE model, in which different classes of genes are recruited in the flower meristem to specify the identity of non-reproductive (sepals and petals) and reproductive organs (stamens, carpels)10,11,12,13,14,15. In Arabidopsis thaliana and other hermaphrodite species, A- and E-class genes control sepal identity and A- combined with B- and E-class genes control petal identity. B- combined with the C- and E-class genes specify stamen identity, and C- and E- and D-class genes specify carpel identity. The ABCDE model has been successfully used to explain flower organ organisation in core eudicots in what seems to be a conserved mechanism16,17,18,19.
The sliding boundaries model20, 21 and the fading borders model22 are modified ABCDE models for the development of flowers of lower eudicots, monocots and basal angiosperms. Both models suggest that the unconventional flower phenotypes of these species are mainly due to alterations in the boundaries of the expression domains of B- and C-class genes. The differential regulation of B and C-class genes has been associated to the development of unisexual flowers by inception in some dioecious species6, 7, 23. In the dioecious Spinacea oleracea, B-class genes are unique or differentially expressed in the male flowers. In transgenic S. oleracea plants, down-regulation of a B-class gene in male flowers originates a conversion of male into female flowers7. Similar results were observed in Thalictrum dioicum, where targeted silencing of a B-class gene by virus-induced gene silencing resulted in homeotic conversion of the male flower into a female flower23. These results suggest that the expansion of B-class gene expression to the carpel whorl in male flowers might be preventing the development of the carpel. Still, there is not enough experimental evidence to explain how potential sex-determinant genes control the regulation of B or C- class expression in the early stages of floral organ determination of unisexual flowers by inception.
Flower unisexuality by inception is particularly common in monoecious tree species such as Carya illinoiensis (pecan), Castanea sativa (chestnut), Juglans regia (walnut), Corylus avellana (hazelnut), Quercus spp., Betula spp., Platanus occidentalis, Persea Americana (avocado) or Cocos nucifera (coconut)24. Despite the economic and ecological importance of these species little is know on the genetic mechanisms controlling their male and female unisexual flower development. Quercus suber L. is a perennial monoecious evergreen oak species abundant in the Mediterranean basin in savannah-type ecosystems. Quercus suber male flowers are contained in catkins that emerge on the branches of the previous growth season. Each individual catkin contains 15 to 25 staminate flowers without any evidence of aborted gynoecia2. Female inflorescences arise in spikes on the axils of new leaves containing three to eight individual flowers, that also do not show any morphological evidence of aborted male organs2, suggesting that both Q. suber male and female flowers are unisexual at inception.
Functional studies in a non-model tree species such as Q. suber are difficult to perform due to several limitations common to many non-model species: unsequenced genome, not amenable to genetic manipulation and a long life cycle. Recently, an high-throughput transcriptomic study was published compiling normalised transcriptomic profiles of several Q. suber organs, including the male and female flowers25. Another RNAseq study revealed the transcriptomic differences between the male and female floral programs at different developmental stages26 and provided a valuable tool for further molecular characterisation of the mechanism controlling flower development of this monoecious species. By comparing female and male non-normalized libraries, Rocheta and colleagues (2014) identified several homologues of transcription factors that showed differential expression. Amongst these were MADS-box transcription factors, thus suggesting a putative role in male and female flower organ identity in Q. suber.
The main objective of this study was to study the potential involvement of MADS-box genes in floral organ identify of the Q. suber flowers, as unisexual flower development may be correlated to readjustments in gene expression programs in the different floral primordia. Q. suber MADS-box homologues genes were identified from available Q. suber cDNA libraries by phylogenetic profiling and its expression determined in male and female flowers at different developmental stages. Protein-protein interaction analysis allowed the identification of putative protein-protein complexes that may be controlling different aspects of flower development including meristem determinacy and reproductive organ identity.
Results
The MADS-box family of genes is conserved in Q. suber
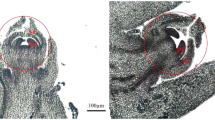
Q. suber male and female flowers develop at different periods during the growing season. The male inflorescence, or catkin, develops in late winter, at the same time that winter-dormant buds break dormancy, containing ten up to twenty-five staminate flowers (Fig. 1A and C). The female inflorescences develop in mid spring, in the axils of newly formed leaves and contain up to eight carpellate flowers (Fig. 1B and D). Morphological observations suggested that neither the male nor the female flowers show aborted organs of the opposite sex.
The involvement of MADS-box genes during the development of unisexual flowers by inception has been reported in several species7, 23, 27. To address the importance of MADS-box genes in the reproductive identity of the Q. suber flowers, homologous genes were identified and their expression characterised during flower development. The highly-conserved MADS domain protein sequence of the Arabidopsis PISTILLATA was used as a query in a blast against the cork oak sequence database (www.corkoakdb.org) resulting in the identification of thirty-nine genes. These genes were then classified by phylogenetic inference using the Arabidopsis closest homologues available in the TAIR10 protein database (Figure S1).
The canonical MADS-box protein has four conserved regions: the M(ADS) domain, the I(ntervening) and K(eratin-like) regions and a variable C-terminal domain with conserved motifs28. MADS-box proteins can be divided into two sub-groups based on the length of the K domain: MIKCc proteins have a shorter K domain whereas MIKC* have a longer one. According to the length of the predicted K domain, thirty-six proteins were classified as MIKCc and three as MIKC*. The more conserved MIKCc proteins were further divided into thirteen clades (GMM13, SQUA, AGL12, AGL17, AGL6, TM3, StMADS11, FLC, AGL15, AG, DEF/GLO and AGL2)9 (Figure S1).
Through phylogenetic inference it was possible to establish an association between the Q. suber MADS-box proteins and corresponding Arabidopsis proteins that are determinant factors in distinct plant organ developmental programs (leaf, root, shoot, flower and fruit organogenesis) (Figure S1). To evaluate which of the Q. suber MADS-box genes may have a role during flower development, a RT-qPCR was performed using a combined cDNA from male and female inflorescences at different developmental stages. No expression was detected for genes associated to the AGL12, GMM13 and AGL17 clades (Fig. 2A). The expression of genes of the AGL6, FLC, TM3, StMADS11, AGL15 and MIKC* clades was detected at lower levels (Fig. 2B). The highest level of gene expression was observed in Q. suber genes of the AG, AGL2, DEF/GLO and SQUA clades (Fig. 2C). These genes are homologous to Arabidopsis genes that have a pivotal role in floral organ identity.
Expression of Quercus suber MADS-box genes in flowers. Gene expression analysis by RT-qPCR of Quercus suber MADS-box genes in a combined sample of male and female Q. suber flowers. (A) Genes associated to the MICK*, AGL12, GMM13, AGL17 clades. (B) Genes associated to the AGL6, TM3, StMADS11, FLC, AGL15 clades. (C) Genes associated to the AG, DEF/GLO, AGL2, SQUA clades. Error bars indicate standard deviation (s.d.) of three biological and technical replicates. QsPP2AA3 was used as reference gene.
The ABCDE MADS-box transcription factors are conserved in Q. suber
The phylogeny of the Q. suber ABCDE-like genes was further analysed using homologues of distinct angiosperm species and, when available, a homologue from a gymnosperm representative species (Fig. 3). In the A-class lineage, QsAPETALA1 (QsAP1) grouped closely with AP1-like proteins from other perennial tree species, particularly with the one from Castanea mollissima, a species phylogenetically close to Q. suber (Fig. 3B). By comparing the protein domain structure and amino acid similarity within each domain of the Q. suber and the Arabidopsis homologue it is predicted that QsAP1 has the four canonical domains and that the level of conservation is very high, particularly in the MADS domain (above 80%) (Fig. 3A).
Phylogenetic profiling of the Quercus suber ABCDE homologous genes. (A) Domains similarity between Q. suber and A. thaliana (or Petunia x hybrida in the case of TM6) ABCDE MADS-box proteins were evaluated by residue identity in the four conserved domains (M - MADS; I - Intervening region; K - Keratin-like domain; C - C-terminal domain). (B) Phylogenetic analysis of the SQUA family. (C) Phylogenetic analysis of the DEF/GLO family. (D) Phylogenetic analysis of the AG family. (E) Phylogenetic analysis of the AGL2 family. Q. suber proteins marked in bold. Independent lineages are distinguishable by different colours (Green – A-class lineage; light purple – paleoAP3 lineage; light blue – euAP3 lineage; dark blue – PI lineage; light pink – C-class lineage; dark pink – D-class lineage; dark purple – E-class lineage).
The B-class clade can be divided into three independent lineages: PI, euAP3 and paleoAP329, 30. Within the PI lineage, QsPISTILLATA was grouped closer to other PI-like proteins (Fig. 3C). QsPI four putative conserved regions are 40–80% identical to the AtPI counterparts (Fig. 3A). Despite a lower level of residue identity (particularly in the MADS domain), QsPI has a completely conserved PI motif (short amino acid sequence that characterises the PI lineage and is essential for protein function29) in the C-terminal region (Figure S2A).
The paleoAP3 and euAP3 lineages diverged after duplication at the base of the core eudicots and the major difference between lineages is a specific motif in the C-terminal domain29. One APETALA3 (AtAP3) homologue (QsAP3) was identified in the euAP3 lineage (Fig. 3C). QsAP3 has a partially conserved euAP3 motif (Figure S2A) and, with the exception of the MADS domain, shows a low degree of residue conservation (less than 60%) when compared to AtAP3, which suggests a possible functional divergence (Fig. 3A). Two paleoAP3 Q. suber proteins (QsTM6.1 and QsTM6.2) were also identified (Fig. 3C). QsTM6.1 differs from QsTM6.2 by having fourteen extra aminoacids in the I-region. Both QsTM6.1 and QsTM6.2 protein domains are very similar to the Petunia x hybrida TM6-like (PhTM6 was used because there is no TM6 homologue in Arabidopsis) (Fig. 3A), however the paleoAP3 motif is not completely conserved (Figure S2A).
The phylogenetic analysis of the AG clade resulted in the grouping of one Q. suber protein with the Arabidopsis D-class protein SHATTERPROOF1 (AtSHP) and three Q. suber proteins with the Arabidopsis D-class protein SEEDSTICK (AtSTK) (Figure S1). Two of the three STK-like Q. suber genes were not expressed in flowers (Fig. 2C). A detailed analysis on the conserved AG motifs (motif I and motif II) in the C-terminal domain31, 32 suggested that QsAG (the putative STK-like gene that is expressed in flowers) has higher proximity to AtAGAMOUS (the canonical C-class protein) than to AtSTK. On the contrary, the AG motifs I and II of QsSHP were more similar to the ones of AtSHP (Figure S2B). A new phylogenetic analysis was performed using AG-like and SHP-like proteins from other species and resulted in the placement of QsAG together with other AG-like proteins (Fig. 3D). QsSHP was placed in the same clade of AtSHP (Fig. 3D), however, a lack of residue conservation, particularly in the I region (Fig. 3A), and a complete deletion of the AG motif II (Figure S2B) may suggest a divergent role for QsSHP during Q. suber flower development.
The E-class includes the SEPALLATA-like genes, which are unique to angiosperms. Four genes were identified in the cork oak database (Figure S1) but QsSEP4 was not expressed in flowers (Fig. 2C). The E-class clade was divided into two different lineages, the SEPALLATA3 (SEP3) (containing one Q. suber protein), and the SEP1/2 lineage (containing two Q. suber proteins, QsSEP1 and QsSEP2) (Fig. 3E). Of the two SEP motifs that characterise the SEP1/2 lineage, only SEP motif II was conserved in both QsSEP1 and QsSEP2 (Figure S2C). QsSEP3 had partially conserved SEP motifs (I and II), which are characteristic of the SEP3 lineage (Figure S2C).
Several Q. suber ABCDE-like genes expression is sex-biased
During flower development, a tight spatiotemporal regulation of MADS-box gene expression is necessary for the development of fully functional floral organs. To determine the expression patterns of Q. suber ABCDE-like genes, a RT-qPCR analysis was performed using two pools of cDNA samples from both male and female flowers, at different developmental stages. One pool contained early flower developmental stages, from early onset to pre-maturation. The other pool contained flowers in late stages of flower development, from post-maturation to pollen shedding (in male flowers), or to early stages of fruit development (in female flowers). Q. suber ABCDE-like genes were predominantly expressed in the flowers, when compared to expression in other tissues tested (leaf, bud, root and fruit). Most of these genes were also significantly expressed in the fruit and some in the axillary bud (Fig. 4).
Some Quercus suber ABCDE gene expression is sex-biased. Gene expression analysis by RT-qPCR of Q. suber ABCDE homologue genes was measured in different tissues: early and late stages of male or female flower development, axillary bud, fruit, leaf, and root. (A) QsAP1; (B) QsSEP1; (C) QsSEP3; (D) QsAP3; (E) QsTM6.1; (F) QsTM6.2; (G) QsPI; (H) QsAG; (I) QsSHP and (J) QsSEP2. Error bars indicate standard deviation (s.d.) of three biological and technical replicates. QsPP2AA3 was used as reference gene.
QsSEP1, QsSEP2 and QsSEP3 were expressed at a similar level in early and late stages of male and female flower development. QsSEP1, QsSEP2 and QsSEP3 were expressed in the fruit but only QsSEP3 had expression in the axillary bud (Fig. 4B,C and J). QsSEP1 and QsSEP2 were the only Q. suber ABCDE-like genes expressed in leaves or roots. QsSHP was expressed at the same level in both male developmental stages and in the early female pool but its expression increased significantly in late stages of female flower development and in the fruit (Fig. 4I). Only QsAP1 was significantly more expressed in the female than in the male flowers, particularly in early stages of female flower development (Fig. 4A). QsAP3, QsTM6.1, QsTM6.2, QsPI and QsAG had higher expression in male flowers than in female, particularly in post-maturation stages. These five genes were also expressed in axillary buds (Fig. 4D–H). Similarly to QsSHP, QsAG was highly expressed in the fruit (Fig. 4H and I). QsPI is the only gene that was uniquely expressed in the axillary buds and male flowers, particularly in late developmental stages, not being detected in female flowers (Fig. 4G). Expression analysis showed that Q. suber ABCDE-like genes are differentially regulated in male and female flowers suggesting an organ-specific gene regulation.
Some Q. suber ABCDE-like genes have a function in flower induction and organ identity
To analyse a potential conservation in the function of cork oak ABCDE-like genes, Arabidopsis transgenic lines overexpressing their coding regions were obtained. QsAP1 and QsAP3 overexpressing plants were similar to the wild type (WT) (Fig. 5A and C). To evaluate whether the absence of phenotypic differences could be related to down-regulation of the transgenes, an RT-qPCR using RNA from floral tissues was performed. QsAP1 was highly expressed in QsAP1 transgenic plants, but the QsAP3 transgene was weakly expressed in the QsAP3 overexpressing plants (Figure S3). Flowers from plants overexpressing QsAP3 were similar to WT, with no organ homeotic conversion (Fig. 6A and B). To assess whether QsAP3 was able to restore the floral defects of the ap3-3 (mutant lacking petals and stamens) (Fig. 6C), mutant plants were complemented with QsAP3 coding region driven by the 35 S promoter or by a 0.5Kbp fragment of the AtAP3 promoter. QsAP3 driven by the AtAP3 native promoter was expressed significantly more that when driven by the 35 S promoter (Figure S3), however, in both strategies, expression of QsAP3 in the ap3-3 background failed to rescue the development of petals and stamens (Fig. 6D and E).
Overexpression of Quercus suber ABCDE homologues genes in A. thaliana generate distinct plant phenotypes. (A) From left to right: wild-type (Col-0), 35 S::QsAP1, 35 S::QsTM6, 35 S::QsAP3 and 35S::QsSHP plants. (B) From left to right: wild-type (Col-0), 35S::QsPI, 35S::QsAG and 35S::QsSEP3 plants. (C) graphic display of the total number of leaves of A. thaliana WT and overexpression plants during flowering. Error bars indicate standard deviation (s.d.). Asterisks indicate p-values ≤ 0.05 determined after performing a Students t-test.
A. thaliana plants overexpressing Q. suber ABCDE genes display distinct flower phenotypes. (A) Wild type (Col-0). (B) 35S::QsAP3. (C) ap3-3 mutant. (D) 35S::QsAP3 in the ap3-3 background. (E) pAtAP3::QsAP3 in the ap3-3 background. (F) 35S::QsTM6 (arrow represent the lack of petal curvature). (G) 35S::QsTM6 flower without a sepal and petal. (H) pAtAP3::QsTM6 in the ap3-3 background. (I) 35S::QsTM6 in the ap3-3 background. (J) 35S::QsPI (arrow represent a sepaloid petal). (K) pAtPI::QsPI (arrow represent a sepaloid petal). (L) pi-1 mutant. (M) pAtPI::QsPI in the pi-1 background (arrow represent ectopic ovules). (N) 35S::QsPI in the pi-1 background. (O) 35S::QsAG. (P) ag-1 mutant. (Q) 35S::QsSHP. (R) 35S::QsSEP3. (S) sep1 sep2 sep3 mutant. (T) 35 S::QsSEP3 in the sep1 sep2 sep3 background. Scale bar: 0.5 mm.
QsTM6.1 overexpressing lines showed mild differences when compared to WT, displaying slight reduced shoot apical dominance (Fig. 5A) and petals that did not curve outwards (Fig. 6F), with no other observed flower defects (Fig. 6G). QsTM6.1 expression failed to complement the floral defects of ap3-3 when driven from the AtAP3 native promoter (Fig. 6H). When driven by the 35 S promoter QsTM6.1 rescued the fertility in 15% of the ap3-3 flowers, despite the majority of the flowers being similar to the mutant ap3-3 (Fig. 6I). Plants overexpressing QsPI flowered significant early under long days when compared to WT plants (Fig. 5B and C) and the flowers displayed homeotic conversions of sepals into petaloid structures (Fig. 6J). Petal cells of the 35::QsPI flowers were similar to the WT (Figure S5B, supporting method) but the flanks of the sepal adaxial epidermis contained typical petal conical cells (Figure S5C), suggesting that petal identity has expanded to the sepal whorl. No alterations were found in the pistils of QsPI overexpressing plants. Complementation of the A. thaliana pi-1 mutant phenotype (mutant flower lacking petals and stamens) (Fig. 6L) was tested using a QsPI coding region controlled by a 1.5 kb fragment of the native Arabidopsis promoter (pPI::QsPI). pPI::QsPI transgenic plants had the same flower defects as the overexpressing line (Fig. 6K). Flowers of pPI::QsPI transgenic plants in the pi-1 background had restored petals and stamen-like structures with ectopic ovules (Fig. 6M). Some of the flowers of pPI::QsPI in the pi-1 background plants had fully functional stamens that enabled self-fertilisation. Complete rescue of stamens was achieved by complementing pi-1 with QsPI controlled by the 35S promoter (Fig. 6N).
Phenotype analysis of QsAG overexpressing plants revealed a variety of phenotypes that could be sorted into three groups. The first included plants with early flowering, early stem termination and small curly leaves (Fig. 5C, 35 S::AG#1 and Figure S4); the second group contained plants with reduced shoot apical dominance and altered phyllotaxy, but no early flowering (Fig. 5C, 35 S::AG#2); and finally a third group of plants that were very similar to the WT (Fig. 5C, 35 S::AG#3). RT-qPCR analysis showed that the QsAG transgene is significantly more expressed in plants of the first and second groups (Figure S3). None of the independent transgenic plants had flowers with homeotic conversion of sepals and petals into carpels and stamens, respectively (Fig. 6O). QsSHP overexpressing plants had slightly early flowering (Fig. 5A and C), but similarly to the 35 S::QsAG plants, there was no organ defect including no conversion of the perianth organs into carpels or stamens (Fig. 6Q). QsAG or QsSHP coding regions driven by the 35S promoter were not able to rescue the defects of the ag-1 mutant (Fig. 6P).
Plants overexpressing QsSEP3 had an extremely early flowering phenotype (Fig. 5B and C). QsSEP3 flowers did not show any kind of homeotic transformation of flower organs (Fig. 6R) but expression of QsSEP3 in the sep1 sep2 sep3 background (mutant containing predominantly sepals, Fig. 6S) restored completely the fertility of the mutant (Fig. 6T).
Different protein-protein interactions might control different aspects of Q. suber flower development
The ability to bind DNA in higher-order complexes is the cornerstone of the MADS-box protein function and determines the identity of flower organs in several species33. Stamen development is dependent on the formation of quaternary complexes of B-, C- and E-class MADS-box proteins, whereas carpels depend on the combinatorial action of C- and E-class MADS-box proteins. Thus, a change on the ability to establish protein-protein interactions could be part of the developmental process that may be involved during unisexual flower formation. The ability of Q. suber ABCDE-like proteins to dimerise was evaluated in a yeast two-hybrid (Y2H) experiment by fusing the coding regions of the Q. suber ABCDE-like genes to the activation or binding domain of the GAL4 transcription factor. QsPI was able to interact with QsAP3 and QsTM6. QsAP3 self-dimerised but did not interact with its closest relative, QsTM6 (Fig. 7, first two columns). Interestingly, QsAP3 and QsPI (B-class proteins) interacted with QsSHP (C-class) but not with QsAG (C-class), suggesting that QsSHP might have retained the C-class function in the formation of higher order complexes in the reproductive whorls (Fig. 7). Furthermore, QsAP3 and QsSHP formed a complex with QsSEP3 (Fig. 7). No interaction was observed between QsAG and QsSEP3, or with any B-class proteins. QsAG only interacted with QsAP1 and QsSEP1. QsSEP1 or QsAP1 fused to the binding domain of GAL4 were not used in this Y2H assay because both proteins were able to activate transcription, as seen in other species34, 35.
The combinatorial activity of Q. suber ABCDE-like proteins is partially conserved. Different interaction combinations between Q. suber ABCDE-like proteins were tested. Q. suber ABCDE-like proteins were fused to either GAD4-activation domain (AD) or to GAL4-binding domain (BD). Double yeast transformations were first tested for plasmid presence by growing cells in Synthetic Defined (SD) medium without tryptophan and leucine (-W-L). Ability to interact was evaluated in SD medium without tryptophan, leucine and histidine (-W-L-H). Protein Interaction strength was assessed by using successive cell culture dilutions (1:10, 1:100 and 1:1000). Each cropped image corresponds to a matching single plate in which the yeast double transformants were selected.
Discussion
Unisexuality by inception likely derives from failure to initiate organ primordia36. Species with this trait represent an excellent opportunity to study the regulatory mechanisms that control the early establishment of male and female flower organ identity. For the past decades, unisexuality by inception has been studied in dioecious species pointing to ABCDE-like genes as one of the molecular switches controlling the development of male or female flowers5,6,7, 23. Differential regulation of ABCDE-like genes involved during the formation of unisexual flowers by inception in dioecious species may be conserved in species with a similar trait but with different sexual strategies (e.g. monoecious), but no such information is presently available. The RNAseq study performed by Rocheta and colleagues (2014) points to several MADS-box genes as being deregulated in male and female libraries of Q. suber but a role for these genes in floral organ identity is still unknown. To bridge this gap, a study was conducted to infer on the role of ABCDE-like genes in the early establishment of the unisexual flowers by inception of Q. suber.
Following the reasoning of the canonical ABCDE model, early establishment of the Q. suber female flower identity should depend on the activity of C- and E-class genes and the male flower identity on C-, B- and E-class genes37, 38. The E-class genes function as co-factors for the establishment of all the flower organs as suggested by the sep1 sep2 sep3 sep4 mutant that develops leaves instead of flowers14, 39. In Q. suber, the expression profile of QsSEP1, QsSEP2 is identical and both genes are expressed during male and female flower development. QsSEP3 is equally expressed in female flowers but is significantly more expressed in late stages of male flower development, suggesting a role in pollen maturation.
Arabidopsis plants overexpressing QsSEP3 display early flowering but show no floral defects, a result consistent with the function of SEP3-like genes in other species34, 40, 41. However, some reports have suggested different roles for SEP3-like genes in floral organ identity. Arabidopsis plants overexpressing AtSEP3 show homeotic transformation of sepals into carpeloid structures with ectopic ovules in the external surface, suggestive of AtAG ectopic activation in the sepal whorl42, whereas the overexpression of the lilium SEP3-like creates indeterminate flowers in Arabidopsis invocative of a compromised C- function43. Thus, the SEP3-like gene function in floral transition is likely conserved but not the ability to promote floral homeotic changes, which may be species-specific and correlated to a divergent evolution of DNA-binding activity of downstream targets or interacting partners.
In the stamen and carpel whorls of Arabidopsis and petunia, interaction of SEP3-like with C-class proteins and with the AP3-like/PI-like heterodimers is necessary to proper organ development35, 44,45,46. In Q. suber, E-class proteins might be fulfilling their role in organ identity because they interact with B- and C-class proteins. Furthermore, it is likely that QsSEP3 is able to interact with native Arabidopsis B-class and C-class proteins because QsSEP3 overexpression rescues the sterile phenotype of the triple sep1 sep2 sep3 Arabidopsis mutant.
There has been some studies reporting interaction between AP1-like and SEP-like proteins in Arabidopsis and rice34, 47, and an Y3H experiment performed in Arabidopsis has shown that AtAP1, AtSEP3 and SEUSS form a higher-order complex that act as repressor of AtAG in sterile floral organs48. In Q. suber, QsSEP1 interacts with QsAG. Interestingly, QsAP1 also interacts with QsAG, which has not been previously reported in any other species. Assuming that QsAP1, QsAG and QsSEP1 are able to interact in vivo, the establishment of a QsAP1-QsAG-QsSEP1 protein complex may be related to an undisclosed mechanism that controls floral meristem development (Fig. 8).
Model on the putative role of MADS-box genes in the reproductive development of Q. suber. (A) The interaction between QsAG/QsAP1/QsSEP1 could be related to the transition from vegetative to the reproductive development. (B) The QsSHP/QsAP3/QsSEP3/QsTM6/QsPI combinatorial complex is involved in male flowering, whereas the QsSHP/QsSEP3 complex is involved in female flowering. Regarding this hypothesis, unisexual flowering in Q. suber could be determined by a MF (male factor) or an FF (female factor) that may control the MADS genes. However, it is possible that an MF or FF could determine sex identity in parallel or independently from the MADS-box genes.
The function of C-class genes in reproductive organs is tightly conserved in angiosperms, and with the notable exception of a basal angiosperm (Illicium)49, in which a C-class gene is expressed in tepals, no other example has been described of a naturally occurring C-class gene expressed in non-reproductive organs. The Arabidopsis C-class gene, AtAG, controls the identity of stamens and carpels, but also the determinacy of the flower meristem50, 51. Contrarily to what happens in Arabidopsis, the majority of the angiosperm species has two or more AG-like genes, and in some species, like maize or rice, different C-class genes share the canonical C-class functions52, 53. Two putative AG-like genes are expressed in the Q. suber flowers, one that clusters with AtAG and the other with AtSHP1 (which has a canonical role in ovule identity15). In Q. suber, the embryo sac development is delayed3, occurring weeks after pollination. Therefore, expression of QsSHP in both male and female flowers is not likely to be associated with ovule development and it is likely that QsAG and QsSHP are both C-class genes.
Arabidopsis plants overexpressing QsAG display severe phenotypic defects such as early flowering and early development arrest. These phenotypes were also observed in plants overexpressing AG-like genes from other species51, 54,55,56. Some of the plants overexpressing QsAG display curly leaves, also observed in 35 S::AtAG plants, a phenotype associated with curly leaf, a mutant in a gene that epigenetically blocks AtAG expression in WT Arabidopsis leaves57. This suggests that QsAG might have retained some of the canonical C-class gene functions. No phenotypic alterations were observed in 35 S::QsSHP plants. Arabidopsis flowers overexpressing QsAG and QsSHP do not display homeotic conversions of petals into stamens and sepals into carpels, as the ones observed in 35S::AtAG Arabidopsis plants or in plants overexpressing AG-like genes of petunia, cucumber, rice or lilium51, 54,55,56. The lack of flower homeotic conversions and the failure to rescue the ag-1 mutant suggests a certain degree of functional divergence. One possibility to explain the lack of expected phenotypes is that both QsAG and QsSHP may be needed to fulfil a complete C-class function as often observed in many core eudicots52, 58, 59, which is supported from the observation that both QsAG and QsSHP have a similar expression pattern in early stages of the Q. suber male and female flower development.
The role of QsSHP and QsAG in Q. suber flower organ identity may be conserved, as they are able to interact with an E-class protein. Unexpectedly, QsSHP also interacts with QsAP3, a B-class protein. The interaction of B-class proteins with C-class proteins without the mediation of an E-like protein has not been previously reported in eudicots. Examples of such interaction have only been shown in gymnosperms, where B- and C-like proteins interact directly to control male cone development60, and in some basal angiosperms species61. The almost complete deletion of the AG motif II from QsSHP protein may allow the interaction of with unusual partners, such as B-class proteins. Direct interaction between B- and C-class proteins may pinpoint the particular nature of the protein complexes that are established during floral organ primordia development in Q. suber.
In Q. suber, four B-class genes were identified based on lineage specific motifs (QsAP3, QsPI, QsTM6.1 and QsTM6.2). The expression profile of the AP3-like and TM6-like genes in flowers is very similar, with significantly higher expression in male flowers, and at a lower level in female flowers. AP3-like genes are usually expressed in the petal and stamen whorls but there are examples of AP3-like genes also weakly expressed in the carpel whorl62. TM6-like genes are usually expressed in petals, stamens and carpels in early stages of flower development and in ovules and transmitting tissue in later stages63, 64, although its function has been shown to be restricted to stamen development65. In Q. suber, the overexpression of QsAP3 failed to rescue petal and stamen development of the ap3-3 mutant or promote homeotic conversion of carpels into stamens, opposite to what happens in 35S::AtAP3 flowers66, suggesting a biochemical divergence within the euAP3 lineage. However, QsAP3 is still able to take part in higher order protein complexes with other Q. suber B- and E-class proteins (as well as with C-class proteins). Similarly to QsAP3, overexpressing the Q. suber TM6-like (QsTM6.1) in A. thaliana failed to promote homeotic conversion of carpels into stamens but was able to partial recover the stamens of the ap3-3 mutant, suggesting that QsTM6.1 may have retained some of the paleoAP3 canonical function.
Obligatory heterodimerisation of AP3- and PI-like proteins controlling petal and stamen development is preserved across the angiosperms67, 68 and is also needed to specify male cones in gymnosperms60. In Q. suber, QsPI interacts with both QsAP3 and QsTM6 suggesting that the obligatory interaction may be preserved in this species. Arabidopsis plants overexpressing QsPI show flowers with homeotic conversion of sepals into a petaloid organ, a phenotype that has been previously described in Arabidopsis plants overexpressing AtPI 69. Furthermore, QsPI expression was able to rescue petal and stamen development in the pi-1 background suggesting a conserved function in organ identity. As expected, no homeotic conversion of carpels into stamens was observed and this may be due to the lack of an AP3-like interactor expressing in the fourth whorl of the A. thaliana wild-type flower, suggesting that QsPI needs to heterodimerise to retain function.
QsPI expression is restricted to the Q. suber male flowers. Lack of QsPI expression in Q. suber female flowers may be tightly interconnected with the lack of male organs in these flowers, as it has been suggested in other unisexual species, where B-class genes need to be transcriptionally repressed to allow the development of female organs5, 7, 27. In Q. suber, obligatory interaction of B-class proteins and establishment of a complex with C- and E-class proteins might be needed in the male flowers (Fig. 8) promoting stamen development and/or inhibiting carpel primordia, similarly to what happens in Arabidopsis when the ectopic expression of AtPI and AtAP3 in the fourth whorl blocks carpel development66. The lack of QsPI expression in female flowers may allow the interaction of C- and E-class proteins to promote carpel development (Fig. 8) not allowing stamen primordia to be initiated. Considering this hypothesis, the mechanisms that establish unisexual flowers in Q. suber might be acting upstream of the regulatory module of the MADS protein complexes, controlling the regulation of QsPI expression. The temporal regulation of QsPI may limit the deployment of a homeotic change to a specific time frame (during male flowering) without causing potentially deleterious effects such as transformation of the carpel into stamens in a later reproductive phase (during female flowering). In the dioecious S. oleracea, there is genetic evidence that regulation of B-class genes might have a direct role in flower organ identity/unisexuality, which might imply that regulation of unisexuality by inception might be conserved. Several questions remain open, in particular the identification of the sex determination factors that may be acting upstream of the MADS-related pathway or in a parallel/independent-signalling pathway (Fig. 8).
The elucidation of the mechanism responsible for the establishment of unisexual flowers has an evolutionary, ecological and economic importance. Although in the past decades several genetic studies have provided a framework to partially explain sex-determining pathways, a common mechanism controlling the onset of male and female flowers at inception is still illusive. Here, we propose a simple model for the regulation of flower organ identity in the monoecious species Q. suber, via the modulation of MADS-box transcription factor function. Taking into consideration the similar nature of flower development of other tree species, it is likely that this model for organ identity may be conserved.
Experimental procedures
Plant material
Arabidopsis thaliana ecotype Columbia (Col-0) and mutant seeds were acquired from the Nottingham Arabidopsis Stock Centre. Seeds were sown in Murashige and Skoog agar medium and incubated under long day conditions (16 hours light/8 hours dark) at 20 °C in controlled environmental growth rooms, with light intensity of 30 μE m−2 s−1. Approximately 10 days after sowing, plantlets were transferred to pots containing a 4:1 (v:v) mixture of turf rich soil and vermiculite. Overexpression constructs were introduced into A. thaliana ecotype Col-0 using the GV3101 agrobacterium strain and the floral dip method70. Transgenic plants were selected in medium supplemented with hygromycin. After checking for single insertion lines, four independent lines were selected from each construct. Functional studies were conducted using plants homozygous for the transgene. Q. suber plant organ samples (root, leaf, flowers, fruit and bud) were collected from three Quercus suber trees located in the Minho University campus. Early and late stages of male and female flower development were selected based on the phenological stages defined by Varela and Valdiviesso (1996).
Phylogenetic methods
The cork oak MADS-box DNA sequences were obtained by performing a BLAST in the cork oak database (http://corkoakdb.org) using the Arabidopsis thaliana PISTILLATA protein sequence as a query. The cork oak MADS-box homologous protein and DNA sequences were obtained by performing a BLAST at the NCBI database (http://www.ncbi.nlm.nih.gov/). The protein sequences were aligned using MUSCLE71; then the peptide alignments were back translated to DNA coding sequences using PAL2NAL72. Alignments were trimmed using GBLOCKS73. Distances were estimated using the Tamura-Nei model of evolution for a maximum-likelihood74 tree with the MEGA6 software package75. To provide statistical support for each node on the tree, a consensus tree was generated from 1000 bootstrap data sets. Protein accessions are contained in Supporting Table S2.
Y2H analysis
Protein–protein interactions were analysed using a GAL4–based yeast hybrid system (Matchmaker two-hybrid system; Clontech). Competent cells of Saccharomyces cerevisiae strain AH10976 were transformed with pGBT9 derivatives (bait vectors; Clontech) using the LiAc/DNA/PEG transformation method77. The resulting yeast cells were subsequently transformed with a pGAD424 derivative (pray plasmid, Contech) dependent on the interaction pair to test. Self-activation assays and selection of positive interactors were performed according to Causier and Davies (2002)78.
RNA extraction and cDNA preparation
Arabidopsis thaliana total RNA was extracted using Trizol reagent (TermoFisher Scientific) according to the manufacturer’s instructions. Quercus suber total RNA was obtained using the CTAB/LiCl extraction method79 with some modifications80 . A. thaliana and Q. suber cDNAs were synthesized according to the Invitrogen cDNA synthesis kit SuperScript® III RT manufacturer’s instructions.
RT-qPCR analysis
cDNA was amplified using SsoFast™ EvaGreen® Supermix (Bio-Rad), 250 nM of each gene-specific primer (listed in Supplementary Table 3.1) and 1 μL of cDNA (1:100 dilution). Quantitative real-time PCR (RT-qPCR) reactions were performed in triplicates on the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). After an initial period of 3 min at 95 °C, each of the 40 PCR cycles consisted of a denaturation step of 10 s at 95 °C and an annealing/extension step of 10 s at the gene specific primer temperature. With each PCR reaction, a melting curve was obtained to check for amplification specificity and reaction contaminations, by heating the amplification products from 60 °C to 95 °C in 5 s intervals. Primer efficiency was analysed with CFX Manager™ Software v3.1 (Bio-Rad), using the Livak calculation method for normalized expression81. Gene expression analysis was established based on three technical and three biological replicates, and normalized with the reference gene QsPP2AA3 82.
Plasmids construction
Overexpression constructs were obtained using Gateway technology (ThermoFisher Scientific) according to the manufacturer’s instructions. The destination vector was pMDC32. For the yeast-two-hybrid assay, open reading frames were cloned using standard molecular biology tools in the pGBT9 or pGAD424 vectors (Clontech). Complementation constructs with native A. thaliana promoters were obtained by substituting the 35 S cauliflower promoter for a fragment of the AtAP3 (0.5 Kbp) or AtPI (1.5 Kbp) promoters83 using standard molecular biology tools. All the primers used are listed in Supporting Table S1.
References
Charlesworth, B. & Charlesworth, D. A Model for the Evolution of Dioecy and Gynodioecy. The American Naturalist 112, 975 (1978).
Varela, M. C. & Valdiviesso, T. Phenological phases of Quercus suber L. flowering. For. Genet. 3, 93–102 (1996).
Boavida, L. C., Varela, M. C. & Feijo, J. A. Sexual reproduction in the cork oak (Quercus suber L.). I. The progamic phase. Sex. Plant Reprod. 11, 347–353 (1999).
Sheppard, L. A. et al. A DEFICIENS homolog from the dioecious tree black cottonwood is expressed in female and male floral meristems of the two-whorled, unisexual flowers. Plant Physiol. 124, 627–640 (2000).
Di Stilio, V. S., Kramer, E. M. & Baum, D. a. Floral MADS box genes and homeotic gender dimorphism in Thalictrum dioicum (Ranunculaceae) - a new model for the study of dioecy. Plant J. 41, 755–66 (2005).
Pfent, C., Pobursky, K. J., Sather, D. N. & Golenberg, E. M. Characterization of SpAPETALA3 and SpPISTILLATA, B class floral identity genes in Spinacia oleracea, and their relationship to sexual dimorphism. Dev. Genes Evol. 215, 132–142 (2005).
Sather, D. N., Jovanovic, M. & Golenberg, E. M. Functional analysis of B and C class floral organ genes in spinach demonstrates their role in sexual dimorphism. BMC Plant Biol. 10, 46 (2010).
Sobral, R., Silva, H. G., Morais-Cecílio, L. & Costa, M. M. R. The Quest for Molecular Regulation Underlying Unisexual Flower Development. Front. Plant Sci. 7, 160 (2016).
Becker, A. & Theißen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 29, 464–489 (2003).
Bowman, J. L., Smyth, D. R. & Meyerowitz, E. M. Genes directing flower development in Arabidopsis. Plant Cell 1, 37–52 (1989).
Coen, E. S. & Meyerowitz, E. M. The war of the whorls: genetic interactions controlling flower development. Nature 353, 31–37 (1991).
Bowman, J. L., Alvarez, J., Weigel, D., Meyerowitz, E. M. & Smyth, D. R. Control of flower development in Arabidopsis thaliana by APETALA1 and interacting genes. Development 119, 721–743 (1993).
Flanagan, C. A., Hu, Y. & Ma, H. Specific expression of the AGL1 MADS-box gene suggests regulatory functions in Arabidopsis gynoecium and ovule development. Plant Journal 10, 343–353 (1996).
Pelaz, S., Ditta, G. S., Baumann, E., Wisman, E. & Yanofsky, M. F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405, 200–203 (2000).
Liljegren, S. J. et al. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404, 766–770 (2000).
Theissen, G. & Melzer, R. Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann. Bot. 100, 603–619 (2007).
Greenup, A., Peacock, W. J., Dennis, E. S. & Trevaskis, B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann. Bot. 103, 1165–72 (2009).
Bowman, J. L., Smyth, D. R. & Meyerowitz, E. M. The ABC model of flower development: then and now. Development 139, 4095–4098 (2012).
Andrés, F. & Coupland, G. The genetic basis of flowering responses to seasonal cues. Nat. Rev. Genet. 13, 627–39 (2012).
Bowman, J. L. Evolutionary conservation of angiosperm flower development at the molecular and genetic levels. J. Biosci. 22, 515–27 (1997).
Kramer, E. M., Di Stilio, V. S. & Schluter, P. M. Complex patterns of gene duplication in the APETALA3 and PISTILLATA lineages of the Ranunculaceae. Int. J. Plant Sci. 164, 1–11 (2003).
Buzgo, M., Soltis, D. E., Soltis, P. S. & Ma, H. Towards a comprehensive integration of morphological and genetic studies of floral development. Trends in Plant Science 9, 164–173 (2004).
Larue, N. C., Sullivan, A. M. & Di Stilio, V. S. Functional recapitulation of transitions in sexual systems by homeosis during the evolution of dioecy in Thalictrum. Front. Plant Sci. 4, 487 (2013).
Sedgley, M. & Griffin, A. R. Sexual Reproduction of Tree Crops. Sexual Reproduction of Tree Crops, doi:10.1016/B978-0-12-634470-7.50012-X (1989).
Pereira-Leal, J. B. et al. A comprehensive assessment of the transcriptome of cork oak (Quercus suber) through EST sequencing. BMC Genomics 15, 371 (2014).
Rocheta, M. et al. Comparative transcriptomic analysis of male and female flowers of monoecious Quercus suber. Front. Plant Sci. 5, 1–16 (2014).
Kazama, Y., Koizumi, A., Uchida, W., Ageez, A. & Kawano, S. Expression of the floral B-function gene SLM2 in female flowers of Silene latifolia infected with the smut fungus Microbotryum violaceum. Plant Cell Physiol. 46, 806–811 (2005).
Kaufmann, K., Melzer, R. & Theißen, G. MIKC-type MADS-domain proteins: structural modularity, protein interactions and network evolution in land plants. Gene 347, 183–198 (2005).
Kramer, E. M., Dorit, R. L. & Irish, V. F. Molecular evolution of genes controlling petal and stamen development: Duplication and divergence within the APETALA3 and PISTILLATA MADS-box gene lineages. Genetics 149, 765–783 (1998).
Kramer, E. M. & Irish, V. F. Evolution of the petal and stamen developmental programs: evidence from comparative studies of the lower eudicots and basal angiosperms. Int. J. Plant Sci. 161, S29–S40 (2000).
Kramer, E. M., Jaramillo, M. A. & Di Stilio, V. S. Patterns of Gene Duplication and Functional Evolution during the Diversification of the AGAMOUS Subfamily of MADS Box Genes in Angiosperms. Genetics 166, 1011–1023 (2004).
Dreni, L. & Kater, M. M. MADS reloaded: Evolution of the AGAMOUS subfamily genes. New Phytologist 201, 717–732 (2014).
Theißen, G., Melzer, R. & Rümpler, F. MADS-domain transcription factors and the floral quartet model of flower development: linking plant development and evolution. Development 143, 3259–3271 (2016).
Pelaz, S., Gustafson-Brown, C., Kohalmi, S. E., Crosby, W. L. & Yanofsky, M. F. APETALA1 and SEPALLATA3 interact to promote flower development. Plant J. 26, 385–394 (2001).
Immink, R. G. H. et al. Analysis of the petunia MADS-box transcription factor family. Mol. Genet. Genomics 268, 598–606 (2003).
Diggle, P. K. et al. Multiple developmental processes underlie sex differentiation in angiosperms. Trends Genet. 27, 368–376 (2011).
Melzer, R., Verelst, W. & Theißen, G. The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in ‘floral quartet’-like complexes in vitro. Nucleic Acids Res. 37, 144–157 (2009).
Smaczniak, C. et al. Characterization of MADS-domain transcription factor complexes in Arabidopsis flower development. Proc. Natl. Acad. Sci. USA 109, 1560–1565 (2012).
Ditta, G., Pinyopich, A., Robles, P., Pelaz, S. & Yanofsky, M. F. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr. Biol. 14, 1935–1940 (2004).
Kang, H. G., Jang, S., Chung, J. E., Cho, Y. G. & An, G. Characterization of two rice MADS box genes that control flowering time. Mol. Cells 7, 559–566 (1997).
Jang, S., Hong, M. Y., Chung, Y. Y. & An, G. Ectopic expression of tobacco MADS genes modulates flowering time and plant architecture. Mol Cells 9, 576–586 (1999).
Castillejo, C., Romera-Branchat, M. & Pelaz, S. A new role of the Arabidopsis SEPALLATA3 gene revealed by its constitutive expression. Plant J. 43, 586–596 (2005).
Tzeng, T. Y., Hsiao, C. C., Chi, P. J. & Yang, C. H. Two lily SEPALLATA-like genes cause different effects on floral formation and floral transition in Arabidopsis. Plant Physiol. 133, 1091–1101 (2003).
Ferrario, S., Immink, R. G. H., Shchennikova, A., Busscher-Lange, J. & Angenent, G. C. The MADS Box Gene FBP2 Is Required for SEPALLATA Function in Petunia. Plant Cell 15, 914–925 (2003).
de Folter, S. et al. Comprehensive interaction map of the Arabidopsis MADS Box transcription factors. Plant Cell 17, 1424–1433 (2005).
Leseberg, C. H. et al. Interaction study of MADS-domain proteins in tomato. J. Exp. Bot. 59, 2253–2265 (2008).
Fornara, F. et al. Functional characterization of OsMADS18, a member of the AP1/SQUA subfamily of MADS box genes. Plant Physiol. 135, 2207–19 (2004).
Sridhar, V. V., Surendrarao, A. & Liu, Z. APETALA1 and SEPALLATA3 interact with SEUSS to mediate transcription repression during flower development. Development 133, 3159–3166 (2006).
Kim, S. et al. Expression of floral MADS-box genes in basal angiosperms: Implications for the evolution of floral regulators. Plant J. 43, 724–744 (2005).
Yanofsky, M. F. et al. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39 (1990).
Mizukami, Y. & Ma, H. Determination of Arabidopsis floral meristem identity by AGAMOUS. Plant Cell 9, 393–408 (1997).
Mena, M. et al. Diversification of C-function activity in maize flower development. Science (80-.). 274, 1537–1540 (1996).
Yamaguchi, T. et al. Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. Plant Cell 18, 15–28 (2006).
Kater, M. M. et al. Multiple AGAMOUS homologs from cucumber and petunia differ in their ability to induce reproductive organ fate. Plant Cell 10, 171–82 (1998).
Kyozuka, J. & Shimamoto, K. Ectopic expression of OsMADS3, a rice ortholog of AGAMOUS, caused a homeotic transformation of lodicules to stamens in transgenic rice plants. Plant Cell Physiol. 43, 130–135 (2002).
Benedito, V. A. et al. Ectopic expression of LLAG1, an AGAMOUS homologue from lily (Lilium longiflorum Thunb.) causes floral homeotic modifications in Arabidopsis. J. Exp. Bot. 55, 1391–1399 (2004).
Goodrich, J. et al. A Polycomb-group gene regulates homeotic gene expression in Arabidopsis. Nature 386, 44–51 (1997).
Causier, B. et al. Evolution in action: Following function in duplicated floral homeotic genes. Curr. Biol. 15, 1508–1512 (2005).
Fourquin, C. & Ferrándiz, C. Functional analyses of AGAMOUS family members in Nicotiana benthamiana clarify the evolution of early and late roles of C-function genes in eudicots. Plant J. 71, 990–1001 (2012).
Wang, Y. Q., Melzer, R. & Theißcen, G. Molecular interactions of orthologues of floral homeotic proteins from the gymnosperm Gnetum gnemon provide a clue to the evolutionary origin of ‘floral quartets’. Plant J. 64, 177–190 (2010).
Melzer, R. et al. DEF- and GLO-like proteins may have lost most of their interaction partners during angiosperm evolution. Annals of Botany 114, 1431–1443 (2014).
Liu, Y. et al. Virus induced gene silencing of a DEFICIENS ortholog in Nicotiana benthamiana. Plant Mol Biol 54, 701–711 (2004).
Pnueli, L. et al. The MADS box gene family in tomato: temporal expression during floral development, conserved secondary structures and homology with homeotic genes from Antirrhinum and Arabidopsis. Plant J. 1, 255–266 (1991).
de Martino, G., Pan, I., Emmanuel, E., Levy, A. & Irish, V. F. Functional analyses of two tomato APETALA3 genes demonstrate diversification in their roles in regulating floral development. Plant Cell 18, 1833–1845 (2006).
Rijpkema, A. S. et al. Analysis of the Petunia TM6 MADS box gene reveals functional divergence within the DEF/AP3 lineage. Plant Cell 18, 1819–1832 (2006).
Jack, T., Fox, G. L. & Meyerowitz, E. M. Arabidopsis homeotic gene APETALA3 ectopic expression: transcriptional and posttranscriptional regulation determine floral organ identity. Cell 76, 703–716 (1994).
Lenser, T., Theissen, G. & Dittrich, P. Developmental robustness by obligate interaction of class B floral homeotic genes and proteins. PLoS Comput. Biol. 5, e1000264 (2009).
Winter, K.-U. et al. Evolution of class B floral homeotic proteins: obligate heterodimerization originated from homodimerization. Mol. Biol. Evol. 19, 587–96 (2002).
Krizek, B. A. & Meyerowitz, E. M. The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122, 11–22 (1996).
Clough, S. J. & Bent, A. F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Edgar, R. C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Suyama, M., Torrents, D. & Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 34 (2006).
Castresana, J. Selection of Conserved Blocks from Multiple Alignments for Their Use in Phylogenetic Analysis. Mol. Biol. Evol. 17, 540–552 (2000).
Guindon, S. & Gascuel, O. A Simple, Fast, and Accurate Algorithm to Estimate Large Phylogenies by Maximum Likelihood. Syst. Biol. 52, 696–704 (2003).
Felsenstein, J. PHYLIP–Phylogeny Inference Package (Version 3.2). Cladistics (1986).
James, P., Halladay, J. & Craig, E. A. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144, 1425–36 (1996).
Gietz, R. D., Schiestl, R. H., Willems, A. R. & Woods, R. A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast 11, 355–60 (1995).
Causier, B. & Davies, B. Analysing protein-protein interactions with the yeast two-hybrid system. Plant Mol. Biol. 50, 855–70 (2002).
Chang, S. J., Puryear, J. & Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Report. 11, 113–116 (1993).
Azevedo, H., Lino-Neto, T. & Tavares, R. M. An improved method for high-quality RNA isolation from needles of adult maritime pine trees. Plant Mol. Biol. Report. 21, 333–338 (2003).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and. Methods 25, 402–408 (2001).
Marum, L., Miguel, A., Ricardo, C. P. & Miguel, C. Reference gene selection for quantitative real-time PCR normalization in quercus suber. PLoS One 7 (2012).
Honma, T. & Goto, K. Complexes of MADS-box proteins are sufficient to convert leaves into floral organs. Nature 409, 525–529 (2001).
Acknowledgements
This work was funded by FCT/COMPETE/FEDER with the project grants FCOMP-01-0124-FEDER-019461/PTDC/AGR-GPL/118508/2010. “Characterization of Reproductive Development of Quercus suber”. R.S. and MMRC were supported by FCT grants with the references SFRH/BD/84365/2012 and SFRH/BSAB/113781/2015, respectively. A special acknowledgment for the John Innes Centre Bioimaging facility and staff for their contribution to this publication and for Sara Laranjeira and Helena Silva for helping revise the manuscript.
Author information
Authors and Affiliations
Contributions
R.S. planned and performed the experiments and wrote the manuscript. M.M.R.C. planned the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sobral, R., Costa, M.M.R. Role of floral organ identity genes in the development of unisexual flowers of Quercus suber L.. Sci Rep 7, 10368 (2017). https://doi.org/10.1038/s41598-017-10732-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10732-0
This article is cited by
-
In silico and expression analyses of fasciclin-like arabinogalactan proteins reveal functional conservation during embryo and seed development
Plant Reproduction (2019)
-
Genome-wide analysis of spatiotemporal gene expression patterns during floral organ development in Brassica rapa
Molecular Genetics and Genomics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.