Abstract
Lack of CD2-associated protein (CD2AP) in mice increases podocyte apoptosis and leads to glomerulosclerosis and renal failure. We showed previously that SHIP2, a negative regulator of the PI3K/AKT signalling pathway, interacts with CD2AP. Here, we found that the expression level and activity of SHIP2 and production of reactive oxygen species (ROS) are increased in cultured CD2AP knockout (CD2AP−/−) mouse podocytes. Oxidative stress was also increased in CD2AP−/− mouse glomeruli in vivo. We found that puromycin aminonucleoside (PA), known to increase ROS production and apoptosis, increases SHIP2 activity and reduces CD2AP expression in cultured human podocytes. PDK1 and CDK2, central regulators of AKT, were downregulated in CD2AP−/− or PA-treated podocytes. Downregulation of PDK1 and CDK2, ROS generation and apoptosis were prevented by CD2AP overexpression in both models. Notably, inhibition of SHIP2 activity with a small molecule inhibitor AS1949490 ameliorated ROS production in CD2AP−/− podocytes, but, surprisingly, further reduced PDK1 expression and aggravated apoptosis. AKT- and ERK-mediated signalling was diminished and remained reduced after AS1949490 treatment in the absence of CD2AP. The data suggest that inhibition of the catalytic activity of SHIP2 is beneficial in reducing oxidative stress, but leads to deleterious increase in apoptosis in podocytes with reduced expression of CD2AP.
Similar content being viewed by others
Introduction
Podocyte (glomerular epithelial cell) injury has been shown in many forms of clinical and experimental glomerular diseases, including, for example, focal segmental glomerulosclerosis (FSGS) and diabetic nephropathy, and is a major contributor to proteinuria1. Various factors may give rise to podocyte injury, including transforming growth factor beta (TGF-β) or oxidative stress resulting from excessive production of reactive oxygen species (ROS)2,3,4. In podocytes in vitro, high glucose induces ROS production through mitochondrial pathways and NADPH oxidase, and activates proapoptotic p38 mitogen-activated protein kinase (MAPK) and caspase-3 and induces apoptosis2. In vivo, reduction of mitochondrial oxidative stress by antioxidant treatment reduces podocyte apoptosis and renal injury3. Thus, therapeutic interventions aiming to reduce oxidative stress could help in preventing podocyte loss and glomerulosclerosis.
CD2-associated protein (CD2AP) is essential for podocyte function, as mice lacking CD2AP develop glomerulosclerosis and die of renal failure at the age of six to seven weeks5. Mutations in CD2AP have also been identified in human patients with FSGS6,7,8. TGF-β expression and apoptosis of podocytes are elevated in the absence of CD2AP in mice9. In line with this, CD2AP is essential for TGF-β-induced early activation of the antiapoptotic phosphatidylinositol-3-kinase/serine/threonine kinase, protein kinase B (PI3K/AKT) pathway, and lack of CD2AP intensifies the activation of the proapoptotic p38 MAPK pathway by TGF-ß19. Heterozygosity of CD2AP in TGF-β1 transgenic mice increases podocyte apoptosis and renal dysfunction further pinpointing an important role for CD2AP in preventing podocyte apoptosis and kidney injury10. Yet, the exact molecular pathways involved in podocyte injury resulting from CD2AP-deficiency are poorly characterized, and ways to prevent the harmful process are lacking.
We found previously that CD2AP forms a complex with SH2 domain-containing inositol phosphatase 2 (SHIP2), a lipid phosphatase of the inositol 5′-phosphatase family11. SHIP2 negatively regulates the PI3K/AKT pathway by hydrolysing phosphatidylinositol (3,4,5)-trisphosphate (PI(3,4,5)P3) to phosphatidylinositol (3,4)-bisphosphate (PI(3,4)P2)12, and overexpression of SHIP2 induces podocyte apoptosis11. Interestingly, SHIP2 is also linked to oxidative stress in apoptosis. In hepatocytes, overexpression of a dominant-negative, inactive form of SHIP2 enhances AKT activation and reduces ROS generation and apoptosis induced by palmitate13. Furthermore, scavenging ROS in hepatocytes overexpressing wild-type SHIP2 ameliorates apoptosis13. These data suggest that inhibiting the activity of SHIP2 could provide a means to ameliorate also podocyte apoptosis induced by elevated oxidative stress.
Thus far the functional interrelationship of CD2AP and SHIP2 in podocytes has remained unclear. Here, we found that CD2AP-deficient podocytes, characterized by increased expression of TGF-β1 and apoptosis9, show elevated level of ROS and increased expression and activity of SHIP2. We then hypothesized that inhibition of SHIP2 activity reduces oxidative stress and apoptosis in the absence of CD2AP. Notably, we observed that inhibition of SHIP2 activity with a specific small molecule inhibitor AS1949490 reduces ROS, but contrary to our expectations, increases podocyte apoptosis in the absence of CD2AP. This suggests that inhibition of the catalytic activity of SHIP2 provides a means to reduce oxidative stress in podocytes, but aggravates rather than ameliorates podocyte injury by increasing apoptosis when CD2AP is depleted.
Results
Absence of CD2AP in mouse podocytes induces ROS production and apoptosis
To analyse whether CD2AP and SHIP2 play a role in oxidative stress-induced apoptosis in podocytes, we first analysed whether lack of CD2AP enhances ROS production. 2′, 7′-Dichlorofluorescein diacetate (DCFH-DA) fluorescent probe assay revealed that lack of CD2AP increases ROS production by 25% (Fig. 1A), and increases apoptosis by 2.8-fold compared to wild type cells (Fig. 1B) as shown by fluorescence-activated cell sorting (FACS) with annexin V and 7-AAD, labelling apoptotic and necrotic cells, respectively. Reintroducing CD2AP back to the knockout cells, even at low level, reduced apoptosis to the same level as in wild type cells (Fig. 1B,C). In line with an increase in apoptosis, we observed that threonine 308 (T308) phosphorylation of AKT was 30% lower at basal state in the absence of CD2AP compared to wild type podocytes (Fig. 1D,E. Supplemental Fig. S1A). In contrast, we did not observe any difference in the phosphorylation of serine 473 (S473) of AKT between WT and CD2AP−/− podocytes (Fig. 1F). We also observed that phosphorylation of extracellular-signal related kinase (ERK), a member of the mitogen-activated protein kinase (MAPK) family that regulates cell survival and apoptosis (reviewed in refs 14 and 15), was significantly lower in podocytes lacking CD2AP (Fig. 1G).
ROS generation and apoptosis are increased in CD2AP−/− podocytes. (A) ROS production is increased in the absence of CD2AP (CD2AP−/−) compared to wild type (WT) podocytes as observed by DCFH-DA fluorescent probe assay. Hoechst 33342 was used for normalization. (B) Flow cytometry with annexin V and 7-AAD double labelling indicates that absence of CD2AP induces apoptosis. Reintroduction of CD2AP into CD2AP−/− podocytes rescues CD2AP−/− podocytes from apoptosis. (C) Representative immunoblot of CD2AP expression in WT podocytes, and in CD2AP−/− podocytes infected with lentiviruses containing an empty vector (EV) or human CD2AP cDNA. (D,E) Immunoblotting and quantification reveals that phosphorylation of AKT on T308 (p-T308) is reduced in CD2AP−/− podocytes compared to WT podocytes. Total AKT (panAKT) is used for normalization. Full width blot of (D) is shown in Supplemental Fig. S1A. (F) In-cell Western and quantification shows no difference in the phosphorylation of AKT on S473 (p-S473) in relation to total AKT (panAKT) between WT and CD2AP−/− podocytes. Both p-S473 and panAKT were normalized to nuclear marker DRAQ5TM. (G) In-cell Western and quantification reveals that the ratio of phosphorylated ERK (p-ERK) to total ERK is significantly lower in CD2AP−/− podocytes compared to WT podocytes. Both p-ERK and total ERK were normalized to nuclear marker DRAQ5TM. The experiments were performed three times with three replicates in each experiment. The bars show the mean expression in arbitrary units (error bars STDEV). *p < 0.05, ***p < 0.001, Student’s t-test.
Inhibition of SHIP2 activity reduces ROS production in CD2AP−/− podocytes
We next defined whether CD2AP and SHIP2 are functionally interrelated, and found that the expression of SHIP2 was increased by 30% in CD2AP−/− podocytes (Fig. 2A,B). In line with this, SHIP2 activity was 26% higher in CD2AP−/− podocytes compared to wild type cells (Fig. 2C). Treatment of CD2AP−/− podocytes with AS1949490, a SHIP2-specific small molecule inhibitor16, reduced SHIP2 activity by 33% compared to wild type podocytes, and by 47% compared to CD2AP−/− podocytes without treatment (Fig. 2C). The differences detected in the activities were not due to different amounts of immunoprecipitated SHIP2 used for the malachite green phosphate assay (Figs 2D and S1B). AS1949490 also reduced ROS production in CD2AP−/− podocytes from 122% back to the level observed in wild type cells (Fig. 2E). Contrary to our expectations, inhibition of SHIP2 did not ameliorate apoptosis caused by lack of CD2AP but rather increased it by 40% (Fig. 2F).
Inhibition of SHIP2 activity reduces ROS production in CD2AP knockout podocytes but does not protect from apoptosis. (A) Representative immunoblot for SHIP2 in wild type (WT) and CD2AP−/− podocytes. Tubulin is included as a loading control. (B) Quantification of SHIP2 expression level in WT and CD2AP−/− podocytes in three replicate blots as in (A) shows an increase in SHIP2 expression. Tubulin was used for normalisation. (C) SHIP2 activity is increased in the absence of CD2AP. Treatment of CD2AP−/− podocytes with SHIP2 inhibitor AS1949490 reduces SHIP2 activity. (D) Representative immunoblot for SHIP2 of immunoprecipitations carried out with SHIP2 IgG (IP SHIP2) or goat IgG (IP IgG) as a control from 500 µg of protein lysates prepared from WT podocytes and CD2AP−/− podocytes treated or not with AS1949490. Similar immunoprecipitations were performed to enrich SHIP2 for activity assays. Full width blot is shown in Supplemental Fig. S1B. (E) Inhibition of SHIP2 prevents the increase in ROS generation induced by the absence of CD2AP, as observed by DCFH-DA fluorescent probe assay. Hoechst 33342 was used for normalization. (F) Flow cytometry with annexin V labelling indicates that AS1949490 treatment of CD2AP−/− podocytes induces apoptosis. (G) Representative immunoblot for PDK1 and CDK2 in WT and CD2AP−/− podocytes. Tubulin is included as a loading control. (H) Quantification of PDK1 and CDK2 in three replicate blots as in (G) in WT and CD2AP−/− podocytes shows a decrease in PDK1 and CDK2 expression in the absence of CD2AP. (I) SHIP2 inhibitor AS1949490 treatment reduces the expression of PDK1 in CD2AP−/− podocytes but not in WT podocytes. The experiments were performed three times with four (A–D,F–I) or 32 (E) replicates in each experiment. The bars show the mean expression in arbitrary units (error bars STDEV). *p < 0.05, ***p < 0.001, Student’s t-test.
To define the reason for this, we investigated the expression of 3-phosphoinositide-dependent protein kinase 1 (PDK1) and cyclin-dependent kinase 2 (CDK2), both shown to induce apoptosis in podocytes when knocked down17, 18. Interestingly, the expression of PDK1 was downregulated by 35% and CDK2 by 65% in CD2AP−/− podocytes when compared to wild type cells (Fig. 2G,H). Notably, AS1949490 treatment led to further downregulation of PDK1 expression in CD2AP−/− podocytes but not in wild type podocytes (Fig. 2I). Collectively, PDK1 and CDK2 are downregulated in the absence of CD2AP and may contribute to increased apoptosis. Inhibition of SHIP2 further reduces the total amount of PDK1 in the absence of CD2AP, which may aggravate apoptosis.
To further investigate the signalling pathways associated with an increase in apoptosis, we determined the activity of AKT and ERK after AS1949490 treatment. T308 phosphorylation of AKT, shown to be mediated by PDK119, was increased in both WT and CD2AP−/− podocytes after the AS1949490 treatment (Fig. 3A,B). In contrast, AS1949490 increased S473 phosphorylation of AKT only in WT podocytes but not in CD2AP−/− podocytes (Fig. 3C). As inhibition of SHIP2 increases the amount of PI(3,4,5)P3 which activates PDK119,20,21, we investigated whether inhibition of SHIP2 increases the activity of PDK1. AS1949490 treatment increased the phosphorylation of serine 241 residue of PDK1, mandatory for the activation of PDK122, in both WT and CD2AP−/− podocytes (Fig. 3D,E). Phosphorylation of ERK was increased in both cell types after AS1949490 treatment but significantly less in CD2AP−/− podocytes compared to WT podocytes (Fig. 3F).
SHIP2 inhibition diminishes activation of AKT and ERK signalling pathways in CD2AP-deficient podocytes. (A) Representative immunoblot used for quantifying T308 phosphorylation of AKT in WT and CD2AP−/− podocytes treated or not with AS1949490. (B) Quantification of three replicate immunoblots as in (A) reveals that AS1949490 treatment increases T308 phosphorylation of AKT (p-T308) in both WT and CD2AP−/− podocytes. (C) In-cell Western and quantification shows that AS1949490 treatment increases S473 phosphorylation of AKT (p-S473) in WT podocytes but not in CD2AP−/− podocytes. Phosphorylated AKT (S473) and total AKT (panAKT) were both normalized to nuclear marker DRAQ5TM. (D) Representative immunoblot used for quantifying p-PDK1 in WT and CD2AP−/− podocytes treated or not with AS1949490. (E) Quantification of three replicate immunoblots as in (D) reveals that AS1949490 treatment increases relative phosphorylation of PDK1 in both WT and CD2AP−/− podocytes. p-PDK1 and total PDK1 were normalized to tubulin before calculation of the ratio. (F) In-cell Western and quantification reveals that AS1949490 treatment increases the phosphorylation of ERK (p-ERK) in both WT and CD2AP−/− podocytes, but the response remains significantly lower in the absence of CD2AP. The experiments were performed three times with three replicates in each experiment. The bars show the mean expression in arbitrary units (error bars STDEV). *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test.
Lack of CD2AP in mice in vivo enhances ROS production in glomeruli
As lack of CD2AP increased oxidative stress in cultured podocytes (Fig. 1A), we stained kidneys of three weeks old wild type and CD2AP−/− mice for 8-hydroxyguanosine (8-OHdG), a marker of oxidative damage (Fig. 4A,B). Quantification of the signal in glomeruli revealed 3.5-fold increase in ROS generation in the absence of CD2AP (Fig. 4C).
Absence of CD2AP in mouse kidneys leads to an increase in ROS. (A) Representative image of a 3 weeks old wild type mouse kidney section stained with anti-8-OHdG IgG. (B) Representative image of a 3 weeks old CD2AP−/− mouse kidney section stained with anti-8-OHdG IgG. (C) Quantification reveals 3.5-fold increase in 8-OHdG staining in glomeruli of CD2AP−/− mice reflecting oxidative damage to DNA and RNA. Scale bar (A,B): 40 µm. In (C), the bars show the difference in arbitrary units between relative mask areas quantified with the HistoQuant module (error bars SEM). ***p < 0.001, Student’s t-test.
PA-treatment downregulates CD2AP, upregulates SHIP2 and increases ROS production and apoptosis in cultured human podocytes
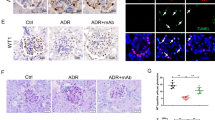
Since puromycin aminonucleoside (PA), a highly potent podocyte toxin, is known to cause podocyte injury by induction of DNA damage mediated via ROS23 and to increase apoptosis in a time- and dose-dependent manner24, we treated differentiated human podocytes in culture with PA and analysed the effect on CD2AP and SHIP2. PA-treatment of podocytes for 48 hours decreased the expression level of CD2AP by 80% (Fig. 5A,B), and increased SHIP2 expression by 34% (Fig. 5C) and its phosphatase activity by 45% (Fig. 5D). PA increased ROS generation by 60% as shown by DCFH-DA fluorescent probe assay (Fig. 5E). FACS analysis with annexin V and 7-AAD double labelling indicated that the level of podocyte apoptosis was increased by 5-fold after PA-treatment (Fig. 5F) confirming previous findings24.
PA-treatment decreases CD2AP expression and increases SHIP2 activity, ROS production and apoptosis in cultured human podocytes. (A) Representative immunoblot for CD2AP after PA-treatment. Tubulin is included as a loading control. (B) Quantification of CD2AP after PA-treatment in immunoblots like in (A) shows a decrease in CD2AP expression. (C) Quantification of SHIP2 expression in podocytes treated with PA by In-Cell Western shows that the expression of SHIP2 is increased by PA-treatment. DRAQ5TM was used for normalization. (D) PA-treatment increases SHIP2 activity in human podocytes. (E) PA increases ROS generation as visualized by an increase in the intensity of DCFH-DA fluorescent probe. Hoechst 33342 was used for normalization. (F) Flow cytometry of cultured human podocytes stained with annexin V and 7-AAD double labelling shows that PA-treatment increases podocyte apoptosis. The experiments were performed three times with four (B,D,F), 24 (C) or 48 (E) replicates in each experiment. The bars (B–F) show the mean expression in arbitrary units (error bars STDEV). ***p < 0.001, Student’s t-test.
SHIP2 inhibition in PA-treated cultured human podocytes does not reduce ROS production or apoptosis
We further tested whether inhibition of SHIP2 reduces ROS production and apoptosis induced by PA. We first confirmed that AS1949490 treatment prevents the increase in the activity of SHIP2 caused by PA (Fig. 6A). As expected, the activity of SHIP2 remained at the level of the control when podocytes were treated simultaneously with PA and AS1949490 for 48 hours (Fig. 6A). However, this did not prevent an increase in ROS production (Fig. 6B) or rescue podocytes from apoptosis (Fig. 6C).
Inhibition of SHIP2 activity does not reduce PA-induced ROS generation or protect cultured human podocytes from apoptosis. (A) PA increases SHIP2 activity of cultured human podocytes. Simultaneous treatment of podocytes with PA and SHIP2 inhibitor AS1949490 prevents PA-induced increase in SHIP2 activity. (B) PA induces ROS generation in cultured human podocytes as observed by DCFH-DA fluorescent probe assay. Hoechst 33342 was used for normalization. Treatment of human podocytes with SHIP2 inhibitor AS1949490 does not prevent PA-induced increase in ROS generation. (C) PA induces apoptosis in cultured human podocytes as visualized by annexin V staining and FACS analysis. Treatment of human podocytes with SHIP2 inhibitor AS1949490 does not prevent PA-induced increase in apoptosis. The experiments were performed three times with four (A), 32 (B) or three (C) replicates in each experiment. The bars show the mean expression in arbitrary units (error bars STDEV). **p < 0.01, ***p < 0.001, Student’s t-test.
CD2AP overexpression reduces ROS production and apoptosis induced by PA in cultured human podocytes
To define whether CD2AP is able to rescue podocytes from the effects of PA, including downregulation of PDK1 and CDK217, we overexpressed CD2AP simultaneously with PA-treatment of cultured human podocytes. PA-treatment reduced the expression of PDK1 by 80% and CDK2 by 40% compared to the empty vector (Fig. 7A,B), and overexpression of CD2AP significantly prevented PA-induced downregulation of both PDK1 and CDK2 (Fig. 7A,B). Overexpression of CD2AP alone had no significant effect on the expression level of any of the analysed proteins (Fig. 7A,B).
CD2AP overexpression restores PDK1 and CDK2 expression and reduces ROS generation and podocyte apoptosis induced by PA in cultured human podocytes. (A) Representative immunoblot for CD2AP, PDK1 and CDK2 in cultured human podocytes infected with lentiviruses containing an empty vector (EV) or human CD2AP cDNA (CD2AP OE), and with or without PA-treatment. Tubulin is included as a loading control. (B) Quantification of CD2AP, PDK1, and CDK2 expression level in Western blots as in (A) shows that PA-treatment reduces the expression of CD2AP, PDK1, and CDK2 and that CD2AP overexpression prevents PA-induced downregulation of CD2AP. Overexpression of CD2AP alone does not affect the expression of PDK1 or CDK2. (C) Overexpression of CD2AP prevents PA-induced increase in ROS generation as visualized by DCFH-DA fluorescent probe assay. Overexpression of CD2AP alone does not affect ROS generation. Hoechst 33342 was used for normalization. (D) Flow cytometry of cultured human podocytes stained with annexin V and 7-AAD confirms that overexpression of CD2AP protects podocytes from PA-induced apoptosis. The experiments were performed three times with three (A–B,D) or 24 (C) replicates in each experiment. The bars (b–d) show the mean expression in arbitrary units (error bars STDEV). *p < 0.05, **p < 0.01, ***p < 0.001, Student’s t-test.
We then defined whether reintroduction of CD2AP rescues podocytes from PA-induced ROS production and apoptosis. PA-treatment increased ROS production by 64% compared to empty vector-transfected podocytes (Fig. 7C), and indeed, overexpression of CD2AP simultaneously with PA-treatment significantly reduced the increase in ROS generation, leading to only 19% increase (Fig. 7C). PA-treatment increased apoptosis by 2.9-fold compared to non-treated podocytes transfected with empty vector (Fig. 7D). The apoptosis rate of PA-treated podocytes overexpressing CD2AP was increased only by 1.5-fold, which did not significantly differ from the rate of apoptosis observed in empty vector-transfected cells (Fig. 7D). Overexpression of CD2AP alone caused no significant difference in ROS generation or apoptosis compared to empty vector-transfected podocytes (Fig. 7C,D). Collectively, the data show that reintroduction of CD2AP back to podocytes treated with PA, showing downregulation of CD2AP, is able to revert an increase in ROS production and apoptosis caused by PA.
PA-treatment reduces CD2AP and increases SHIP2 expression in PA-induced nephrotic rats
To define whether PA-treatment leads to downregulation of CD2AP and upregulation of SHIP2 in vivo, we immunostained kidneys of rats with PA-induced nephrosis. The rats had significantly increased proteinuria 9 days after PA administration25, and as previously shown25, greatly diminished expression of nephrin 10 days after induction of nephrosis (Fig. 8B,E,H,K). Double immunofluorescence staining for nephrin and CD2AP or SHIP2 revealed that the expression of CD2AP appears diminished (Fig. 8A–F) and SHIP2 increased (Fig. 8G–L) in podocytes in rats with PA-induced nephrosis, confirming the data obtained in cultured cells.
CD2AP expression appears to be reduced and SHIP2 expression increased in kidneys of PA-induced nephrotic rats. (A–C) In normal rat glomerulus CD2AP is expressed in podocytes and partially co-localizes with nephrin. (D–F) In PA-induced nephrotic rats 10 days after PA administration, both CD2AP and nephrin appear weak and show patch-like accumulation of the signal. (G–I) In normal rat glomerulus weak signal for SHIP2 is observed in podocytes as visualized by double labelling for nephrin. (J–L) In PA-induced nephrotic rats 10 days after PA administration, SHIP2 staining appears in diffuse, patch-like accumulations similarly as nephrin. Scale bar: 20 µm.
Discussion
CD2AP-deficient podocytes are more prone to undergo apoptosis than wild type podocytes9. Here, we show that lack of CD2AP in podocytes induces oxidative stress, which may be one of the contributing factors leading to an increase in apoptosis. We also observed that the activity of SHIP2, an interaction partner of CD2AP11, is increased in the absence of CD2AP. Notably, we further found that inhibition of SHIP2 activity ameliorates oxidative stress caused by lack of CD2AP. However, SHIP2 inhibition alone was not able to rescue CD2AP-deficient podocytes from apoptosis.
We showed previously that CD2AP associates with SHIP211, and here we found that SHIP2 expression and activity are upregulated in CD2AP−/− podocytes. Together these data suggest that CD2AP has an inhibitory role on SHIP2. Our previous data revealed that overexpression of SHIP2 in podocytes reduces AKT activation and enhances apoptosis11, suggesting that increased expression of SHIP2 could contribute to increased apoptosis in CD2AP−/− podocytes. Elevated SHIP2 increases apoptosis also in neuronal cells in SHIP2 overexpressing mice26 and hepatocytes13. In line with the elevated activity of SHIP2 in CD2AP−/− podocytes, we observed that the activity of the AKT and ERK cell survival pathways at basal state were reduced (as visualized by reduced AKT and ERK phosphorylation) in the absence of CD2AP reflecting the increased rate of apoptosis. This is in line with earlier literature showing that CD2AP interacts with p85 subunit of PI3K and, together with podocyte proteins nephrin and podocin, stimulates the PI3K/AKT pathway27. The data are also consistent with a previous report revealing reduced base-line activation of ERK in the absence of CD2AP28. Reduced ERK activation may be due to an increase in SHIP2 expression, as a previous study showed decreased insulin-stimulated ERK activation in SHIP2 overexpressing cells12.
Interestingly, we found that lack of CD2AP reduces the expression of PDK1, a central activator of AKT19, providing a further link between CD2AP and cell survival signalling (Fig. 9). Pancreatic β cells depleted of PDK1 are more prone to undergo apoptosis29 and in line with this, knockdown of PDK1 in podocytes increases apoptosis by inhibiting antiapoptotic and stimulating proapoptotic pathways18. Here, we observed that lack of CD2AP also reduces the expression of CDK2, a cell cycle regulatory protein previously shown to activate AKT during cell cycle progression30. Notably, knockdown of CDK2 downregulates PDK1, decreases activation of AKT and increases apoptosis17. Furthermore, knockdown of PDK1 downregulates CDK2 and thus proposes a regulatory loop between the proteins17. The data suggest that PDK1 and CDK2 play central roles in CD2AP-mediated control of the AKT cell survival pathway, but the exact mechanisms leading to their downregulation in the absence of CD2AP require further studies.
Schematic model illustrating the effect of SHIP2 inhibition in CD2AP-deficient podocytes. (A) Wild type podocytes in basal state. CD2AP associates with SHIP2. CD2AP inhibits the production of ROS, and SHIP2 negatively regulates the pathways leading to AKT and ERK phosphorylation. The balance between the prosurvival and proapoptotic signals is maintained. (B) CD2AP−/− podocytes in basal state. The expression and activity of SHIP2 and generation of ROS are increased in the absence of CD2AP. Increased SHIP2 activity leads to low phosphorylation level of AKT and ERK. The expression of PDK1 is downregulated contributing to degreased phosphorylation of T308 of AKT. Decrease in prosurvival signalling manifests as increased apoptosis of the cells. (C) Inhibition of SHIP2 activity with AS1949490 in CD2AP−/− podocytes. Inhibition of the activity of SHIP2 leads to reduced generation of ROS. AS1949490 attenuates the potential of SHIP2 to negatively regulate AKT and ERK activity, yet the phosphorylation of S473 of AKT does not increase. Despite of low expression level of PDK1, the phosphorylation of T308 of AKT increases. As phosphorylation of both sites of AKT is required for its full activity, remains the balance between prosurvival and proapoptotic signalling disrupted and podocytes undergo apoptosis. It is also possible that increased ERK activation may contribute to an increase in apoptosis (see Discussion for details).
Excess generation of ROS has been shown to lead to apoptosis and progression of kidney injury31, 32. Our study shows that an increase in ROS may be one of the mediators of podocyte injury in the absence of CD2AP, as we observed increased ROS in CD2AP−/− kidneys at the age of three weeks, which coincides with an increase in podocyte apoptosis and the development of albuminuria and glomerular lesions reminiscent of FSGS9. A recent study proposed that podocytes bind plasminogen and activate it, which then evokes production of superoxide anion (a type of ROS) that further contributes to podocyte injury33. Interestingly, the authors found increased concentrations of plasminogen in the urine of CD2AP knockout mice which could lead to increased ROS in CD2AP-deficient kidneys33, in line with our findings. Resembling CD2AP-deficient podocytes, PA-treatment leads to downregulation of CD2AP and PDK1 (this study) and increases ROS generation23.
The key finding of this study is that inhibition of SHIP2 activity with a small-molecule inhibitor AS1949490 in the absence of CD2AP reduces ROS generation (Fig. 9). AS1949490 is highly specific towards SHIP2 and does not inhibit other phosphatases that regulate the PI3K-pathway such as SHIP1, a close homologue of SHIP2, or phosphatase and tensin homolog (PTEN), a lipid 3′-phosphatase16. Previous studies in hepatocytes indicated that catalytically inactive form of SHIP2 protects against palmitate or high glucose -induced excessive production of ROS and apoptosis13, 34. In the absence of CD2AP, however, inhibition of SHIP2 activity alone was not able to rescue podocytes from apoptosis, but rather increased it. This could be due to the fact that SHIP2 inhibition increased only T308 but not S473 phosphorylation of AKT in CD2AP-deficient cells (Fig. 9), which is not enough to fully activate AKT35. Phosphorylation of T308 may sound contradictory as phosphorylation of this site occurs by PDK119 and PDK1 is downregulated in the absence of CD2AP. However, as the catalytic activity of SHIP2 is to hydrolyse PI(3,4,5)P3 to PI(3,4)P2, SHIP2 inhibition is expected to lead to an increase in PI(3,4,5)P3 in cells similarly as after SHIP2 knockdown36. Increase in PI(3,4,5)P3, in turn, may lead to activation of PDK1 (as we observed here), in line with studies showing that PDK1 is activated by both PI(3,4,5)P3 and PI(3,4)P2 19,20,21, but that it binds PI(3,4,5)P3 more strongly than PI(3,4)P2 21. PI(3,4,5)P3 also binds AKT, apparently altering its conformation, and thereby enhances its binding to PDK1 contributing to an increase in T308 phosphorylation37. Furthermore, even though AS1949490 treatment slightly increased the phosphorylation of ERK in the absence of CD2AP, its activity remained lower compared to WT podocytes treated with AS1949490. In a similar fashion, a previous study showed an increase in AKT and ERK phosphorylation by AS1949490 in lymphatic endothelial cells38. Interestingly, the authors found that knockdown of SHIP2 in these cells, contrary to expectations, increased apoptosis38, resembling our finding here that SHIP2 inhibition in the absence of CD2AP increases apoptosis. As ERK activation may also trigger apoptosis14 and ERK activation has been associated with podocyte injury39, it is also possible that SHIP2 inhibition in the absence of CD2AP triggers proapoptotic pathways leading to reduced cell survival (Fig. 9).
The inability of SHIP2 inhibition to rescue CD2AP-deficient podocytes from apoptosis does not rule out the potential of SHIP2 inhibitors to reduce ROS and apoptosis in proteinuric diseases presenting normal expression levels of CD2AP. Increased levels of ROS have been observed, for example, in podocytes in diabetic kidney disease2. Our previous finding that SHIP2 is upregulated in glomeruli in diabetic rodent models11 together with this study suggests that elevated SHIP2 could contribute to an increase in ROS in podocytes in diabetes. In future studies it will be of interest to define whether inhibition of SHIP2 in diabetic kidney disease reduces the increased levels of ROS, and thereby potentially prevents or slows down the progression of the disease.
Reduced expression of CD2AP apparently associates with multiple pathways leading to podocyte injury, shown here with both CD2AP-deficient podocytes and the PA model of podocyte injury. The latter was our model of choice to be used side by side with CD2AP−/− podocytes, as the PA-induced nephrosis in rats is a commonly used model for FSGS40, and the glomerular changes observed in CD2AP knockout mice resemble those observed in human FSGS5. In addition, mutations in CD2AP, detected in a subgroup of patients with FSGS, lead to reduced expression of CD2AP6,7,8. The molecular changes, including decrease of CD2AP, PDK1 and CDK2 expression, and increase of SHIP2 activity, oxidative stress and apoptosis, were shared between the two models. In the harsh PA injury model, apparently affecting a multitude of proteins in addition to CD2AP, inhibition of SHIP2 did not rescue podocytes from excess ROS generation and apoptosis, whereas overexpression of CD2AP did. In CD2AP−/− podocytes, SHIP2 inhibition did reduce ROS but aggravated apoptosis. Resembling this, we observed previously increased activity of β-catenin in podocytes in the absence of CD2AP, but found that inhibition of the Wnt/β-catenin pathway did not ameliorate but further aggravated kidney injury41. These data reinforce the central role of CD2AP as a regulator of multiple pathways in podocytes and reveal that inhibition of the harmful pathways may have unexpected adverse effects.
Collectively, SHIP2 inhibitors may be effective in preventing excessive generation of ROS. However, this may not be a suitable approach to treat kidney diseases associated with reduced expression or lack of CD2AP. Further studies are necessary to define the applicability of SHIP2 inhibition as a part of therapy in kidney diseases presenting with oxidative stress, such as diabetic kidney disease.
Materials and Methods
Cell culture
Mouse wild type and CD2AP−/− podocytes, HEK 293FT cells (Sigma-Aldrich, St. Louis, MO) and conditionally immortalized human podocytes (AB 8/13) were maintained as decribed42. Media and foetal bovine serum (FBS) were from Sigma-Aldrich, ITS from Gibco (Paisley, UK) and ultraglutamine from Lonza (Basel, Switzerland).
Measurement of ROS production
Cells cultured in 96-well plates were treated where indicated with 10 µM AS1949490 (Tocris Biosciences, Bristol, UK), a small molecule inhibitor of SHIP216, for 24 hours. Cells were washed with phenol red-free RPMI 1640 (Sigma-Aldrich) and incubated with 50 µM 2′,7′-Dichlorofluorescein diacetate (DCFH-DA) fluorescent probe and 2 µM Hoechst 33342 (Fluka, Sigma Aldrich) (100 μl/well) at 37 °C for 60 min. After washing twice with phenol red-free RPMI 1640, the fluorescence intensity was measured at 485 nm excitation and 525 nm emission wavelength with a Fluoroskan Ascent FL lumino/fluorometer (Thermo Fisher Scientific, Waltham, MA, USA).
Induction and detection of apoptosis
Differentiated human podocytes were exposed to PA (Sigma-Aldrich, 50 µg/ml) for 48 hours. After 24 h, podocytes were treated with 10 µM AS1949490 or DMSO (diluent) as a control. CD2AP−/− and wild type podocytes were treated with 10 µM AS1949490 or DMSO for 24 h prior to detection of apoptosis. Apoptosis was detected by flow cytometry using annexin V-FITC and 7-AAD double staining with FACSAria (BD Biosciences, Franklin Lakes, NJ) or CyAn ADP (Beckman Coulter, Brea, CA). Cells positive for annexinV-FITC and negative for 7-AAD were deemed apoptotic. A total 1 × 104 cells were detected by FACS in each sample.
Immunoblotting and antibodies
Immunoblotting was performed as described previously11. Primary antibodies used were mouse monoclonal anti-8-hydroxyguanosine (8-OHdG) (15A3), rabbit polyclonal anti-CD2AP H-290, mouse monoclonal anti-CDK2 and goat anti-SHIP2 I-20 IgGs (Santa Cruz Biotechnology, Dallas, Texas, USA), rabbit polyclonal, anti-phospho-PDK1 (Ser241), anti-PDK1, anti-phospho-p44/p42 MAPK (T202/Y204), anti-p44/p42 MAPK (referred to as ERK), and anti–phospho-AKT (Thr308) IgGs, and mouse monoclonal anti-phospho-AKT (Ser473) IgG (Cell Signaling Technology, Danvers, MA), mouse monoclonal anti–Pan AKT IgG (R&D Systems, Minneapolis, MN) and mouse monoclonal anti-tubulin IgG (Sigma-Aldrich). Rabbit polyclonal anti-CD2AP IgG raised against mouse CD2AP was described previously43. Alexa Fluor 680 (Invitrogen, Carlsbad, CA, USA) and IRDye 800 (LI-COR, Lincoln, NE) donkey anti-rabbit, anti-goat or anti-mouse IgGs were used as secondary antibodies. Detection and quantification was performed with an Odyssey Infrared Imager (LI-COR). Full-length lanes of blots probed with antibodies against SHIP2, CD2AP, panAKT, p-AKT (Thr308), PDK1 and p-PDK1 (Ser241) are shown in Supplemental Fig. S2.
Lentiviral infection
CD2AP was overexpressed in podocytes by lentiviral infection. Untagged human CD2AP cDNA (OriGene Technologies, Rockville, MD, USA) was cloned into pSIN18.cppt.hEF1_p.WPRE vector44. Empty pSIN18.cppt.hEF1_p.WPRE vector was used as control. Virus production and infections were performed as previously described11.
SHIP2 activity measurement
CD2AP−/− and wild type podocytes treated with 10 µM AS1949490 for 24 h when indicated were lysed with 1% NP-40, 20 mM Tris-HCl pH 7.5, 150 mM NaCl supplemented with 10 µM AS1949490. SHIP2 was immunoprecipitated from 500–700 µg of lysate with 5 µg of SHIP2 I-20 IgG (Santa Cruz Biotechnology) or 5 µg of purified goat IgG (Invitrogen) as a control. Protein complexes were harvested using protein G sepharose beads (Invitrogen). Beads were washed three times with malachite green assay buffer (10 mM HEPES pH 7.5, 250 mM sucrose, 6 mM MgCl2, 0,25 mM EDTA, 0,1% CHAPS) supplemented with 10 µM AS1949490. The phosphatase activity of SHIP2 was measured in malachite green assay buffer containing 100 µM PI(3,4,5)P3 (Echelon Biosciences, Salt Lake City, UT), 10 µM AS1949490 and 5 µl of immunoreaction beads to initiate the breakdown of PI(3,4,5)P3 to PI(3,4)P2, with 50 µl total reaction volume. Samples were incubated at RT for 20 min. 100 µl of Biomol Green (Enzo Life Sciences, Farmingdale, NY) was added followed by 25 min incubation at RT to stop the enzymatic reaction. Absorbance at 630 nm was measured using Fluoroskan Ascent FL (Thermo Fisher Scientific) to detect free phosphate concentration.
In-Cell Western
In-Cell Western was performed as previously described18. Shortly, after permeabilization cells were incubated with goat anti-SHIP2 I-20, rabbit anti-phospho-AKT (Thr308), mouse anti-phospho-AKT (S473), rabbit anti-Pan AKT, anti-phospho-p44/p42 MAPK (T202/Y204) or anti-p44/p42 MAPK IgGs at RT for 1 h, followed by IRDye 800 donkey anti-goat IgG (LI-COR) and 1 µM DRAQ5TM (Thermo Fisher Scientific) at RT for 1 h. Detection and quantification was performed with an Odyssey Infrared Imager (LI-COR). The signal for each protein was normalized with DRAQ5TM.
Immunohistochemistry
Generation of the CD2AP−/− knockout mouse model has been described earlier5. Kidney samples of 3 weeks old wild type and CD2AP−/− mice were embedded in Tissue-Tek® O.C.T. Compound (Sakura, Leiden the Netherlands) and fixed with 2% paraformaldehyde (PFA) after sectioning. Sections were stained with anti-8-OHdG (15A3) with Mouse on Mouse Polymer IHC Kit (Abcam, Cambridge, UK). Slides were counterstained with haematoxylin and digitally scanned with 3DHistech Pannoramic 250 FLASH II (3DHISTECH Ltd., Budapest, Hungary). Quantification of the signal in glomeruli (n = 50–100 glomeruli per kidney) of three wild type and four CD2AP−/− mouse kidneys was performed with HistoQuant module (3DHISTECH Ltd.).
Induction of nephrosis
PA-nephrosis was induced to young male Sprague-Dawley rats weighing 250–280 g by a single intraperitoneal injection of PA (15 mg/100 g, Sigma-Aldrich) in 0.9% saline. Control rats were injected with 0.9% saline. Animals had access to standard chow and water ad libitum. Urine was collected in metabolic cages 0, 3, 6 and 9 days after PA/saline injections. Level of albuminuria was measured by nephelometry (Behring Nephelometer 100 analyzer, Behringwerke AG, Marburg, Germany). The nephelometry results are published earlier25. Animals treated with PA or saline were sacrificed 10 days after PA injection (n = 3 per group). The National Animal Experiment Board approved the protocols, and all animal experiments were performed according to the approved guidelines.
Immunofluorescence microscopy
Rat kidneys were snap-frozen and embedded in Tissue-Tek® O.C.T. Compound (Sakura). Cryosections were fixed with 3.5% PFA, permeabilized with 0.1% Triton X-100 in phosphate-buffered saline (PBS) at RT for 15 min and blocked with CAS-block (Invitrogen). Sections were incubated with rabbit anti-CD2AP (Santa Cruz Biotechnology), quinea pig anti-nephrin IgGs (Sigma Aldrich) and goat anti-SHIP2 I-20 IgGs (Santa Cruz Biotechnology) overnight at +4 °C in ChemMate (Dako Cytomation, Glostrup, Denmark), followed by Alexa Fluor 555 donkey anti-rabbit, Alexa Fluor 488 donkey anti-quinea pig and Alexa Fluor 555 donkey anti-goat IgGs (Invitrogen) together with Hoechst (Fluka, Sigma Aldrich) for 1 h in ChemMate (Dako Cytomation). Samples were mounted in Mowiol and examined with Leica SP2 confocal microscope (Leica Microsystems CMS GmbH, Mannheim, Germany).
Statistical methods
All cell culture experiments were carried out at least three times. All variables were presented as mean ± SD or ±SEM. The significance of differences between groups was determined by ANOVA or Students t-test (GraphPadPrism6, GraphPad Software Inc, La Jolla, CA, USA), and p-values of less than 0.05 were considered statistically significant.
Data availability
No datasets were generated or analysed in the current study.
References
P. M. & Reiser, J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 77, 571–580 (2010).
Susztak, K., Raff, A. C., Schiffer, M. & Böttinger, E. P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55, 225–233 (2006).
Daehn, I. et al. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J. Clin. Invest. 124, 1608–1621 (2014).
Schiffer, M. et al. Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Invest. 108, 807–816 (2001).
Shih, N.-Y. et al. Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286, 312–315 (1999).
Gigante, M. et al. CD2AP mutations are associated with sporadic nephrotic syndrome and focal segmental glomerulosclerosis (FSGS). Nephrol. Dial. Transplant. 24, 1858–1864 (2009).
Kim, J. M. et al. CD2-associated protein haploinsufficiency is linked to glomerular disease susceptibility. Science 300, 1298–1300 (2003).
Löwik, M. M. et al. Focal segmental glomerulosclerosis in a patient homozygous for a CD2AO mutation. Kidney Int. 72, 1198–1203 (2007).
Schiffer, M., Mundel, P., Shaw, A. S. & Böttinger, E. P. A novel role for the adaptor molecule CD2-associated protein in transforming growth factor-β-induced apoptosis. J. Biol. Chem. 279, 37004–37012 (2004).
Xavier, S. et al. TßRI independently activated Smad- and CD2AP-dependent pathways in podocytes. J. Am. Soc. Nephrol. 20, 2127–2137 (2009).
Hyvönen, M. E. et al. Lipid phosphatase SHIP2 downregulates insulin signalling in podocytes. Mol. Cell. Endocrinol. 328, 70–79 (2010).
Ishihara, H. et al. Molecular cloning of rat SH2-containing inositol phosphatase 2 (SHIP2) and its role in the regulation of insulin signaling. Biochem. Biophys. Res. Commun. 260, 265–272 (1999).
Gorgani-Firuzjaee, S., Adeli, K. & Meshkani, R. Inhibition of SH2-domain-containing inositol 5-phosphatase (SHIP2) ameliorates palmitate induced-apoptosis through regulating Akt/FOXO1 pathway and ROS production in HepG2 cells. Biochem. Biophys. Res. Commun. 464, 441–446 (2015).
Cagnol, S. & Chambard, J.-C. ERK and cell death: Mechanisms of ERK-induced cell death - apoptosis, autophagy and senescence. FEBS J 277, 2–21 (2009).
Lu, Z. & Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 58, 621–631 (2006).
Suwa, A. et al. Discovery and functional characterization of a novel small molecule inhibitor of the intracellular phosphatase, SHIP2. British J. Pharmacol. 158, 879–887 (2009).
Saurus, P. et al. Cyclin-dependent kinase 2 protects podocytes from apoptosis. Sci. Rep. 6, 21664 (2016).
Saurus, P. et al. Podocyte apoptosis is prevented by blocking the Toll-like receptor pathway. Cell Death Dis. 6, e1752 (2015).
Alessi, D. R. et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 (1997).
Alessi, D. R. et al. 3´-phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7, 776–789 (1997).
Currie, R. A. et al. Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337, 575–583 (1999).
Casamayor, A., Morrice, N. A. & Alessi, D. R. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem. J. 342, 287–292 (1999).
Marshall, C. B., Pippin, J. W., Krofft, R. D. & Shankland, S. J. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 70, 1962–1973 (2006).
Sanwal, V. et al. Puromycin aminonucleoside induces glomerular epithelial cell apoptosis. Exp. Mol. Pathol. 70, 54–64 (2001).
Luimula, P. et al. Nephrin in experimental glomerular disease. Kidney Int. 58, 1461–1468 (2000).
Soeda, Y. et al. The inositol phosphatase SHIP2 negatively regulates insulin/IGF-I actions implicated in neuroprptection and memory function in mouse brain. Mol. Endocrinol. 24, 1965–1977 (2010).
Huber, T. B. et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol. Cell. Biol. 23, 4917–4928 (2003).
Tossidou, I. et al. CD2AP/CIN85 balance determines receptor tyrosine kinase signaling response in podocytes. J. Biol. Chem. 282, 7457–7464 (2007).
Hashimoto, N. et al. Ablation of PDK1 in pancreatic β cells induces diabetes as a result of loss of β cell mass. Nature Gen. 38, 589–593 (2006).
Liu, P. et al. Cell-cycle-regulated activation of Akt kinase by phosphorylation at its carboxyl terminus. Nature 508, 541–548 (2014).
Inagi, R. Endoplasmic reticulum stress as a progression factor for kidney injury. Curr. Opin. Pharmacol. 10, 156–165 (2010).
Kawakami, T. et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J. Am. Soc. Nephrol. 26, 1040–1052 (2015).
Raij, L., Tian, R., Wong, J. S., He, J. C. & Campbell, K. N. Podocyte injury: the role of proteinuria, urinary plasminogen, and oxidative stress. Am. J. Physiol. Renal Physiol. 311, F1308–F1317 (2016).
Gorgani-Firuzjaee, S. & Meshkani, R. SH2 domain-containing inositol 5-phosphatase (SHIP2) inhibition ameliorates high glucose-induced de-novo lipogenesis and VLDL production through regulating AMPK/mTOR/SREBP1 pathway and ROS production in HepG2 cells. Free Radic. Biol. Med. 89, 679–689 (2015).
Alessi, D. R. et al. Mechanism of activation of protein kinase B by insuin and IGF-1. EMBO J. 15, 6541–6551 (1996).
Zhuang, G., Hunter, S., Hwang, Y. & Chen, J. Regulation of EphA2 receptor endocytosis by SHIP2 lipid phosphatase via phosphatidylinositol 3-kinase-dependent Rac1 activation. J. Biol. Chem. 282, 2683–2694 (2007).
Stokoe, D. et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 (1997).
Agollah, G. A. et al. Evidence for SH2 domain-containing 5′-inositol phosphatase-1 (SHIP2) contributing to a lymphatic dysfunction. PloS ONE 9, e112548 (2014).
Liu, S., Ding, J., Fan, Q. & Zhang, H. The activation of extracellular signal-regulated kinase is responsible for podocyte injury. Mol. Biol. Rep. 37, 2477–2484 (2010).
Pippin, J. W. et al. Inducible rodent models of acquired podocyte diseases. Am. J. Physiol. Renal Physiol. 296, F213–F229 (2009).
Kuusela, S., Wang, H., Wasik, A. A., Suleiman, H. & Lehtonen, S. Tankyrase inhibition aggravates kidney injury in the absence of CD2AP. Cell Death Dis 7, e2302, doi:10.1038/cddis.2016.2217 (2016).
Wasik, A. A. et al. Septin 7 forms a complex with CD2AP and nephrin and regulates glucose transporter trafficking. Mol. Biol. Cell 23, 3370–3379 (2012).
Lehtonen, S. et al. In vivo interaction of the adapter protein CD2-associated protein with the type 2 polycystic kidney disease protein, polycystin-2. J. Biol. Chem. 275, 32888–32893 (2000).
Gropp, M. et al. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol. Ther. 7, 281–287 (2003).
Acknowledgements
We thank Leena Saikko, Biomedicum Imaging Unit and Biomedicum FACS Core Facility (University of Helsinki, Helsinki, Finland) for excellent technical assistance and Vincent Dumont (University of Helsinki, Helsinki, Finland) for critical reading of the manuscript. Dr. Andrey S. Shaw (Washington University School of Medicine, St. Louis, MO, USA) is thanked for the CD2AP−/− podocyte cell line and CD2AP−/− knockout kidney samples, and Dr. Moin Saleem (University of Bristol, Bristol Royal Hospital for Children, Bristol, UK) for the human podocyte cell line AB 8/13. This work was supported by the European Research Council (242820; S.Le.), the Academy of Finland (131255, 218021, 255551; S.Le.), the Jane and Aatos Erkko Foundation (S.Le.), the European Foundation for the Study of Diabetes/Boehringer Ingelheim Research programme (S.Le.), the Sigrid Jusélius Foundation (S.Le.), the Päivikki and Sakari Sohlberg Foundation (S.Le.), the Diabetes Research Foundation (S.Le., P.S., T.A.T.), the Finnish Society of Sciences and Letters (P.S.), the Finnish Kidney Research Foundation (S.Li.), the Finnish-Norwegian Medical Research Foundation (S.Li.), the Faculty of Medicine, University of Helsinki (S.Le.) and the Doctoral Programme in Biomedicine (P.S., T.A.T.).
Author information
Authors and Affiliations
Contributions
P.S., T.A.T., S.Li. and S.K. performed experiments and analysed data. H.H., E.L. and S.Le. participated in interpreting the results. P.S., T.A.T. and S.Le. wrote the main manuscript and P.S., T.A.T. and S.Li. prepared the figures. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saurus, P., Tolvanen, T.A., Lindfors, S. et al. Inhibition of SHIP2 in CD2AP-deficient podocytes ameliorates reactive oxygen species generation but aggravates apoptosis. Sci Rep 7, 10731 (2017). https://doi.org/10.1038/s41598-017-10512-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10512-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.