Abstract
To estimate the prevalence of hyperuricemia and its major risk factors among Chinese preschool children. A cross-sectional survey was carried out in six central urban districts of Tianjin from March to June 2015. A cluster sampling was employed to obtain a random sample of preschool children. A total of 4073 children aged from 3 to 6 years participated in this survey. Logistic regression was used to obtain odds ratios and 95% confidence intervals. The overall mean serum uric acid concentrations was 243.0 ± 53.2 μmol/L, corresponding to a mean concentrations of 247.3 ± 53.7 μmol/L among boys and 238.3 ± 52.4 μmol/L among girls. The overall prevalence of serum uric acid ≥ 310 μmol/L among children was 10.1%. Boys, obesity, diastolic blood pressure, and serum triglyceride concentrations were associated with the increased risk of hyperuricemia in univariable models, although the statistically significant association between diastolic blood pressure, serum triglyceride concentrations and hyperuricemia disappeared in multivariable models. The prevalence of hyperuricemia among children aged from 3 to 6 years was relatively high. Several metabolic syndrome components were associated with the risk of hyperuricemia.
Similar content being viewed by others
Introduction
Serum uric acid and the prevalence of hyperuricemia in adults have been rising over the past decades1,2,3. The overall prevalence of hyperuricemia among United States (US) adults has increased from 18.2% in 1988–1994 to 21.4% in 2007–20083. A few studies showed that hyperuricemia in adults was associated with metabolic syndrome and its components, including type 2diabetes and coronary artery disease3,4,5,6,7,8. The US National Health and Nutrition Examination Survey (NHANES) of 1988–1994 found that the prevalence of metabolic syndrome increased substantially with increasing serum uric acid levels3. Meanwhile, some studies showed that the indices of blood pressure (BP), triglyceride, and obesity were important risk factors of serum uric acid7, 8.
Some studies also reported the association between the elevated serum uric acid level and its risk for metabolic syndrome and its components in school age children population. Recently, a cross-sectional prevalence study indicated that hyperuricemia among 148 obese or overweight adolescents was strongly associated with metabolic syndrome9. Another cross-sectional analysis of 1370 US children and adolescents aged 12 to 17 years also found that serum uric acid concentrations were strongly associated with the prevalence of the metabolic syndrome and several of its components10. However, to date, studies on the prevalence of hyperuricemia in preschool children are lacking and the relationship between hyperuricemia in preschool children and the components of metabolic syndrome is unknown. The aim of the present study was to estimate the prevalence of hyperuricemia and its related risk indicators among the preschool children in urban districts of Tianjin, China.
Results
We recruited 4073 children between 3 and 6 years old with an average age of 5.6 ± 0.8 years old and an average BMI of 15.9 ± 2.2 kg/m2, 9.4% were overweight and 12.9% were obese.
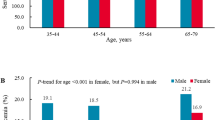
The overall mean serum uric acid concentration was 243.0 ± 53.2 μmol/L. The averages of the data studied were: systolic blood pressure: 97.8 ± 10.9 mmHg, diastolic blood pressure: 59.5 ± 8.4 mmHg, fasting glucose: 4.87 ± 0.37 mmol/L, total cholesterol 4.28 ± 0.70 mmol/L, triglycerides: 0.75 ± 0.33 mmol/L. 2.5% had an impaired fasting glucose, and 9.6% and 1.4% were diagnosed with high total cholesterol and high triglycerides, respectively (Table 1). There were no significantly differences on education levels (mother or father) and family income between children with normal serum uric acid (<310 μmol/L) and higher serum uric acid (≥310 μmol/L). The history of gout of child’s mother and father were about 0.05% (2/4059) and 1% (40/4073), respectively, and there was also no significantly difference between children with normal serum uric acid (<310 μmol/L) and higher serum uric acid (≥310 μmol/L). The distributions of uric acid levels by age, sex and BMI are summarized in Table 2. The mean level of uric acid in boys (247.3 ± 53.7 μmol/L) was significantly higher than that in girls (238.3 ± 52.4 μmol/L), especially among those children aged more than 5 years old. Compared with children with normal weight, children with overweight or obesity had a higher concentrate of serum uric acid in both boys and girls.
The prevalence of hyperuricemia among children in different subgroups is shown in Table 3. The overall prevalence of hyperuricemia among preschool children was 10.1%. The prevalence of hyperuricemia among boys (11.8%) was significantly higher than that among girls (8.3%) (P < 0.001). Compared with the prevalence of hyperuricemia among children with normal weight (8.7%), the prevalence increased among children with overweight (9.9%) and obesity (18.9%) (P for trend <0.001). Children with higher diastolic BP or higher serum triglyceride concentrations (≥1.70 mmol/L) had higher prevalence of hyperuricemia (P < 0.05).
Table 4 shows the association between the potential risk factors and hyperuricemia risk. Boys (OR: 1.47, 95% CI: 1.19–1.81), obesity (OR: 2.43, 95% CI: 1.89–3.13), higher diastolic BP (OR: 1.54, 95% CI: 1.04–2.28), and higher serum triglyceride concentrations (OR: 2.67, 95% CI: 1.43–5.00) were positively associated with the risk of hyperuricemia risk in univariable analysis model (model 1). After adjustment for all potential influencing factors, boys and obesity remained significantly associated with the risk of hyperuricemia risk (model 2).
Discussion
In this study in Tianjin, China, the prevalence of hyperuricemia (≥310 μmol/L (5.2 mg/dL)) in preschool children was 10.1%. Our results have shown that BMI and triglycerides were the most significant contributing factors to hyperuricemia prevalence, which was in accordance with the previous study11.
Most of previous studies focused on the prevalence of hyperuricemia among school age children. A cross-sectional analysis using data from the Nation Health and Nutrition Examination Survey(NHANES)1999–2002 reported that the mean concentration of uric acid among children and adolescents aged 12 to 17 years was 301.9 μmol/L (5.1 mg/dL), and the percentage of serum uric acid ≥ 327 μmol/L (5.5 mg/dL) was 30.2%10. Another study reported that the median serum uric acid level in risperidone-naïve children and adolescents with Autism Spectrum Disorder was 5.35 mg/dL and the prevalence of hyperuricemia (>5.5 mg/dL) was 44.70%, higher than normal children and adolescent12. The mean serum uric acid level of children aged less than 7 years in our study was 243.0 μmol/L (4.1 mg/dL), which was a little higher than that of Hispanic children aged 5 years (3.9 ± 0.8 mg/dL), but lower than previous results of the NHANES study and risperidone-naïve children and adolescents with Autism Spectrum Disorder10, 12, 13. Civantos Modino, S. et al. studied the prevalence of hyperuricemia in children with overweight or obesity and analyzed the relation between metabolic syndrome and hyperuricemia9. They proposed uric acid level which most is the likely diagnosis of metabolic syndrome corresponded to 5.4 mg/dl in children9. Although our results found that serum uric of preschool children was lower than school age children, it was noteworthy that there were still 7.6% of children in our study who had serum uric acid level higher than ≥ 321 μmol/L (5.4 mg/dL).
There was no universally accepted threshold to define hyperuricemia in children and adolescents. Most of the previous studies have used the criteria of hyperuricemia for children and adolescents with their own cut-off values9, 10, 14, 15. Among children with overweight and obesity, uric acid’s level, from which a diagnosis of metabolic syndrome was most likely, corresponded to 5.4 mg/dl (area under the curve 0.66, sensitivity: 64% and specificity 62%)9. An early report14 found that a serum uric acid value > 5.5 mg/dL strongly supported the diagnosis of primary hypertension in children (sensitivity: 87% and specificity 86%). In a Japanese study15, hyperuricemia was defined as exceeding the mean plus one standard deviation based on the reference value of each age group. Namely, the cut-off values were 5.9 mg/dL for 6–8 years (both genders), 6.1 mg/dL for 9–11 years (both genders), 7.0 mg/dL (males), and 6.2 mg/dL (females) for 12–14 years. In our study, we used 90th percentile of serum uric acid value (5.2 mg/dL) in our sample as the cut-off value of hyperuricemia. In the further study, it is necessary to determine and evaluate the optimal cut-off values of hyperuricemia in preschool children in Chinese and other population.
Several studies concluded that hyperuricemia was a risk factor for the development of diseases such as obesity, hypertension, hyperlipidemia, and diabetes16, 17. In concordance with the previous report10, our study also found a significant difference in the prevalence of hyperuricemia between obesity and normal weight children. A longitudinal data from the Bogalusa Heart Study indicated that obesity in childhood was a predictor of elevated serum uric acid in adulthood18. Other studies also showed a positive association between obesity and uric acid17, 19, and some of them hypothesized that obesity is the main determinant of high serum uric acid values in the general population20. It is considered that insulin resistance caused by accumulation of fat is the underlying mechanism between obesity and hyperuricemia21. On the other hand, it has also been observed that hyperuricemia precedes the development of obesity17. Sautin et al.22 speculated uric acid contributed to the development of obesity by giving rise to inflammatory and oxidative changes in adipocytes.
Many studies showed that hyperuricemia appeared as a common component in subjects with metabolic syndrome5, 8, 10. It is known that adult patients with hyperuricemia had a higher risk of developing metabolic syndrome and this prevalence increased with elevation of plasma uric acid levels5. In the present study, elevated serum uric acid level was associated with a higher risk of higher diastolic BP and higher serum triglyceride concentrations. Several large studies reported an elevated serum uric acid could predict the development of hypertension in children14, 23. However, seldom studies reported which type of hypertension was associated with serum uric acid level. Daniel evaluated 125 children aged 6 to 18 years and concluded that uric acid levels were directly positive correlated with systolic and diastolic blood pressure14. Similarly, our results also found an association between hyperuricemia and higher diastolic blood pressure. Some studies stated the mechanism for the elevated serum uric acid level in children with primary hypertension. For example, hyperuricemia may affect arterial pressure by activating the renin-angiotensin system and reducing nitric oxide synthase activity23. Furthermore, a recent review including many clinical and experimental studies, summarized the association between serum uric acid concentration and a variety of cardiovascular and renal diseases, particularly in hypertension24. This review clearly demonstrated that an increasing serum uric acid level may be a useful biomarker of hypertension.
In our study, we also found hyperuricemia was associated with higher serum triglyceride concentrations, which was similar to previous studies25, 26. Those studies reported that hypertriglyceridemia was related to hyperuricemia independent of obesity and central body fat distribution. It is speculated that the synthesis of triglycerides needs NADPH, which results in increased uric acid production27. The biological mechanism of the relationship between serum triglyceride and uric acid is still unclear, and further studies in vitro and clinical are needed.
The major strength of our study is to report the prevalence of hyperuricemia in children aged from 3 to 6 years. To our knowledge, we are the first to examine the relationship between hyperuricemia and its risk factors, including BMI, SBP and DBP, the level of glucose, cholesterol and triglyceride in a preschool children population. Our study has certain limitations. First, we used the data from a cross-sectional survey to assess the risk indicators of hyperuricemia. Second, we cannot interpret well the causality between these risk indicators and hyperuricemia. In addition, the sample size of children aged less than 5 years was relatively small, which may influence the prevalence of hyperuricemia among little children. Further investigation on hyperuricemia and children aged less than 5 years is necessary. Third, the presence of hyperuricemia in parents was a better influencing factor than their history of gout for children hyperuricemia risk. However, in China, it is not universal for people to know clearly that they have hyperuricemia, but gout, as a kind of clinical symptoms of the disease, is easier to be known than hyperuricemia. So we investigated the history of gout in parents but not the presence of hyperuricemia in our study.
In conclusion, our findings of children aged from 3 to 6 years indicated that the prevalence of hyperuricemia among preschool age children in China was relatively high, especially in obese children. Several metabolic syndrome components were associated with the risk of hyperuricemia. Future studies in different population should be conducted to clarify the association between hyperuricemia and metabolic syndrome components among preschool age children.
Methods
Study population and settings
The city of Tianjin, located in Northern China, is the fourth largest city with a population of 14.1 million28 and is directly under the administration of the central government of China. Tianjin consists of 16 county-level administrative areas, including six central urban districts, one new urban district, four suburban districts and five rural districts. A cross-sectional survey was carried out in six central urban districts of Tianjin from March to June 2015. A cluster sampling was employed to obtain a random sample of preschool children. Three to six kindergarten schools were randomly selected from each central urban district and finally about 10% of kindergartens were included (26 kindergartens of 263). In Tianjin, children in kindergarten schools aged from 3 to 6 years old. All children (n = 7794) in selected kindergartens were invited to take part in our survey. Finally, a total of 4457 children, including 3391 (95.6% of 3547) children aged ≥ 5 years and 1066 (25.1% of 4247) children aged 3− < 5years, agreed to participate.
We sequentially excluded 24 children who did not have serum uric acid test and 360 children with missing variables required for this analysis. Finally, 4073 children were included in the analysis. The study protocols were guided by the Ethical Principles and Guidelines for the Protection of Human Subjects of Research and the study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center. An informed consent was obtained from all participants. The methods were carried out in accordance with the Declaration of Helsinki.
Measurements
A questionnaire was completed by children’s parents at home. The questionnaire included information on the child’s birth date, sex, history of illness status; current health status, and parents’ socioeconomic factors (such as occupation, education, and family income), and parents’ history of gout and current health status (such as diabetes, dyslipidemia and hyperuricemia). Children who had tuberculosis, hepatitis, chronic bronchial catarrh, asthma of the lungs, or nephritis diagnosed by a doctor were classified as having a history of illness status. Current disease status was classified as yes for participants who reported having pneumonia, cold or fever during the past 30 days.
Weight, height and BP were measured by specially-trained pediatricians using the standardized protocol at the kindergarten clinic unit. Weight was measured to the nearest 0.01 kg using a digital scale (TCS-60, Tianjin Weighing Apparatus, China). Standing height was measured to the nearest 0.1 cm using a stadiometer (SZG-180, Shanghai Zhengdahengqi, China). Body mass index (BMI) was calculated by dividing weight in kilograms (kg) by the square of height in meters (m). Z-scores for weight, height, and BMI were calculated based on the World Health Organization (WHO) child growth standards (2–5 years old) and WHO child growth reference (5–19 years old)29. In order to make the data most useful for comparison, and to contribute to the understanding of international standard definitions of overweight and obesity, we used BMI to classify each child as “normal,” “overweight,” or “obesity”. Normal weight was defined as a BMI less than the 85th percentiles for age and gender using the WHO growth reference (<1035 Z score), overweight and obesity was defined as a BMI above the 85th percentile (≥1.035 Z score), and obesity was defined as a BMI above the 95th percentile (≥1.645 Z score)29. Sitting BP was measured from the right arm using automated sphygmomanometers (OMRON HBP-1300)30 after at least 5 minutes’ rest. The fifth Korotkoff sound was adopted for diastolic BP recording. Mean BP was calculated from 3 readings. Hypertension was defined based on the criteria recommended for Chinese children, i.e., systolic or diastolic BP ≥ 95th percentile for age and sex31.
All children provided a 0.5 mL peripheral blood sample from the ring finger after at least 8 hours of fasting at the kindergarten clinic unit32. The blood samples were immediately centrifuged and processed with an automatic device. Serum levels of total cholesterol, triglycerides, glucose and uric acid were measured enzymatically using an automatic analyzer (RX Daytona; Randox Laboratories Ltd, Antrim, Ireland)33. Due to the lack of practical guideline for hyperuricemia in preschool children, hyperuricemia was defined as 310 μmol/L (5.2 mg/dL, 90th percentile in our sample) or higher. Hyperglycemia was defined as a fasting glucose level of 5.6 mmol/L or higher34. High total cholesterol and high triglycerides was defined as 5.18 mmol/L or higher and 1.70 mmol/L or higher, respectively35.
Statistical analysis
As for the statistical analysis, quantitative variables were expressed as mean ± standard deviation and the quantitative variables as frequencies. Since the sample corresponds to a normal distribution, the One-way analysis of variance (ANOVA) was used to detect the difference among groups. To compare the difference of general characteristics between hyperuricemia and normal-level children, Chi-square test was used for categorical variables. Binary logistic regression was used to assess the associations between hyperuricemia and the related risk factors in univariable and multivariable analyses. The related risk factors included children’s age, sex, BMI, BP, serum levels of total cholesterol, triglycerides and glucose, gout history of parents, education of parents, family income and health history of parents. All statistical analyses were performed with PASW for Windows, version 20.0 (Statistics 20, SPSS, IBM, Armonk, NY). A p value of less than 0.05 for a two-tailed test was considered to be statistically significant.
References
Chang, H. Y., Pan, W. H., Yeh, W. T. & Tsai, K. S. Hyperuricemia and gout in Taiwan: results from the Nutritional and Health Survey in Taiwan (1993-96). J Rheumatol 28, 1640–1646 (2001).
Rathmann, W., Haastert, B., Icks, A., Giani, G. & Roseman, J. M. Ten-year change in serum uric acid and its relation to changes in other metabolic risk factors in young black and white adults: the CARDIA study. Eur J Epidemiol 22, 439–445, doi:10.1007/s10654-007-9132-3 (2007).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum 63, 3136–3141, doi:10.1002/art.30520 (2011).
Choi, H. K. & Curhan, G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 116, 894–900, doi:10.1161/CIRCULATIONAHA.107.703389 (2007).
Choi, H. K. & Ford, E. S. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 120, 442–447, doi:10.1016/j.amjmed.2006.06.040 (2007).
Kim, S. Y. et al. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res (Hoboken) 62, 170–180, doi:10.1002/acr.20065 (2010).
Guan, S. et al. Prevalence of hyperuricemia among Beijing post-menopausal women in 10 years. Arch Gerontol Geriatr 64, 162–166, doi:10.1016/j.archger.2016.02.002 (2016).
Wang, B., Lin, L. & Zhao, C. Related factors of serum uric acid in patients with primary hypertension and hyperhomocysteinemia. Clin Exp Hypertens, 1-5, doi:10.3109/10641963.2015.1107088 (2016).
Civantos Modino, S. et al. Hyperuricemia and metabolic syndrome in children with overweight and obesity. Endocrinol Nutr 59, 533–538, doi:10.1016/j.endonu.2012.06.010 (2012).
Ford, E. S., Li, C., Cook, S. & Choi, H. K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 115, 2526–2532, doi:10.1161/CIRCULATIONAHA.106.657627 (2007).
Qiu, L. et al. Prevalence of hyperuricemia and its related risk factors in healthy adults from Northern and Northeastern Chinese provinces. BMC Public Health 13, 664, doi:10.1186/1471-2458-13-664 (2013).
Vanwong, N. et al. Hyperuricemia in Children and Adolescents with Autism Spectrum Disorder Treated with Risperidone: The Risk Factors for Metabolic Adverse Effects. Frontiers in pharmacology 7, 527, doi:10.3389/fphar.2016.00527 (2016).
Lurbe, E., Garcia-Vicent, C., Torro, M. I., Aguilar, F. & Redon, J. Associations of birth weight and postnatal weight gain with cardiometabolic risk parameters at 5 years of age. Hypertension 63, 1326–1332, doi:10.1161/HYPERTENSIONAHA.114.03137 (2014).
Feig, D. I. & Johnson, R. J. Hyperuricemia in childhood primary hypertension. Hypertension 42, 247–252, doi:10.1161/01.HYP.0000085858.66548.59 (2003).
Tang, L., Kubota, M., Nagai, A., Mamemoto, K. & Tokuda, M. Hyperuricemia in obese children and adolescents: the relationship with metabolic syndrome. Pediatr Rep 2, e12, doi:10.4081/pr.2010.e12 (2010).
Kodama, S. et al. Association between serum uric acid and development of type 2 diabetes. Diabetes Care 32, 1737–1742, doi:10.2337/dc09-0288 (2009).
Ishizaka, N. et al. Changes in waist circumference and body mass index in relation to changes in serum uric acid in Japanese individuals. J Rheumatol 37, 410–416, doi:10.3899/jrheum.090736 (2010).
Muntner, P. et al. Impact of childhood metabolic syndrome components on the risk of elevated uric acid in adulthood: the Bogalusa Heart Study. Am J Med Sci 335, 332–337, doi:10.1097/MAJ.0b013e31815574a4 (2008).
Abdullah, A. R., Hasan, H. A. & Raigangar, V. L. Analysis of the relationship of leptin, high-sensitivity C-reactive protein, adiponectin, insulin, and uric acid to metabolic syndrome in lean, overweight, and obese young females. Metab Syndr Relat Disord 7, 17–22, doi:10.1089/met.2008.0045 (2009).
Bos, M. J., Koudstaal, P. J., Hofman, A., Witteman, J. C. & Breteler, M. M. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 37, 1503–1507, doi:10.1161/01.STR.0000221716.55088.d4 (2006).
Hikita, M. et al. Relationship between hyperuricemia and body fat distribution. Intern Med 46, 1353–1358 (2007).
Sautin, Y. Y., Nakagawa, T., Zharikov, S. & Johnson, R. J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. American journal of physiology. Cell physiology 293, C584–596, doi:10.1152/ajpcell.00600.2006 (2007).
Khosla, U. M. et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 67, 1739–1742, doi:10.1111/j.1523-1755.2005.00273.x (2005).
Susic, D. & Frohlich, E. D. Hyperuricemia: A Biomarker of Renal Hemodynamic Impairment. Cardiorenal Med 5, 175–182, doi:10.1159/000381317 (2015).
Skak-Nielsen, H. et al. Uric acid as a risk factor for cardiovascular disease and mortality in overweight/obese individuals. PLoS One 8, e59121, doi:10.1371/journal.pone.0059121 (2013).
Su, P., Hong, L., Zhao, Y., Sun, H. & Li, L. Relationship Between Hyperuricemia and Cardiovascular Disease Risk Factors in a Chinese Population: A Cross-Sectional Study. Medical science monitor: international medical journal of experimental and clinical research 21, 2707–2717, doi:10.12659/MSM.895448 (2015).
Wun, Y. T., Chan, C. S. & Lui, C. S. Hyperuricaemia in Type 2 diabetes mellitus. Diabetes, nutrition & metabolism 12, 286–291 (1999).
Xiao, Y. et al. Trends in the Prevalence of Overweight and Obesity among Chinese Preschool Children from 2006 to 2014. PLoS One 10, e0134466, doi:10.1371/journal.pone.0134466 (2015).
Mercedes de Onis. et al. The WHO Multicentre Growth Reference Study Group. The WHO Child Growth Standards. Available: http://www.who.int/childgrowth/standards/en/ (2006).
Takahashi, H., Yokoi, T. & Yoshika, M. Validation of the OMRON HBP-1300 upper arm blood pressure monitor, in oscillometry mode, for clinic use in a general population, according to the European Society of Hypertension International Protocol revision 2010. Available from: http://www.dableducational.org/Publications/2014/ESH-IP 2010 Validation of Omron HBP-1300.pdf (2014).
Mi, J. et al. Development of blood pressure reference standards for Chinese children and adolescents. Clin J Evld Based Pediatr 5, 4–14 (2010).
Krleza, J. L., Dorotic, A., Grzunov, A. & Maradin, M. Capillary blood sampling: national recommendations on behalf of the Croatian Society of Medical Biochemistry and Laboratory Medicine. Biochem Med (Zagreb) 25, 335–358, doi:10.11613/BM.2015.034 (2015).
Tian, Z. et al. Sleep duration and hyperglycemia among obese and nonobese children aged 3 to 6 years. Archives of pediatrics & adolescent medicine 164, 46–52, doi:10.1001/archpediatrics.2009.233 (2010).
Diagnosis and classification of diabetes mellitus. Diabetes Care 32 Suppl 1, S62-67, doi:10.2337/dc09–S062 (2009).
Special Committee for Children of China Practitioner’s Association.Expert: control of hyperlipid in children and adolescents in China. Chin J Pediatr 47, 426–428, doi:10.3760/cma.j.issn.0578-1310.2009.06.007 (2009).
Acknowledgements
This project was supported by Tianjin Public Health Bureau of Science and Technology Fund (Grant number: 14KG130).
Author information
Authors and Affiliations
Contributions
N.L. designed the study, acquired the data, performed the statistical analysis, interpreted the data, drafted the article and approved the final version to be published. J.L. designed the study, acquired the data, reviewed and critically revised the article and approved the final version to be published. N.L., S.Z., W.L., L.W., H.L., W.L., T.Z., G.L., and Y.D. performed the field work and critically revised the article, and approved the final version to be published. J.L. was responsible for the integrity of the work as a whole.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, N., Zhang, S., Li, W. et al. Prevalence of hyperuricemia and its related risk factors among preschool children from China. Sci Rep 7, 9448 (2017). https://doi.org/10.1038/s41598-017-10120-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10120-8
This article is cited by
-
Associations of overweight and obesity with cardiometabolic risk factor clusters among Korean adolescents
Scientific Reports (2024)
-
Distribution of serum uric acid concentration and its association with lipid profiles: a single-center retrospective study in children aged 3 to 12 years with adenoid and tonsillar hypertrophy
Lipids in Health and Disease (2023)
-
Associations between body mass index in different childhood age periods and hyperuricemia in young adulthood: the China Health and Nutrition Survey cohort study
World Journal of Pediatrics (2022)
-
Metabolic Syndrome in Children and Adolescents: Looking to New Markers
Current Treatment Options in Pediatrics (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.