Abstract
We compared the performance of the Roche Diagnostics Elecsys immunoassay for the detection of Treponema pallidum specific antibodies in patient serum samples with that of the Abbott Laboratories Architect chemiluminescent microparticle immunoassay and the InTec and KHB enzyme-linked immunosorbent assays, which are commonly used in China. We tested 13,767 serum samples collected from 13 independent laboratories throughout China, which included samples from 999 previously confirmed syphilis cases and 158 ‘borderline’ samples previously identified using the Architect, InTec, and KHB tests. The Mikrogen Syphilis Immunoblot was used to confirm positive test results. The consistency between the four different assays was 100%. The sensitivity of Elecsys immunoassay was 100% versus 98.26% for Architect, 99.11% for InTec; and 98.56% for KHB. The specificity of the Elecsys immunoassay was 99.81% versus 99.74% for Architect; 99.93% versus 99.80% for InTec; and 99.85% versus 99.77% for KHB. For borderline samples, the Elecsys immunoassay yielded no false-negative results and fewer false-positive results, compared to the other tests. Considering the ease-of-use, automation, high speed, and high throughput capacity of the Elecsys assay, the higher sensitivity and specificity indicate it is superior for routine screening of serum samples for syphilis diagnosis.
Similar content being viewed by others
Introduction
In 2008, China had 278,215 officially reported cases of syphilis, which represented a three-fold increase, compared to the prevalence of syphilis in 2004, and an approximate ten-fold increase over the prevalence reported in the 1990s1,2,3. Guangdong Province, the largest province in southern China, reported more syphilis cases in 2008 than all of the countries in the European Union combined. Guangzhou City, the provincial economic center, was most affected, where 43.4 latent cases per 100,000 were reported in 2013 for all patient groups aged 20 years and older4 and 31 cases per 100,000 were reported the following year5. The control of Treponema pallidum transmission and infection is, therefore, a public health priority in China4.
Factors affecting the immunogenicity of T. pallidum include the relative scarcity of outer membrane proteins and antigenic variation of the putative surface-exposed TprK protein6. However, syphilis can be successfully treated, and early diagnosis is crucial to prevent transmission and avoiding delays in treatment7, 8. Testing for syphilis is common in antenatal care, screening of blood and organ donors, and surveillance of sexually transmitted infections8,9,10,11. However, there is no commonly accepted gold standard for syphilis diagnostic testing, and the algorithms used for initial screening and diagnosis confirmation vary between different countries8,9,10,11. The fastidious nature of T. pallidum prevents culturing using in vitro methods, and direct testing of swabs of primary lesions is often not possible because the short-lived lesions may occur internally. As a result, serologic testing is regarded as the preferred method for syphilis diagnosis and formmonitoring treatment outcomes8.
With the increased burden of syphilis and the dramatic increase in the number of patients requiring testing in China, a critical demand exists for a simple, accurate, rapid, and high-throughput test for T. pallidum detection. Currently, enzyme-linked immunosorbent assays (ELISA) are the most widely used methods for identifying antibodies to T. pallidum in both clinical and blood bank settings. These include the InTec (Xiamen, China)12 and KHB (Shanghai Kehua Bioengineering, Shanghai, China)13 double-antigen sandwich (DAGS) ELISA tests. These tests are, however, not high-throughput methods, and chemiluminescence-based automated assays offer advantages in terms of high-throughput capacity and sensitivity, which is important for early-stage syphilis cases14. The Architect syphilis TP system (Abbott Laboratories, Abbott Park, IL, USA) uses paramagnetic microparticle capture of T. Pallidum specific combined with chemiluminescent detection15. However, the Architect system is not fully automated. By contrast, the newly developed Elecsys syphilis assay (Roche Diagnostics, Indianapolis, IN, USA) is a fully automated electrochemiluminescence immunoassay that detects T. pallidum specific antibodies in human serum samples16.
We performed a multicenter study to compare the performance of the Elecsys immunoassay with that of the Architect, InTec, and KHB syphilis tests based on the analysis of serum samples collected at various locations throughout China. Our results indicated that the Elecsys immunoassay is superior to these other methods for the routine detection of T. pallidum in human blood samples.
Materials and Methods
Study design
This multicenter evaluation involved 13 independent laboratories in 10 different regions in China, including Beijing, Xi’an, Fuzhou, Jinan, Guangzhou, Chengdu, Kunming, Nanjing, Hangzhou, and Changchun. The main study objective was to compare the sensitivity and specificity of the Elecsys syphilis assay with that of the Architect, InTec, and KHB tests, which are commonly used in China. Our study was conducted in accordance with the Declaration of Helsinki with regard to ethical principles for research involving human subjects, and our study protocol was approved by the ethics committee of each participating institution (West China Hospital, Xijing Hospital, The First Hospital of Jilin University, Peking University Third Hospital, The First Affiliated Hospital of Nanjing Medical University, The Second Affiliated Hospital of Zhejiang University School of Medicine, The Third People’s Hospital of Kunming, Beijing Youan Hospital, Shandong Province Hospital, Nanfang Hospital, Fujian Provincial Hospital, The First Affiliated Hospital of Fujian Medical University, and The Second Affiliated Hospital of Guangzhou University of Chinese Medicine). All participants were 18 years of age or older, and written informed consent was obtained from each participant prior to our analysis.
Samples
Samples from 999 patients with clinically and laboratory confirmed syphilis and 158 samples with borderline results for the Architect, InTec, and KHB tests (n = 158) were included in our analysis (Table 1). All of the samples (n = 13,767) were assigned an anonymous identifier prior to testing, and were screened in parallel. The samples comprised routine specimens that were collected from patients scheduled for surgery, persons at high risk of syphilis infection, pregnant women, and other types of common diagnostic requests.
Assays
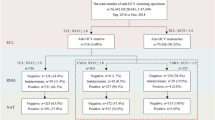
All samples were tested according to the manufacturer’s instructions for each assay. The manufacturers’ recommended cut-off indices (COIs) were <1.00 and ≥1.00 for negative and positive results, respectively. The borderline samples and all other samples for which the results between the different assays were inconsistent were further subjected to examination using the Mikrogen Syphilis Immunoblot (Mikrogen Diagnostik, Neuried, Germany) to confirm the presence of T. pallidum specific antibodies. Using this approach, the outcomes of the Elecsys, Architect, InTec, and KHB assays were categorized as positive, false-positive, negative, or false-negative according to the flow diagram in Fig. 1. Samples with an indeterminate result for immunoblotting were excluded from the interassay comparisons (Fig. 1).
Elecsys Syphilis assay
The Elecsys Syphilis assay was performed using the Cobas e 601 platform (Roche Diagnostics). The automated Elecsys immunoassay simultaneously detects anti-treponemal IgG and IgM antibodies in serum or plasma. A 10-µL aliquot of each sample was incubated with a mixture of biotin- and ruthenium-labeled recombinant TpN15, TpN17, and TpN47 antigens to form a DAGS immune complex, after which streptavidin-coated paramagnetic microparticles were added. Following biotin-streptavidin binding, the immune complex attached to the microparticles was then magnetically sequestered on the electrode. Chemiluminescence was electrically induced and measured. The analyzer software automatically calculated the results, and the average testing duration was approximately 18 min.
Architect Syphilis TP assay
The Architect syphilis TP system uses paramagnetic microparticles coated with recombinant TpN15, TpN17, and TpN47 to bind and purify IgG and IgM antibodies specific for T. pallidum. After incubating the microparticles with 30 µL of serum, murine anti-IgG/anti-IgM acridinium-labeled antibody conjugates were added, and chemiluminescence was measured. The average testing duration was approximately 29 min.
InTec and KHB ELISA tests
Both of these tests are two-step DAGS ELISA tests in which the assay plates have been coated with the recombinant T. pallidum antigens, TpN15, TpN17, and TpN47. After incubating 100 µL of serum in each well, T. pallidum specific IgG and IgM antigen-antibody complexes were detected by the addition of horseradish-peroxidase-labeled TpN15, TpN17, and TpN47 antigens, which were visualized using 3,3′,5,5′-Tetramethylbenzidine (TMB) as the chromogenic substrate. The average testing duration was approximately 2 h.
Mikrogen Syphilis Immunoblot
The recomLine Treponema IgG and IgM kits were used to confirm the detection of IgG and IgM antibodies against T. pallidum by immunoblotting (TPIB). Nitrocellulose membrane test strips containing the Tp47, TmpA, Tp257, Tp453, Tp17 and Tp15 antigens were incubated with 20-µL serum samples, which was followed by incubation with horseradish-peroxidase-labeled secondary antibodies specific for human IgG or IgM. The immune complexes were visualized as dark bands on the strips after the addition of TMB. The intensity of each antigen band was assessed by comparison with the intensity of the cut-off control band. A test has been considered as positive when at least two of six diagnostic bands, which corresponded to
TmpA, Tp47, Tp257, Tp453, Tp15 or Tp17 were recognized as reactive. Using initial serum incubation times of 1 or 3 h, the average testing duration was approximately 4 or 6 h.
Results
Interassay comparisons of consistency, sensitivity, and specificity
We first compared the sensitivities of the various assays for samples from the 999 previously confirmed syphilis cases according to disease stage (Table 2). No differences were observed in the results for these samples between the Elecsys, Architect, InTec, and KHB tests. A comparison of consistency between the results of the various tests for the routinely screened samples revealed no significant interassay variation (Table 3), with consistencies of >99.30% for each of the methods evaluated. The results of interassay comparisons for the routinely screened samples revealed sensitivities for the Elecsys test of 100% versus 98.26% for the Architect test, 99.11% for the InTec ELISA, and 98.56% for the KHB ELISA. The specificity of the Elecsys immunoassay was also higher than those of the Architect, InTec, and KHB tests (Table 4).
Subgroup analysis of the borderline samples
The Elecsys syphilis assay was also assessed based on the analysis of 158 borderline samples previously identified using the Architect, InTec, and KHB tests (Table 5). The TPIB analysis revealed the following results: 28 false-positives among the 72 Architect samples versus 6 false-positives for the Elecsys assay using the same false-positive sample COI range as the Architect; 19 false-positives and 2 false-negatives among the 71 InTec ELISA samples versus 15 false-positives and no false-negatives for the Elecsys immunoassay with 5 samples in different false-positive COI ranges; and 3 false-positives among the 15 KHB ELISA samples versus 3 false-positives for the Elecsys immunoassay, 2 of which were misdiagnosed by both assays using the same COI range.
Discussion
The recent resurgence of syphilis has become a serious threat to public health worldwide, and is a particular problem in China. Its prevalence must be determined by comprehensive screening of blood donations, pregnant women, and organ transplantation donors. Therefore, an accurate, rapid, high-throughput laboratory method of T. pallidum detection is needed to aid in the diagnosis of syphilis.
Serologic tests for T. pallidum detection are classified as non-treponemal or treponemal assays. Treponemal tests detect T. pallidum specific antibodies14, 17. By contrast, non-treponemal tests, such as the Rapid Plasma Reagin (RPR) or Venereal Disease Research Laboratory (VDRL) tests, detect antibodies against host-cell lipids, such as cardiolipin and lecithin, which are released from cells upon T. pallidum infection. Non-treponemal tests can, however, produce false-positive results due to viral infections, pregnancy, and certain autoimmune disorders18, 19, and have limited sensitivity for primary, late, or latent stage syphilis cases20.
Current guidelines leave the choice of first-line laboratory testing for syphilis diagnosis to the discretion of the physician or clinical laboratory. The results of our study revealed that the Elecsys immunoassay demonstrated superior specificity and sensitivity for routine sample screening, compared to that of the Architect, InTec, and KHB tests (Table 4). In the analysis of the borderline samples, the Elecsys immunoassay produced no false-negatives and fewer false-positive results than the other tests (Table 5), with the Architect, InTec, and KHB tests producing at total of 50 false-positives and 2 false-negatives for the borderline samples, whereas the Elecsys test produced only 24 false-positives and no false-negatives. The use of the TpN15, TpN17, and TpN47 antigens in all of the assays evaluated suggest that the differences in the technologies used by the various tests contributed to the differences in the false-positive rates.
The fully automated Elecsys syphilis assay is well suited for providing results rapidly in high-volume clinical laboratories. With a sample assay volume of only 10 μL and an average testing duration of only 18 minutes, the Elecsys test delivers results faster than the Architect, InTec, and KHB tests. The specificity and sensitivity of the Elecsys syphilis assay were also superior to the Architect, InTec, and KHB tests, which indicates that it represents a good choice for routine first-line screening of blood samples for syphilis diagnosis in China. Given that confirmatory treponemal or non-treponemal tests are recommended for reverse algorithms, our results revealed that false-negatives can be completely eliminated by using the Elecsys immunoassay with the Architect, InTec, or KHB immunoassay.
In conclusion, based on its high levels of sensitivity and specificity for T. pallidum screening, the Elecsys syphilis assay represents an excellent first-line laboratory diagnostic test for syphilis diagnosis in China. For the routine screening of blood samples, it has the advantage of being fast, easy to use, and can be used in a high-throughput platform. However, like other treponemal assays, the Elecsys syphilis assay is suitable for the diagnosis of recently acquired and previously treated infections only. Its use in the diagnosis of advanced disease requires other laboratory results and clinical findings.
References
Organization, W. H. One stone to kill two birds. An interview with Xiang-Sheng Chen. World Health Organization, Geneva, Switzerland, http://www.who.int/bulletin/volumes/87/11/09-041109/en/ (2009).
Chen, Z. Q. et al. Syphilis in China: results of a national surveillance programme. Lancet 369, 132–138 (2007).
Tucker, J. D. et al. Scaling up syphilis testing in China: implementation beyond the clinic. Bull World Health Organ 88, 452–457 (2010).
Zhang, W. et al. Syphilis in the economic center of South China: results from a real-time, web-based surveillance program. BMC Infect Dis 15, 318 (2015).
Prevention., C. C. f. D. C. a. Annual infectious disease report. Center for Disease Control and Prevention, China, http://www.nhfpc.gov.cn/jkj/s3578/201502/847c041a3bac4c3e844f17309be0cabd.shtml (2015).
Lafond, R. E. & Lukehart, S. A. Biological basis for syphilis. Clin Microbiol Rev 19, 29–49 (2006).
Mattei, P. L., Beachkofsky, T. M., Gilson, R. T. & Wisco, O. J. Syphilis: a reemerging infection. Am Fam Physician 86, 433–440 (2012).
Janier, M. et al. 2014 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol 28, 1581–1593 (2014).
Organization, W. H. Screening donated blood for transfusion-transmissible infections. World Health Organization, Geneva, Switzerland, http://www.who.int/bloodsafety/ScreeningDonatedBloodforTransfusion.pdf (2010).
Prevention, C. f. D. C. A. Sexually transmitted diseases treatment guidelines. 2015, 1–137 (2015).
Administration, F. a. D. Guidance for Industry: Recommendations for screening, testing, and management of blood donors and blood components based on screening tests for syphilis. Food and Drug Administration, USA http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/UCM340993.pdf (2014).
Wang, L. N. & Li, J. M. Evaluation of immunoglobulin M and G Western blot and ELISA for screening antibodies to Treponema pallidum in blood donors. Sex Transm Dis 36, 413–416 (2009).
Zhang, Q. L., Zhou, J. & Guo, Y. Q. Kappa consistency analysis between Kehua TP-ELISA and Murex TP-ELISA. Shanxi Medical Journal 15, 1747–1749 (2015).
Sena, A. C., White, B. L. & Sparling, P. F. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis 51, 700–708 (2010).
Young, H., Pryde, J., Duncan, L. & Dave, J. The Architect Syphilis assay for antibodies to Treponema pallidum: an automated screening assay with high sensitivity in primary syphilis. Sex Transm Infect 85, 19–23 (2009).
Enders, M. et al. Performance evaluation of the Elecsys syphilis assay for the detection of total antibodies to Treponema pallidum. Clin Vaccine Immunol 22, 17–26 (2015).
Ho, E. L. & Lukehart, S. A. Syphilis: using modern approaches to understand an old disease. J Clin Invest 121, 4584–4592 (2011).
Larsen, S. A., Steiner, B. M. & Rudolph, A. H. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev 8, 1–21 (1995).
Ortel, T. L. Antiphospholipid syndrome: laboratory testing and diagnostic strategies. Am J Hematol 87(Suppl 1), S75–81 (2012).
van der Sluis, J. J. Laboratory techniques in the diagnosis of syphilis: a review. Genitourin Med 68, 413–419 (1992).
Acknowledgements
The authors would like to thank Hai Huang, Dandan Gao, Liyan Cui, Huaguo Xu, Songzhao Zhang, Songqin Lu, Jinli Lou, Jianwen Zou, Shuai Yuan, Jing Shen, Jing Chen, Wujiao Huang, and Zhen Zeng for their valuable assistance for sample collections, the collection of patient information, sample and data transport, and data source verification. Funding for this study was provided by Roche Diagnostics Limited (Shanghai, China; grant no. RD002178).
Author information
Authors and Affiliations
Contributions
C.T., X.H., W.X., J.Z., S.P., Z.T., X.L., J.C., B.Z., Y.Q., Y.W., Q.O., X.H., and L.W. were responsible for the conception and design of the study and the acquisition of the data. C.T. and L.W. performed the data analysis. All of the authors participated in interpretation of the findings. C.T. and L.W. drafted the manuscript. All of the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
C.T., X.H., J.Z., S.P., Z.T., Y.Q., Q.O., X.H., and L.W. have previously received fees from Roche Diagnostics for professional speaking engagements that were not related to this study. The remaining authors have no conflicts of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tao, C., Hao, X., Xu, W. et al. Evaluation of the Elecsys syphilis immunoassay for routine screening of serum samples in China. Sci Rep 7, 9559 (2017). https://doi.org/10.1038/s41598-017-10103-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10103-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.