Abstract
Honey was used to treat wounds since ancient times till nowadays. The present study aimed at preparing a honey-based hydrogel and assay its antimicrobial properties and wound healing activity; in-vitro and in-vivo. Topical honey hydrogel formulations were prepared using three honey concentrations with gelling agents; chitosan and carbopol 934. The prepared formulae were evaluated for pH, spreadability, swelling index, in-vitro release and antimicrobial activity. The pH and spreadability were in the range of 4.3–6.8 and 5.7–8.6 cm, respectively. Chitosan-based hydrogel showed higher in-vitro honey release with diffusional exponent ‘n ≤ 0.5 indicates Fickian diffusion mechanism. Hydrogel formulae were assessed for in-vitro antimicrobial activity using Disc Diffusion antibiotic sensitivity test against common burn infections bacteria; Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumonia and Streptococcus pyogenes. The 75% honey-chitosan hydrogel showed highest antimicrobial activity. This formula was tested for in-vivo burn healing using burn-induced wounds in mice. The formula was evaluated for burn healing and antibacterial activities compared to commercial product. 75% honey-chitosan hydrogel was found to possess highest healing rate of burns. The present study concludes that 75% honey-chitosan hydrogel possesses greater wound healing activity compared to commercial preparation and could be safely used as an effective natural topical wound healing treatment.
Similar content being viewed by others
Introduction
Burns are a common health problem in both developing and industrialized countries. Burns in developing countries, however, may result in serious complications due to the low public hygiene measures which might lead to secondary bacterial infections with need to urgent use of antimicrobial agents. However, the wide use of antibiotics has led to a rise in microbial drug resistance, resulting in poor treatment efficacy and significant economic loss1.
A variety of commercially available wound dressings were launched in recent years, yet they possess certain critical limitations such as addition of antimicrobial agents, which might possess cytotoxic effects, especially on prolonged treatment period, leading to delayed wound healing. A large number of the marketed dressings lose their moisturizing effect which makes them adhere to the surface of the wound and damage the newly formed epithelium. The need for effective natural products, such as honey, is essential to overcome the problems of using chemotherapeutics.
Honey is a nutritious thick carbohydrate-rich syrup, which was effectively used since ancient times in traditional medicine. Nowadays, honey is widely used due to its evidenced broad therapeutic uses. It is a well-known antibacterial2, anti-parasitic3, pain-reliever4 and it has proven efficiency against respiratory tract infections5. Honey has the ability to stimulate human monocytes to produce cytokine. Recently, honey showed antineoplastic activity using an induced bladder cancer model6.
Using honey in treating burns has the advantage of creating a moist environment, it saves the integrity of the burn surface as it is non- adherent, it provides a bacterial barrier that prevents cross-infection and prevents infecting bacteria. Antibacterial property of honey is attributed to its high osmolarity, low pH and hydrogen peroxide production7. Many reports address the antibacterial properties of honey against a wide variety of microbes which accelerates wound healing process7,8,9. The phytochemical components of honey; flavonoids and phenolic acids, play a major role as antioxidants due to their free radical scavenging activities which guards cells from the damage due to free radicals and eventually decrease the inflammatory response8, 9. Honey has anti-inflammatory properties as it debrides the wound, inhibits scarring and it also encourages wound healing by stimulating tissue regeneration in addition to reducing the need for skin grafting. There are no adverse effects from using honey in burn healing10.
The physical properties of honey makes it difficult for direct application on an affected area, as it may liquefy at skin burn temperature as it becomes more fluid at higher temperatures; this restricts the body location on which it can be used, due to the problem of liquefaction and leakage also leading to difficulties in maintaining the required therapeutic concentration for enough time.
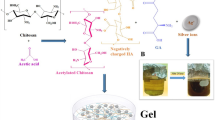
The previous limitations can be solved by incorporating honey in a hydrogel formula. Literatures have reported that hydrogel technology deliver products appropriate for medical use particularly in wound management11,12,13. Providing a moist environment and good fluid absorbance which are needed for a successful wound healing process are generously provided by hydrogels11. Hydrogel pharmaceutical preparation is preferred over creams because of the high water content in gels which help in pain reduction at the time of application, particularly to mucous membranes and in injured or burned skin14. Hydrogels have several favorable properties over ointments when intended for dermatological use such as being emollient, thixotropic and greaseless. Hydrogels also spread more easily and are easily removable, non-staining, compatible with several excipients and water-soluble or miscible15. Therefore, developing hydrogel-based wound dressings became of major interest using a wide variety of biocompatible matrices and biologically active substances such as chitin, chitosan, alginate and the neutralized polyacrylic acid (PAA) (carbopol)16,17,18,19. Chitosan, a widely used natural polymer, is obtained from the skeletal materials of crustaceans, cuticles of insects, and cell walls of many fungi. It is a polysaccharide comprising copolymers of glucosamine and N acetylglucosamine. Chitosan can be prepared by N-deacetylation of chitin20, 21. Chitosan is biodegradable22 biocompatible23, 24, non-toxic and have bio-adhesive features25, 26 making it a great choice for integration in topical burn healing pharmaceutical dosage preparations. Chitosan enhances the epithelisation process27 and thus possess an accelerating effect on wound healing progress. Scientists are currently in the process of synthesizing the chitosan based hemostatic dressing as it has a promising outcome on decreasing pre-operative and post-operative bleeding28. In the utilization of chitosan material, hydrogels is a major branch, since chitosan hydrogel showed promising results in wound dressing, and bone fracture fixation devices29. The basic way to prepare a chitosan hydrogel is solubilization of chitosan in an acidic aqueous medium30.
PAA are used as ion exchange resins and adhesive polymers31. They are also known for being thickening, dispersing, suspending and emulsifying agents in pharmaceuticals, cosmetics and paints32. The neutralized PAA gels are appropriate to obtain biocompatible media for dermatological applications such as gels for skin care or treatment products for skin diseases33, 34.
The present study aims at overcoming the disadvantages of direct application of honey by formulation of honey-chitosan or honey-cabopol 934 hydrogels, to finally obtain a pharmaceutical honey topical preparation with desirable healing effects and antibacterial properties.
The present study measured the antibacterial activity of different honey formulations in vitro against Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pyogenes and Klebsiella pneumonia. The most effective formulation was assessed for activity in vivo. Burn induced mice models were used as an assessment for wound healing and antibacterial effect of the honey – chitosan hydrogel in vivo.
Materials and Methods
Honey samples
Honey was purchased from local Emtinan Stores Giza, Egypt, carbopol 934 (Goodrich Chemicals Co., Cleveland, USA), chitosan low molecular weight was purchased from Sigma Chemical Company (Saint Louis, MO). Glacial acetic acid, triethanolamine (TEA), methyl paraben were purchased from Fisher Scientific, U.K. Analytical grade chemicals were used.
Six honey formulations were tested; 25%, 50% and 75% w/w; each concentration was formulated as a hydrogel twice; once with carbopol 934 and the other with chitosan.
Honey hydrogel formulation
Six formulae of Honey hydrogel were prepared by cold mechanical method19. Polyacrylic acid polymer (carbopol 934) and chitosan hydrogel were prepared by dissolving the calculated amount of polymer in purified water with continuous stirring using a magnetic stirrer for 1 h till the polymer soaked in water. TEA was added with continuous stirring to neutralize the carbopol hydrogel to maintain the pH of the hydrogel. Followed by addition of the necessary amount of methyl paraben as a preservative and then left for 24 h for complete swelling and equilibration of polymer. Finally honey with different concentrations were added to the previous mixture with uninterrupted stirring till honey totally dispersed in the hydrogel. The final weight was completed to 100 g with the aqueous solution. The final formulations were packed in wide mouth glass containers covered with screw plastic lid and placed in refrigerator to complete the formation of hydrogel20.
Physicochemical Evaluation of Honey hydrogel Formulae
Visual examination
The prepared honey formulations were examined for their color, consistency, homogeneity35 and existence of lumps by visual check after they were set in the container.
pH determination
One g of each hydrogel formulae was weighed and mixed with 25 ml of purified water. The pH of the mixture was measured using pH meter (Orion Research, Inc., USA), which was calibrated prior to each use with buffer solution 4.0, 7.0 and 9.0. Experiments were carried out in triplicates and average values were calculated36.
Swelling index
The swelling capacity is an important characteristic of wound healing formulations especially in exudating wounds. Due to their high fluid holding capacity they can absorb a moderate amount of the wound exudates by swelling which leads to formation of a dry bed of wound which further aids into healing process. To determine the swelling index of the prepared topical hydrogel, one gram sample from each formula was soaked into 5 ml phosphate buffer pH 5.5 and left for a specific time, and then the excess buffer was removed and the samples were weighted again. This test was done at time intervals after one and three hours. The following formula will be used to calculate the swelling ratio37.
where Ws is the weight of the swollen hydrogel at time t and W0 is the initial weight.
Spreadability measurement
The spreadability of the hydrogel formulation was determined, by pressing 0.5 g hydrogel between two horizontal plates (20 × 20 cm), then 5 g standardized weight was put on the upper plate and left for about 5 minutes where no more spreading was expected38. Diameters of spread circles were measured in cm and were taken as comparative values for spreadability. The results obtained were the average of three determinations39.
In vitro release studies
Honey release from prepared hydrogels was studied using a dialysis bag technique40. Dialysis bags (Spectra/PorR Dialysis Membrane, MWCO:3.500, Spectrum Laboratories Inc., USA) were soaked before use in distilled water at room temperature for 12 h to remove the preservative, followed by rinsing thoroughly with distilled water. The dialysis bags were fitted on the dissolution tester apparatus I by attaching a stainless steel part to allow fixing the dialysis bags. A specific weight of honey hydrogel was placed in a measured volume (100 ml) of 5.5 pH phosphate buffer at 37 ± 0.5 °C and the quantity of released honey was measured at different time intervals by taking the absorbance of test sample using UV-Vis spectrophotometer (Shimadzu 1800, Japan). The absorbance of the released honey was measured at λ max 340 nm. All experiments were done in triplicates, the average of results were taken. Blank experiments were done using plain bases.
In vitro antimicrobial examination of honey formulations
Disc Diffusion antibiotic sensitivity test (Kirby-Bauer) technique was employed as previously described41. The tests were done against the most strains known to be isolated from burn infections. The tests were run in Mueller Hinton agar (Oxoid, UK) for Pseudomonas aeruginosa, Staphylococcus aureus and Klebsiella pneumonia; Blood agar for Streptococcus pyogenes. All were prepared according to the manufacturer’s instructions. Cheesbrough42 method was used for preparing filter paper disks (6 mm diameter). Disks saturated with the six different honey formulations were employed in the study. The test was done a second time using discs impregnated with F3 (honey 75% - chitosan formula), pure honey and pure chitosan. The method of Koneman et al.43 was used to prepare 5 mL 0.5 McFarland standard in a sterile test tube. From the corresponding bacterial suspension an inoculum of each bacterial isolate was prepared. Briefly, 5–6 bacterial isolate colonies were emulsified in sterile distilled water and the turbidity was adjusted to 1.5 × 108 CFU/mL (equivalent to 0.5 McFarland standard). This was followed by dipping a sterile cotton swab into the standardized bacterial suspension and inoculated on the corresponding agar plates evenly. Then plates were left to dry for 5 minutes. This was followed by placement of all formulation impregnated disks on the agar plates and forced lightly to confirm thorough contact with agar. A space of 20 mm was kept from the plates‘ edges to avoid intersection of inhibition zones. Amoxicillin disk (15 g) was used as a positive control. After 15 minutes all plates were incubated at 37 °C for 3–5 days. The plates were inspected and the diameter of the zone of inhibition was measured.
In vivo burn healing evaluation
Animals and Experimental Design
A total of 10 albino mice (weight 30–35 g) were used. The animals were almost 8 weeks old. Animals were separately kept in clean polyethylene cages under usual experimental conditions of temperature 23 ± 2 °C. Mice were divided into 2 equal groups; males and females then anaesthetized with anaesthetic ether and shaved on the back with electric clipper then with a shaving cream. The shaved area was disinfected with 70% (v/v) ethanol. The burn wounds were induced as stated in literature44. Briefly, a cylindrical metal rod (10 mm diameter) was heated over an open flame for 30 seconds and pressed to the shaved and disinfected dorsal mouse skin surface for 20 seconds under light ether anesthesia. Four burning positions were induced on the back of each mouse; the four positions were treated with the following: F (Honey 75% - chitosan formula), H (Pure honey 100%), P (Positive control, standard wound healing cream, silver sulphadiazine) and N (Negative control, normal saline). Using a sterile cotton swab, all treatments (100 mg) were applied topically on each mouse once daily in a rotational manner (FHPN, HPNF, PNFH,……). Treatments were continued daily for nine days.
The mice were fed on commercial pellet and water ad libitum throughout the study. The experimental protocol was approved by the Animal Care and Use Committee of The British University in Egypt and the study was done according to the animal welfare guidelines and regulations.
The assessed wound healing parameters were the measurement of burn edge contraction, continuous monitoring of possible bacterial contamination and histopathological findings of the burned skin autopsy taken after nine days of treatment, in addition to the continuous assessment of any wound bacterial infection.
Measurement of wound area
The changes in burn edge diameter was measured (mm) using a digital caliper every day before treatments application. Burn edge contraction was expressed as the decrease of the original burn diameter. All measurements were recorded for further analysis.
Microbial infection assessment of the induced burns
Surface swabs were collected from burn wounds before the daily application of the topical treatments. The total burned area was swabbed using a sterile cotton swab. Swab samples were taken from the burned wound area where the degree of burn is highest. Samples were homogenized in 4 mL sterile saline. Samples were immediately cultured on blood agar and MacConkey agar plates. MacConkey agar was used for the isolation of Gram negative bacteria while the blood agar was used for isolation and identification of Gram positive bacteria. After inoculation, plates were incubated at 37 °C for 24–48 h.
Histopathological analysis
Autopsy samples were taken from the skin of mice in both groups; males and females. 4 samples from each mouse representing the positions of application, with a total of 40 samples; 20 males and 20 females, representing 10 of each application. The samples were fixed in 10% formalin solution for 24 h. Tap water was used to wash the samples then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used to dehydrate the samples. Xylene was used to clear the specimens then embedded in paraffin at 56 °C in hot air oven for 24 h. Tissue blocks in paraffin bees wax were prepared for sectioning at 4 microns thickness using a sledge microtome. Prepared tissue sections were collected on glass slides, de-paraffinized and stained by hematoxylin & eosin stain (H&E) for examination through the light electric microscope45. Digital photomicrographs were captured at representative locations and the burns were evaluated for the extent of acanthosis, new blood capillaries formation and regeneration.
Statistical analysis
The results are expressed as mean of triplicate measurements of inhibition zones of in vitro antimicrobial activity. The results of the in vivo wound diameter are expressed as means of 10 measurements groups daily for the 9 days ± SD. In the latter case Two way ANOVA was used.
Day 9 measurements were statistically analyzed using one way ANOVA test followed by Tukey post-hoc test. The difference was considered significant at p < 0.05.
Data availability
The data generated and/or analysed during the current study are available from the corresponding author on request.
Results and Discussion
Preparation and Physicochemical Evaluation of Honey hydrogel Formulae
Hydrogels are high-water content materials prepared from cross-linked polymers that are able to provide sustained, local delivery of a variety of therapeutic agents46,47,48,49. The development of hydrogels from a variety of synthetic materials like carbopol 934 has been widely explored for the controlled delivery of many therapeutics with diversity in mechanical strengths and biological responses50,51,52,53. Additionally, polysaccharides as category of natural polymers have been interestingly used as the structural material in hydrogels. This is due to polymers biocompatibility, low toxicity, low immunogenicity and susceptibility to enzymatic degradation54. Of these, hydrogels using the natural polymer, chitosan, have received a great deal of attention due to their well-documented biocompatibility, low toxicity55, 56, and degradability by human enzymes57.
In the current study different honey hydrogel formulations were prepared using cold mechanical method utilizing two gel forming polymers either synthetic or natural naming carbopol 934 and chitosan, respectively to compare the effect of polymer type regarding the physicochemical characteristics of prepared hydrogel (Table 1).
All formulations prepared represented homogenous, transparent three-dimensional networks which is considered as a good point to assist the monitoring process of wound healing progression. Water-soluble linear polymers of both natural and synthetic origin are either physically associated or chemically cross-linked to form the hydrogel. However, simple mixing the components of gel under the appropriate conditions usually produce polymer-based physical gel31. This process has an advantage over other crosslinking methods since it can be performed at room temperature and in physiological pH without using toxic and hard to remove crosslinking agents58. Therefore, it is always safe for clinical applications. Polysaccharides such as chitosan are reported in literature for the preparation of physically crosslinked hydrogels by hydrophobic modification59. Because the network formation by this interaction is purely physical, gel formation can be reversed60.
This is important for the biodegradation and drug release kinetics of therapeutics from hydrogels.
The result of drug content varies as shown in Table 2. The results indicated that the process employed to prepare hydrogel formulations in this study was capable of producing formulations with uniform drug content and minimal variability. Honey was dispersed uniformly throughout the hydrogel. The pH value of each honey-hydrogel is shown in Table 2. The pH of formulated hydrogels was found to be in the range of 4.3 to 6.8, which lies in the normal pH range of the skin61, which indicates the suitability of the formulations for application on the skin to avoid any irritation upon application. Hydrogel containing 75% honey was slightly acidic (pH 4.3, 4.7) compared to other 25, 50% honey hydrogels. This could be due to the acidic property of honey itself; pH being between 3.2 and 4.518. Uniform application of paste to the skin is dependent on the spreadability parameter, where a good paste takes less time to spread and will have high credibility and spreadability62. Spreadability is important in patient compliance and helps in uniform application of gel to the skin. Spreadability of ideal formulation was found to be 8.6 ± 0.03 which was met by F3 containing 75% honey with chitosan gelling agent as shown in Table 2.
Swelling index was performed for all formulations (F1-F6) initially after 1 h and up to 3 hrs (Table 3). It was noticed that although all formulations showed rapid swelling due to the porous nature of the hydrogels offering large surface area allowing rapid uptake of the solvent but it was found that swelling index was inversely proportional to the concentration of honey. As the honey concentration increases there is decrease in swelling percentage. This is may be attributed to the viscosity of the polymer used which had major influence on swelling process. Results reflected also relation between swelling and type of the polymer, degree of cross-linking, ionic strength and presence of water63. Increase in cross–linker concentration shows a direct negative effect on hydrogel swelling index due to formation of tighter structure64. This is in agreement with our results as the lowest swelling index values ranged from 15% to 33%w/w belonged to hydrogels based on high cross-linker polymer carbopol 934.
In vitro release studies
In vitro release study showed that the release of honey from the hydrogel varied according to the type of gelling agent used and percentage of honey in the hydrogel. The results showed a concentration-dependent increase in the amount of honey diffused through cellophane bag of all hydrogel formulations (i.e., from 25% to 75%). It was also observed that among the prepared hydrogels the release of honey increased with increasing its concentration regardless the polymeric hydrogel used in the formulation. This may be attributed to the viscosity of the gel matrix in the hydrogel which is a major issue to address in evaluation of drug penetration from gels through the skin or artificial film and is used to measure the extrudability of a gel18. The decreasing viscosity of the hydrogels with increasing honey concentrations (from F1 to F3 and from F4 to F6) not only indicates that these hydrogel formulations would be easily extruded from their containers but also shows potential enhancement in the diffusivity of honey within the hydrogel network which consequently would ease flux65.
Also it is worth saying that the method by which the therapeutics is loaded into the hydrogels has direct impact on drug release behavior66. The hydrogels prepared by direct addition method as adopted in this study represents a typical release profile with rapid burst release of the drugs (up to 70%) during initial hydrogel swelling after transfer to in vitro media, followed by the extended release of the remaining drugs loaded within the gel network. However, highly cross-linked polymer showed lower burst release of drugs (10–25%)67, 68.
All formulations based on chitosan gelling agent showed an initial burst effect. This may be attributed to honey diffusion due to rapid gel swelling as previously mentioned and release of adsorbed honey in the direction of the surface of the gel matrix69, 70.
On the other hand, hydrogel formulae based on carbopol 934 produced the least percent of honey released from the hydrogels. This is justified since carbopol 934, like other high molecular weight polymers, demonstrates multiple cross-linkages per polymer producing more robust hydrogels with rheology similar to mayonnaise71, 72. Among all hydrogel formulations, F4 carbopol 934 based hydrogel showed superior sustained drug release. Carbopol 934 is a hydrophilic polyacrylic acid polymer and its carboxyl groups become highly ionized after neutralization with TEA, forming a gel due to electrostatic repulsion among charged polymer chains. Increasing pH of the prepared hydrogel to be suitable for skin application resulted in uncoiling of the polymer chains due to ionization of its carboxyl groups and subsequently forming a rigid gel73.
Hydrogels are a common form of topical application. They control the release of loaded therapeutics by diffusion through the hydrogel microporous structure into the skin74,75,76. Kinetic study of the in vitro results of all prepared honey hydrogels revealed that the hydrogels acquire diffusion controlled-release kinetics. In other words, the release of honey from prepared hydrogels is diffusion rate limited. As the release exponent ‘n’ was found to be ≤ 0.5 regarding all preparations which indicates Fickian diffusion mechanism.
From the results (Fig. 1) it is seen that F3 formulation showed better in vitro honey release when compared with other formulations.
In vitro antimicrobial examination of honey formulations
The antibacterial activity of the six prepared formulations (F1, F2, F3, F4, F5 and F6) was tested against the most common burn bacterial infections; Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumonia and Streptococcus pyogenes (Table 4). The test was done using Disc Diffusion antibiotic sensitivity technique. All formulas prepared with chitosan showed larger zones of inhibition than those prepared with carbopol, thus better antibacterial activity. Among the three best chitosan formulations the diameter of zone of inhibition decreased with decreasing honey concentration. As the concentration of honey increased, the mean diameter of zone of inhibition increased till reaching maximum with honey 75% - chitosan gel formula where F3 formula showed the highest zone of inhibition compared to the others. Therefore, F3 formula was chosen for further investigations.
A second Disc Diffusion antibiotic sensitivity test was done to compare the antibacterial activity of the chosen formula F3 with that of blank chitosan and pure honey (Table 5). Blank chitosan gel and pure honey showed minimum bacterial inhibition compared to F3 (Fig. 2). This can be explained by the known antimicrobial property of chitosan and the hygroscopic property of honey which dehydrate bacteria, also its high sugar content and low level pH can also prevent the microbes from growth77. It was also reported in literature that the 75% honey solution exerted higher antibacterial activity than pure honey against common respiratory tract infections5.
Therefore, it is evident that both chitosan and honey contribute to the antibacterial property in the prepared gel and the addition of honey increases the zone of inhibition.
In vivo burn healing evaluation
Burns were induced in albino mice using a preheated metallic rod which gave a final diameter in all burned positions of about 10 mm (Fig. 3). Treatments were applied on daily basis for nine days.
Measurement of wound area
Measurement of wound diameter was a main criteria for indication of progressive healing where burn edge contraction was expressed as a decrease of the original burn diameter. All measurements were recorded daily for nine days. The positions treated with F3 formula on all mice showed continuous burn edge contraction during the course of treatment. At the end of the treatment period the maximum burn diameter contraction was reached by F3. This shows that honey 75% - chitosan formula showed the best healing properties compared to the pure honey and the commercial product tested (Table 6).
Microbial infection assessment of the induced burns
Skin swabs were taken continuously from the surface of the burn to investigate any bacterial infection of the wound. All swabs from all positions did not show any bacterial growth during the course of treatment, which means that no bacterial infection occurred to the induced burns. This may be attributed to the already known antibacterial properties of the treatments (honey, chitosan and commercial product). It was reported that during treatment with honey the wounds become sterile within 7–10 days and promote the formation of healthy granulation tissue78. The slight acidic pH of the prepared formula (pH 4.3–4.7), similar to that of healthy skin, makes it most comfortable to wear and this low pH creates an unfavorable environment for bacterial growth79. In previous experimentally induced burns, there was no obvious infection, but honey continued to cause a decrease in inflammation, this shows that the anti-inflammatory activity of honey is a direct action and not a side effect of eliminating infection by antibacterial activity80.
Swabs from positions treated with normal saline (0.9% NaCl) also did not show bacterial growth this may be attributed to the fact that normal saline is isotonic and it does not induce tissue damage, cause sensitization or allergies, or alter the normal bacterial flora of the skin which makes it the favored wound cleansing solution for non-infected wounds because it does not interfere with the normal healing process77.
Histopathological analysis
Forty autopsy samples were cut and divided into four groups according to the treatment applied. All skin samples were compared to a control group.
The skin autopsy of the control group showed no histopathological alteration where the skin showed the normal histological structure of the epidermis, dermis, subcutaneous tissue with adipose one and underlying musculature.
The resulting burn was a third degree type which showed a focal wide area of necrosis in the epidermis, dermis and underlying subcutaneous tissue associated with massive inflammatory cells infiltration in the subcutaneous tissue as well as adipose tissue and musculature (Fig. 4a).
Representative photomicrographs of tissue sections stained with H&E. (a) Day 1 directly after induced third degree burn showing necrosis of the epidermal and dermal layers in focal manner with inflammatory cells infiltration in subcutaneous tissue and musculature (16x mag). (b) Day 9 after burn. Skin of mice treated with silver sulphadiazine showing focal necrosis of the epidermis with acanthosis in the adjacent one and inflammatory cells infiltration in the underlying dermis and subcutaneous tissue (16x mag). (c) Day 9 after burn. Skin of mice treated with pure honey showing few newly formed blood capillaries and few fibroblastic cell proliferation as granulation tissue in subcutaneous tissue with inflammatory cell infiltration (40x mag). (d) Day 9 after burn. Skin of mice treated with honey 75%-chitosan formula showing regeneration of the epidermal layer with separation of the necrosed areas as scales and formation of new blood capillaries. (40x mag).
The epidermal layer of the group of thermally inducted wound of mice and treated by silver sulphadiazine showed focal necrosis with acanthosis in the adjacent one. Inflammatory cells infiltration was detected in the underlying dermis and the deep subcutaneous tissue as well as the adipose tissue. Inflammatory cells infiltration was also detected with necrosis and congestion in the blood vessels in the underlying musculature (Fig. 4b).
On the other hand, the group treated by 100% honey, there was rejection of the necrosed area from the epidermis (scales) associated with acanthosis in the prickle cell layer of the adjacent epidermis. The subcutaneous tissue showed inflammatory cells infiltration as well as few fibroblastic cells proliferation and few newly formed capillaries as beginning of granulation tissue formation. Inflammatory cells infiltration was detected in between the degenerated musculature (Fig. 4c).
Regarding the group treated by 75% honey-chitosan formula, the epidermis and dermis showed regeneration with hyperkeratosis and acanthosis in the epidermis replacing the necrosed one. These findings are consistent with previous studies which showed that honey promotes epithelization of wounds81,82,83,84,85 where epithelium regeneration is one of the most important indices for wound healing rate evaluation. Few inflammatory cells infiltration was detected in the dermis and subcutaneous tissue associated with fibroblastic cells proliferation and newly formed blood capillaries which is an indication of the beginning of healing process as stated by previous researches86,87,88,89,90,91 (Fig. 4d).
From the histopathological sections it is clear that treatment with honey 75%-chitosan (F3) treatment resulted in best healing results indicated by the regeneration of the epidermis tissue and the formation of new blood capillaries. This was followed by pure honey treatment (H) also showed a promising healing effect but to a lesser extent compared to the F3 formula. Finally the least effect was the commercial formulation where no epithelial regeneration or new blood capillaries were formed.
Statistical analysis
There was a significant healing of wounds by time in all groups (p < 0.0001), significant difference between groups (p < 0.0001) using two way ANOVA, as well as a significant time x treatment interaction (p < 0.0018) at day 9 of treatment.
Conclusion
This study shows that honey plays a positive role in modulating wound healing when incorporated into chitosan-based hydrogel matrix. The prepared hydrogel wound dressing containing 75% of honey will not function merely as coverage to provide clean moist environment for healing but also directly contribute to enhanced tissue regeneration and recovery.
These design parameters established an alternative cheap, non-toxic, natural and efficient honey-chitosan hydrogel delivery system that can move closer to clinical availability for wound healing.
References
Huang, X., Bao, X., Liu, Y., Wang, Z. & Hu, Q. Catechol-Functional Chitosan/Silver Nanoparticle Composite as a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. Sci. Rep. 7, 1860 (2017).
Gulfraz, M. et al. Compositional analysis and antimicrobial activity of various honey types of Pakistan. Int. J. Food Sci. Technol. 46, 263–267 (2011).
Azim, M. K. & Sajid, M. Evaluation of nematocidal activity in natural honey. Pak. J. Bot. 41, 3261–3264 (2009).
Azim, M. K., Perveen, H., Mesaik, M. A. & Simjee, S. U. Antinociceptive activity of natural honey in thermal-nociception models in mice. Phytother. Res. 21, 194–197 (2007).
El-Kased Reham, F. Natural antibacterial remedy for respiratory tract infections. Asian Pac. J. Trop. Biomed. 6, 270–274 (2016).
Tonks, A. J. et al. A 5.8-kDa component of manuka honey stimulates immune cells via TLR4. J. Leukoc. Biol. 82, 1147–1155 (2007).
Swellam, T. et al. Antineoplastic activity of honey in an experimental bladder cancer implantation model: in vivo and in vitro studies. Int. J. Urol. 10, 213–219 (2003).
Alfonso, R. Gennaro. Remington, The Science and Practice of Pharmacy (Lippincott Williams & Wilkins) 856 (2000).
Honrao, M. S. & Pabari, R. Gels. The Indian Pharmacist. 5, 16–21 (2004).
Temu, M. J., Damian, F., Kinget, R. & Mooter, G. V. D. Intra-vaginal gels as drug delivery systems. J. Women. Health. 13, 834–844 (2004).
Cooper, R. A., Molan, P. C. & Harding, K. G. Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med. 92, 283–285 (1999).
Visavadia, B. G., Honeysett, J. & Danford, M. H. Manuka honey dressing: An effective treatment for chronic wound. Br. J. Oral. Maxillofac. Surg. 6, 55–56 (2008).
Lusby, P. E., Coombes, A. L. & Wilkinson, J. M. Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res. 36, 464–467 (2005).
Henriques, A., Jackson, S., Cooper, R. & Burton, N. Free radical production and quenching in honeys with wound healing potential. J. Antimicrob. hemother. 58, 773–777 (2006).
Swarbrick, J. & Boylan, J. C. Encyclopedia of Pharmaceutical Technology. (Marcel Dekker, Inc.) (1992).
Klich, C. M. Jels & Jellies. In: Encyclopedia of Pharmaceutical Technology. (Swarbrick J, Boylan JC, eds.) 415–439 (Marcel Dekker Inc; 1992).
Zohdi, R. M., Zakaria, Z. A. B., Yusof, N., Mustapha, N. M. & Abdullah, M. N. H. Sea cucumber (Stichopus hermanii) based hydrogel to treat burn wounds in rats. J. Biomed. Mater. Res. Part B. 98, 30–37 (2011).
Yang, J. M., Su, W. Y., Leu, T. L. & Yang, M. C. Evaluation of chitosan/PVA blended hydrogel membranes. J. Membrane Sci. 236, 39–51 (2004).
Cho, W. J., Heang, O. S. & Ho, L. J. Alginate film as a novel post-surgical tissue adhesion barrier. J. Biomater. Sci. 21, 701–713 (2010).
Jayakumar, R., Prabaharan, M., Nair, S. V. & Tamura, H. Novel chitin and chitosan nanofibers in biomedical applications. Biotechnol. Adv. 28, 142–150 (2010).
Bhuvaneshwari, S., Sruthi, D. & Sivasubramanian, V. Niranjana kalyani and Sugunabai J. Development and characterization of chitosan film. Int. J. Eng. Res. Appl. 1, 292–299 (2011).
Guerrero, L. I. & de la Caba, K. Functional Properties of chitosan-based films. Carbohydr Polym. 93, 339–346 (2013).
Xu, J., McCarthy, S. P., Gross, R. A. & Kaplan., D. L. Chitosan Film Acylation and Effects on Biodegradability. Macromolecules. 29, 3436–3440 (1996).
In-Yong, K. et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 26, 1–21 (2008).
Shelma, R., Paul, W. & Sharma, C. P. Chitin nanofibre reinforced thin chitosan films for Wound healing application. Trends Biomater. Artif. Organs. 22, 107–111 (2008).
Dutta, P. K., Tripathi, S., Mehrotra, G. K. & Dutta, J. Perspectives for chitosan based antimicrobial films in food applications. J. Food Chem. 114, 1173–1182 (2009).
Emir, B. D. & Raphael, M. O. Perspectives on: Chitosan Drug Delivery Systems Based on their Geometries. J. Bioact. Compat. Polym. 21, 351–368 (2006).
Marie, P. & Arockianathan, S. Evaluation of biocomposite films containing alginate and sago starch impregnated with silver nano particles. Carbohydr Polym. 90, 717–724 (2012).
Mercy, H. P., Halim, A. S. & Hussein, A. R. Chitosan-Derivatives As Hemostatic Agents: Their Role In Tissue Regeneration. Regen. Med. 1, 38–46 (2012).
Pu, X.-M. et al. Fabrication of chitosan/hydroxylapatite composite rods with a layer-by-layer structure for fracture fixation. J. Biomed. Mater. Res. B. 100B, 1179–1189 (2012).
Nie, J., Wang, Z. & Hu, Q. Chitosan Hydrogel Structure Modulated by Metal Ions. Sci. Rep 6, 36005 (2016).
“Acrylates”. The Macrogalleria. Polymer Science Learning Center. Retrieved 2015-06-25 (2005).
Orwoll, Robert A.; Yong, Chong S. Poly (acrylic acid). (Mark, James E. Polymer Data Handbook). 252–253 (Oxford University Press, Inc. 1999).
Kaiser, N., Klein, D., Karanja, P., Greten, Z. & Newman, J. (2009). Inactivation of chlorhexidine gluconate on skin by incompatible alcohol hand sanitizing gels. Am. J. Infect. Control. 37, 255–256 (2008).
Todica, M. et al. UV-Vis fluorescence investigation of some poly (acrylic) gels. Studia UBB Chemia. 1, 7–17 (2015).
Guptal, A., Mishra, A. K., Sinngh, A. K., Gupta, V. & Bansal, P. Formulation and evaluation of topical gel of diclofenac sodium using different polymers. Drug Invent. Today. 2, 250–253 (2010).
Anumolu, S. S et al. Doxycycline hydrogels with reversible disulfide crosslinks for dermal wound healing of mustard injuries. Biomaterials. 1–14 (2010).
Contreras, M. & Sanchez, M. Application of factorial design to the study of the flow behavior, spreadability, transparency of carbopol ETD 2020ge1. Part II. Int. J. Pharm. 234, 149–157 (2002).
Rao, N. G. R., Rao, K. P. & Muthalik, S. Clinical studies and antimicrobial activity of ciprofloxacin hydrochloride medicated dental gels for periodontal infection. Asian J. Pharmacol. 3, 125–134 (2009).
Gonullu, U., Uner, M. I., Yener, G., Kara man, E. M. F. & Gmus, Z. A. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm 65, 1–13 (2015).
Bauer, A. W., Kirby, W. M., Sherris, J. C. & Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45, 493–496 (1966).
Cheesbrough, M. District Laboratory Practice in Tropical Countries. Part II. 1933–1934 (Cambridge University Press 2000).
Koneman, E. W., Allen, S. D., Janda, W. M., Schreckenberger, P. C. & Winn, W. C. Diagnostic Microbiology, (5th edn, Philadelphia, Lippincott) 803–841(1997).
Rozaini, M. Z., Zuki, A. B. Z., Noordin, M., Norimah, Y. & Hakim, A. N. The effects of different types of honey on tensile strength evaluation of burn wound tissue healing. Int. J. Appl. Res. Vet. M. 2, 290–296 (2004).
Banchroft, J. D., Stevens, A. & Turner, D. R. Theory and Practice of Histological Techniques. (Fourth Ed. Churchil Livingstone, New York, London, San Francisco, Tokyo) 273–292 (1996).
Ahmed, E. M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 6, 105–121 (2015).
Sun, Y. et al. Self-assembly of a 5-fluorouracil-dipeptide hydrogel. Chem. Commun. 52, 5254–5257 (2016).
Kim, S. H., Sun, Y., Kaplan, J. A., Grinstaff, M. W. & Parquette, J. R. Photo-crosslinking of a self-assembled coumarin-dipeptide hydrogel. New J. Chem. 39, 3225–3228 (2015).
Verhulsel, M. et al. A review of microfabrication and hydrogel engineering for micro-organs on chips. Biomaterials 35, 1816–1832 (2014).
Hoffman, A. S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 54, 3–12 (2002).
Peppas, N. A., Hilt, J. Z., Khademhosseini, A. & Langer, R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 18, 1345–1360 (2006).
Peppas, N. A., Bures, P., Leobandung, W. & Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 50, 27–46 (2000).
Jeong, B., Kim, S. W. & Bae, Y. H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 54, 37–51 (2002).
Nie, J. et al. Orientation in multi-layer chitosan hydrogel: morphology, mechanism, and design principle. Sci. Rep. 5, 7635 (2015).
Knapczyk, L. et al. Requirements of chitosan for pharmaceutical and biomedical applications, in: G. Skak-Braek, T. Anthonsen, P. Sandford (Eds), Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications, Elsevier, London, 657–663 (1989).
Hirano, H. S. S., Akiyama, I. & Nonaka, I. Chitosan: a biocompatible material for oral and intravenous administration, in: C. G. Gebelein, R. L. Dunn (Eds.), Progress in Biomedical Polymers, Plenum Press, New York, pp. 283–289 (1990).
Muzzarelli, R. A. A. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cell. Mol. Life Sci. 53, 131–140 (1997).
Hennink, W. E. & Nostrum, C. Fvan Department of Pharmaceutics, Utrecht University. Adv. Drug Deliv. Rev. 54, 13–36 (2002).
Bhattarai, N., Gunn, J. & Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 62, 83–99 (2010).
Martin, A. Martin’s Physical Pharmacy and Pharmaceutical Sciences: Physical Chemical and Biopharmaceutical Principles in the Pharmaceutical Sciences; 565–569 (Fifth edition, Lippincott Williams & Wilkins (2006).
Molan, P. C. The role of honey in the management of wounds. J. Wound Care. 8, 415–418 (1999).
Kumar, L. & Verma, R. In vitro evaluation of topical gel prepared using natural polymer. International Journal of Drug Delivery. 2, 58–63 (2010).
Hiremath, J. N. & Vishalakshi, B. Effect of crosslinking on swelling behavior of IPN hydrogels of Guar Gum & Polyacrylamide. Der Pharma Chemica. 3, 946–955 (2012).
Rozaini, M. Z., Zuki, A. B. Z., Noordin, M. M., Norimah, Y. & Abdullah, M. N. H. The effect of topical application of Malaysian honey on burn wound healing. J. V. M. 16, 47–50 (2004).
Kim, S. W., Bae, Y. H. & Okano, T. Hydrogels: swelling, drug loading, and release. Pharm. Res. 9, 283–290 (1992).
Huang, X. & Brazel, C. S. Analysis of burst release of proxyphylline from poly(vinyl alcohol) hydrogels. Chem. Eng. Commun. 190, 519–532 (2003)
jeong, B., Bae, Y. H. & Kim, S. W. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J. Control. Release 63, 155–163 (2000).
Subrahmanyam, M. Topical application of honey for burn wound treatment-an overview. Ann. Burns Fire Disasters. 20, 322–333 (2007).
Wang, Z., Nie, J., Qin, W., Hu, Q. & Tang, B. Z. Gelation process visualized by aggregation-induced emission fluorogens. Nat. Commun. 7, 12033 (2016).
Aljady, A. M., Kamaruddin, M. Y., Jamal, A. M. & Mohd-Yassim, M. Y. Biochemical study on the efficacy of Malaysian Honey on inflicted wounds: an animal model. Med. J. Islamic World Acad. Sci. 13, 125–132 (2000).
Khoo, Y. T., Halim, A. S., Singh, K. B. & Mohamad, N. A. Wound contraction effects and antibacterial properties of Tualang honey on full-thickness burn wounds in rats in comparison to hydrofibre. BMC Complement. Altern. Med. 10, 1–8 (2010).
Roopa, G. & Bhat, R. S. Wound healing activity of a prepared long acting gel loaded with ciprofloxacin HCL microspheres in albino rat. Pharmacology 2, 1010–1016 (2010).
Canal, T. & Peppas, N. A. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J. Biomed. Mater. Res. 23, 1183–1193 (1989).
Amsden, B. Solute diffusion within hydrogels. Mechanisms and models, Macromolecules. 31, 8382–8395 (1998).
Raja, R., Abbulu, K., Sudhakar, M., Roopakarki, S. & Rajkumar, B. Design and in vitro evaluation of modified release valsartan hydrogels. Int. J. of Drug Delivery 3, 648–660 (2011).
Chauhan, A., Pandey, V., Chacko, K. M. & Khandal, R. K. Antibacterial activity of raw and processed honey. Electron. J. Biol. 5, 58–66 (2010).
Armon, P. J. The use of honey in the treatment of infected wounds. Trop, Doct. 10, 91 (1980).
Fernandez, R. & Griffiths, R. Water for wound cleansing. Cochrane Database Syst, Rev. 23, (2008).
Parsons, D., Bowler, P. G., Myles, V. & Jones, S. Silver antimicrobial dressings in wound management: a comparison of antibacterial, physical, and chemical characteristics. Wounds 17, 222–232 (2005).
Molan, P. C. The evidence supporting the use of honey as a wound dressing. Int. J. Low Extrem. Wounds 5, 40–54 (2006).
Oladejo, O. et al. A comparative study of the wound healing properties of honey and Ageratum conyzoides. Afr. J. Med. Sci 32, 193–196 (2003).
Hejase, M., Bihrle, R. & Coogan, C. Genital Fournier’s gangrene: experience with 38 patients. Urology 47, 734–739 (1996).
Yang, K. The use of honey in the treatment of chilblains, non-specific ulcers, and small wounds. Chin. Med. J. 62, 55–60 (1944).
Hutton, D. Treatment of pressure sores. Nurs Times 62, 1533–1534 (1966).
Iftikhar, F. et al. Effects of acacia honey on wound healing in various rat models. Phytother Res. 24, 583–586 (2010).
Subrahmanyam, M. A prospective randomized clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns 24, 157–161 (1998).
Kumar, A., Sharma, V., Singh, H., Prakash, P. & Singh, S. Efficacy of some indigenous drugs in tissue repair in buffaloes. Indian Vet J 70, 42–44 (1993).
Gupta, S., Singh, H., Varshney, A. & Prakash, P. Therapeutic efficacy of honey in infected wounds in buffaloes. Indian J Anim Sci 62, 521–523 (1992).
Bergman, A., Yanai, J., Weiss, J., Bell, D. & David, M. Acceleration of wound healing by topical application of honey. An animal model. Am J Surg 145, 374–376 (1983).
Bulman, M. Honey as a surgical dressing. Middlesex Hosp J 55, 188–189 (1955).
Suguna, L., Chandrakasan, G. & Joseph, K. T. Influence of honey on collagen metabolism during wound healing in rats. J Clin Biochem Nutr, 13, 7–12 (1992).
Author information
Authors and Affiliations
Contributions
R.F.E. designed the study, wrote the manuscript and performed microbiological experiments. R.I.A. performed pharmaceutics experiments. D.A. and M.M.E. supervised the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Kased, R.F., Amer, R., Attia, D. et al. Honey-based hydrogel: In vitro and comparative In vivo evaluation for burn wound healing. Sci Rep 7, 9692 (2017). https://doi.org/10.1038/s41598-017-08771-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08771-8
This article is cited by
-
Combination of stem cell-derived secretome from human exfoliated deciduous teeth with Yemeni Sidr honey on cell viability and migration: an in vitro study
BDJ Open (2024)
-
Advances in the Study of Liposomes Gel with Stimulus Responsiveness in Disease Treatment
Journal of Cluster Science (2024)
-
Gellan gum/guar gum films incorporated with honey as potential wound dressings
Polymer Bulletin (2024)
-
A fabricated hydrogel of hyaluronic acid/curcumin shows super-activity to heal the bacterial infected wound
AMB Express (2023)
-
Phytochemicals, Biodegradation, Cytocompatibility and Wound Healing Profiles of Chitosan Film Embedded Green Synthesized Antibacterial ZnO/CuO Nanocomposite
Journal of Polymers and the Environment (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.