Abstract
The aim of this study was to explore the possible antibacterial components of Salvia miltiorrhizae on Pseudomonas aeruginosa using a combination of chemical fingerprint and bioactivity evaluation. The chemical fingerprints of 32 batches of S. miltiorrhizae samples from different sources were developed using high-performance liquid chromatography with diode array detection, and then were evaluated by similarity analysis and hierarchical clustering analysis. Anti-P. aeruginosa activity was determined by microcalorimetry. Some crucial thermokinetic parameters obtained from the heat-flow power-time curves of P. aeruginosa growth in the absence or presence of these S. miltiorrhizae samples were evaluated using principal component analysis. Thereafter, multiple linear regression analysis was used to analyze the fingerprint-activity relationship between the chemical fingerprints and anti-P. aeruginosa activity. This established the related equation between the inhibition ratio (I, %) of S. miltiorrhizae samples on P. aeruginosa and the peak areas of the common peaks. The results showed that the 32S. miltiorrhizae samples could be grouped into three clusters according to their chemical fingerprints and anti-P. aeruginosa activities. Protocatechualdehyde, salvianolic acid B, together with three unidentified compounds might be the major components that contributed largely to the antibacterial properties of S. miltiorrhizae and should be the focus of S. miltiorrhizae quality control. Thus, this study provided a preferred way for exploring the bioactive components of medicinal plants.
Similar content being viewed by others
Introduction
Salvia miltiorrhizae (family Lamiaceae), called “Danshen” in Chinese, has been officially listed in the Chinese pharmacopoeia (2015 edition), United States Pharmacopeia (USP39-NF34) and European Pharmacopoeia (EP6.1) as an important and well-known medicinal plant. With pronounced antioxidative, antibacterial and anticoagulant properties, it has been widely used to treat cerebrovascular1 and cardiovascular diseases2, as well as to prevent inflammation3. The main bioactive constituents of S. miltiorrhiza include phenolic compounds and lipophilic diterpenoid tanshinones, which are typically evaluated during quality control of S. miltiorrhizae and its related products4. Several chromatographic techniques including liquid chromatography (LC)5,6,7, gas chromatography (GC)8, high-speed counter-current chromatography (HSCCC)9, and thin-layer chromatography (TLC)10, have been used to construct chemical fingerprints of S. miltiorrhizae, which allow researchers to visualize and identify as many components as possible11. Among these techniques, LC (used in tandem with various detectors) is typically employed to establish the chemical fingerprint characteristics of S. miltiorrhizae. S. miltiorrhizae samples derived from different geographical sources, produced by different manufacturers, or processed by different methods, will have differences in their fingerprints that reflect the differences in their ingredients, as well as their relative contents. Fingerprints have proved to be a useful tool in quality control of S. miltiorrhizae and related products12, 13. However, it is not clear which components are responsible for the pharmacological activities of S. miltiorrhizae in curing many diseases. Chemical fingerprints alone only improve quality control and standardization, but cannot determine the antibacterial efficacy of S. miltiorrhizae.
To monitor microbial growth, isothermal microcalorimetry can be used. Isothermal microcalorimetry is a sensitive technique that has been used successfully to evaluate the effects of various substances on microbial metabolism14. It provides a continuous measurement of heat-production by variety of microbes in a complex medium, and thereby giving both qualitative and quantitative thermodynamic information on the biological activity of the microbes15. By evaluating some crucial thermokinetic parameters such as rate constant and heat-flow power, isothermal microcalorimetry can be used to evaluate the efficacy of other substances on the microbes16. This method only requires knowledge of the initial and final energetic states of the system, and is independent of the organisms studied or reaction pathways involved17. As a powerful and appealing tool, isothermal microcalorimetry has been used to describe the effects of many medicinal plants on various types of microbes18,19,20.
One of the microbes is the Pseudomonas aeruginosa. It is a ubiquitous, non-fermentative gram-negative microbe21 that causes many kinds of human infections22, such as bacteraemia in burn victims, urinary-tract infections in catheterized patients, and hospital-acquired pneumonia in patients on respirators23. These infections are difficult to eradicate, in part because of the natural resistance of the bacterium to antibiotics, and can ultimately lead to pulmonary failure and death24. Therefore, it is necessary to screen for highly effective anti-P. aeruginosa compounds with low toxicity using appropriate techniques. Recently, the antibacterial effects of S. miltiorrhizae have attracted growing attention. Nevertheless, to date, no research has focused on identifying the major constituents of S. miltiorrhizae that mainly contribute to its anti-P. aeruginosa activity.
Therefore, this study combined the chemical fingerprints and anti-P. aeruginosa effects of S. miltiorrhizae to establish an integrated evaluation system for exploring the putative active components of this medicinal plant representing its biothermal activity. First, the chemical fingerprints were developed from 5% methanol extracts of 32 batches of S. miltiorrhizae from different sources by high-performance liquid chromatography with diode array detection (HPLC-DAD). This allowed us to characterize as many components as possible, and the peak area of each component was recorded. Then, the anti-P. aeruginosa activity of each S. miltiorrhizae sample was determined by microcalorimetry. Specifically, some crucial thermokinetic parameters obtained from the heat-flow power-time curves of P. aeruginosa growth in the absence and presence of each S. miltiorrhizae sample were evaluated. The chemical fingerprints and biothermal activities of S. miltiorrhizae from different sources were combined to establish fingerprint-activity relationships with the help of chemometrics by carrying out similarity analysis, principal component analysis and multiple linear regression analysis. Understanding these relationships allowed us to further explore the active components of S. miltiorrhizae that contributed largely to the anti-P. aeruginosa activity. The approach described in this study provides a preferred model for future studies. It illustrates how chemical fingerprints can be combined using LC method and biothermal activity by introducing microcalorimetry technique to study the active components of medicinal plants and other valuable materials.
Results and Discussion
HPLC chemical fingerprints
Method validation
The results showed that the relative standard deviation (RSD) values of peak areas for evaluating the precision, repeatability and stability of the method were less than 8.79%, 8.28% and 6.82% respectively. This indicates that the HPLC method was precise and sensitive enough for the qualitative analysis and the establishment of HPLC fingerprints. Thus, the results have proved satisfactory stability and reproducibility of the sample solution.
HPLC fingerprinting and similarity analysis
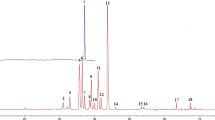
Chemical fingerprints are strongly recommended by many international organizations, including Chinese materia medicas, to accurately profile integrities of complicated matrices for the authentication and identification of their components. The evidence described above shows that the validated HPLC-DAD method was valid and suitable for stable and reproducible chemical fingerprints of S. miltiorrhizae samples. Solutions of 32 batches of S. miltiorrhizae samples collected from different sources were prepared under the optimum chromatographic conditions (see Materials and Methods) and loaded into the HPLC system. Typical HPLC fingerprints were recorded (Fig. 1A), indicating that these samples had similar chemical profiles with some common chemical components. A reference fingerprint was generated by comparing samples’ DAD spectra and HPLC retention times (Fig. 1B). This reference fingerprint was created with the assumption that all the common peaks should be observed in every sample. Eighteen common characteristic peaks were identified in the reference chromatogram. Table S1 listed the average peak area and relative retention time of the 18 common characteristic peaks in the 32 samples.
Although all samples shared similar HPLC fingerprints in Fig. 1A, the average peak areas of the 18 common peaks varied between samples. The coefficients of variance (C.V.%) for peak area ranged from 65.9–178.0%. This indicated that, in addition to qualitative differences between samples, there were significant differences in the amount of each component between samples.
Next, the professional similarity evaluation software was used to perform similarity analysis on the data reported in Table S1. The similarities between the reference fingerprint and each chromatographic profile of the 32 S. miltiorrhizae samples were evaluated by calculating the correlation coefficients, which for the 32 samples were as follows: 0.876, 0.872, 0.808, 0.856, 0.824, 0.867, 0.962, 0.962, 0.963, 0.385, 0.445, 0.302, 0.447, 0.96,0 0.966, 0.844, 0.919, 0.881, 0.935, 0.96, 0.966, 0.982, 0.953, 0.967, 0.963, 0.921, 0.877, 0.820, 0.797, 0.933, 0.961, and 0.922. Significant variations of correlation coefficients ranging from 0.302 to 0.982 further indicated that the chemical fingerprints and internal qualities of S. miltiorrhizae samples vary greatly between samples derived from different sources.
Hierarchical Clustering Analysis
In order to explore the natural clustering of S. miltiorrhizae samples from different sources, hierarchical clustering analysis (HCA) was performed on HPLC fingerprints. Thirty-two samples were analyzed using HCA of the peak areas of the 18 common analytical markers in the HPLC fingerprints. The between-groups linkage method was chosen as average linkage and the squared Euclidean distance was selected to establish clusters, and to assess the resemblance of the 32 batches of samples. The resulting hierarchical cluster heat-map in Fig. 2 produced well-defined clusters and showed the peak area profile of the 18 common peaks. Samples were obviously grouped into three main clusters. Samples S2 and S3 collected from Sichuan province, S7–S9 from Henan province, and S14 and, S15 from Beijing city were categorized into cluster I; Samples S4–S6 collected from Sichuan province, S10–S13 from Shandong province, and S20 and S21 from Guizhou province were grouped into a second distinct cluster (cluster II). The remaining samples including S16–S19, S22–S32 from Guizhou province and S1 from Sichuan province were put into cluster III. As shown in Fig. 2, samples from the same source were typically grouped together. The distances between samples in the same cluster were shorter than distances between samples not in the same cluster, indicating that HPLC chemical fingerprints and internal qualities were more similar within clusters compared to samples in other clusters. The distance between cluster II and III was shorter than the distances between these clusters and cluster I. This result could be explained by the fact that samples were collected different geographic settings, which have different cultivated climates, soil quality, longitude and latitude. These results agreed with the findings from the similarity analysis, and with a simple visual comparison of HPLC chromatograms, including comparison of color variation between the 18 common peaks (Fig. 1A), which might reflect their different bioactivity. Therefore, in the next section, the biothermal activities of the 32 samples on P. aeruginosa growth were studied.
Biothermal activity evaluation
Metabolic power-time curves of P. aeruginosa growth
Using the microcalorimeter, the normal power-time curve of P. aeruginosa growth at 37 °C without any substance was recorded and shown in Fig. 3A. Because P. aeruginosa was grown under airtight conditions in the glass ampoules, bacterial consumption of nutrients and oxygen was limited. Therefore, the growth curve of P. aeruginosa was divided into five phases: lag phase (A-B), first exponential phase (B-C), transition phase (C-D), second exponential phase (D-E), and decline phase (E-F).
Growth rate constant (k) of P. aeruginosa
The heat-flow power-time curves of P. aeruginosa growth in the LB culture medium at 37 °C monitored by the microcalorimeter showed that the bacteria growth can be described by an exponential model as follows:
where P 0 and P t represent the heat-flow power at time 0 and t, respectively. The exponential phase of P. aeruginosa growth corresponded to Eq. (1). Using this equation, the growth rate constants (k) including k 1 and k 2, which corresponded to the first and second exponential phase of P. aeruginosa growth, could be calculated by fitting ln P t and t to a linear equation. The values of k 1 and k 2 in the absence of other substances of three reduplicative experiments were k 1 = (0.00850 ± 0.00134) min−1 and k 2 = (0.01468 ± 0.00246) min−1, with correlation coefficients greater than 0.99284, indicating good reproducibility of the microcalorimetry measurements.
Biothermal activity of S. miltiorrhizae on P. aeruginosa
Addition of different concentrations of S. miltiorrhizae sample solution to the internal system of P. aeruginosa growth in the glass ampoule would influence bacterial growth. As shown in Fig. 3(B), addition of 1000 µg/mL of S. miltiorrhizae sample solution produced a strong inhibitory effect on P. aeruginosa growth by expressing satisfactory power-time curve, as well appropriate peak height and appearance time of the first and second highest peaks. Therefore, a final concentration of 1000 µg/mL was used for all S. miltiorrhizae sample solutions added to the ampoule.
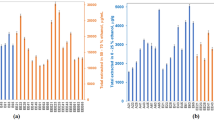
The heat-flow power-time curves of P. aeruginosa growth at 37 °C in the presence of 32 S. miltiorrhizae sample solutions are shown in Fig. 4. It was found that the shape of these power-time curves was similar to the control without S. miltiorrhizae sample, i.e., the five phases of the curves still existed; however, the first and second peaks’ height of the treatment sample was lower and the appearance time was prolonged relative to control, illustrating that the 32 S. miltiorrhizae samples all had inhibitory effects on P. aeruginosa growth. The samples’ anti-P. aeruginosa activity could also be quantitatively evaluated by examining the changes in some thermokinetic parameters (Table 1) obtained from the power-time curves. These parameters included the growth rate constants k 1 and k 2 (min−1) for the first and second exponential phase, the two maximal heat-flow powers P 1 m and P 2 m (mW), the appearance time t 1 m and t 2 m (min) of the two highest peaks, and the heat outputs Q 1 and Q 2 for the two growth stages associated with the total heat output Q t.
The thermokinetic parameters reported in Table 1 were different for all the samples. In general, the smaller the values of k 1, k 2, P 1 m, P 2 m are, and the bigger the values of t 1 m, t 2 m are, the stronger anti-bacterial activity a substance possesses25. From Table 1 and Figure S1, it could be figured out that the nine parameters exhibited different increasing or decreasing trends to various degrees. Thus, examination of a single parameter was not helpful for accurate assessment of the biothermal activities of the 32 S. miltiorrhizae samples. Therefore, it was necessary to identify the most important parameter(s) for quick and accurate assessment. To solve this problem, PCA was introduced.
Results of PCA
PCA can summarize and contract the information residing in the multi-dimensional original data, i.e., either the chemical descriptors, the biological responses, or both, into a few descriptive dimensions that are denoted as principal components to represent the main variation in the data26. Therefore, PCA is recommended to reduce the computation burden. Here, the values of the nine thermokinetic parameters in Table 1 were set as variables, and the 32 samples were set as observations. Then, the variables were centered and scaled to “Unit Variance” before performing the PCA by SIMCA-P. The results in Fig. 5 show that the first three principal components (t1/t2/t3) explained 80.7% of the total variance. Moreover, the 32 S. miltiorrhizae samples were divided into three groups in Fig. 5A: group I included samples S1–S6 from Sichuan province, group II contained samples S16–S32 from Guizhou province, and group III contained samples S7–S15 from Henan and Shandong provinces, and Beijing city. The samples of interest were clearly grouped into distinct classes of anti-P. aeruginosa activity according to their collection site. This might be due to the differences in harvesting time, longitude and latitude of the growth site, or differences in climate, soil and water conditions, and other factors. The results obtained from PCA based on the anti-P. aeruginosa activities of S. miltiorrhizae samples were in accordance with that of HCA of the chemical fingerprints.
Additionally, the 3D loading plots (p1/p2/p3 loading plot in Fig. 5B) indicate that t 2 m and k 2 were the two most important thermokinetic parameters that could be used to access the anti-P. aeruginosa activity of S. miltiorrhizae samples. These two parameters could be used to calculate an inhibition ratio to quantitatively compare the biothermal activities between samples.
Inhibition ratio (I, %) of S. miltiorrhizae samples on P. aeruginosa
To quantitatively describe the inhibitory effects of S. miltiorrhizae samples on P. aeruginosa, the inhibition ratio (I, %) is calculated according to the following formula:
where k (2,0) is the growth rate constant for the second exponential growth phase of P. aeruginosa in the absence of any substance (the control) and k (2,s) is the growth rate constant in the presence of an S. miltiorrhizae sample. I reflects the inhibition extent and effects of S. miltiorrhizae on the bacteria’s growth. Higher the values of I represent stronger the inhibitory effects of S. miltiorrhizae on P. aeruginosa growth.
Based on the parameter k 2 in Table 1, I values were calculated according to Eq. (2) (Table 1). The maximum value of I is 65.99% for sample S13 collected from Shandong province, which is almost four times greater than the minimum value of I (18.72%) for sample S3 from Sichuan province. These results further illustrate the substantial differences in the anti-P. aeruginosa activity of the 32 S. miltiorrhizae samples from various sources. Box and whisker plots in Fig. 6 show the markedly wide distribution of I values, as well as the changing trends, for all samples. The minimum and maximum values (which indicate the range of I), the median value (which represent the average), and the 25% and 75% quartile values of I (depicted as a box to indicate the variability around this average) demonstrated that samples collected from Beijing city had the greatest inhibitory effect, followed by samples from Shandong, Henan, Guizhou and Sichuan provinces. Taking the trending line to show the median values into account, it can be concluded that the S. miltiorrhizae samples from Beijing city exhibited the strongest anti-P. aeruginosa activities, while the samples from Sichuan province had the smallest inhibitory effects. Combining these findings with the groupings of samples in Fig. 5A, we further predicted that the magnitude of the three groups’ anti-P. aeruginosa activities should be ordered as follows: group III > group II > group I. Furthermore, within group III, the order of greatest inhibitory effect would be: Beijing city > Shandong province > Henan province. Different harvesting time, longitudes and latitudes location of growth sites, and different climates, soil and water conditions might account for the different anti-P. aeruginosa activities between samples, which should be explored in detail in future studies.
Results of MLRA
The results described above demonstrated that S. miltiorrhizae samples from various sources had different chemical profiles and different anti-P. aeruginosa activities. However, which of the main active components within the chemical profile contributed largely to the anti-P. aeruginosa activities of S. miltiorrhizae samples were not clear. Therefore, MLRA was used to help in establishing the fingerprint-activity relationships by comparing chemical fingerprints to biothermal activity.
MLRA generalizes directly to multiple predictor variables and the multiple linear regression equation relates the continuous response variables (Y) to predictor variables (x). In this study, it was found that a strong linear relationship exists between \(\sqrt{I}\) (the square root of I/%) and the normalized peak area values. Therefore, the value of \(\sqrt{I}\) in Table 1 was set as the dependent variable (Y) and the normalized peak area values of 18 chromatographic peaks (described in Table S1) were set as independent variables x 1, x 2, x 3, … x 18 by fitting a linear equation with multiple linear regression model. The results showed that the F value was 1.658 and the corresponding P value was 0.000 (ie., less than 0.05), demonstrating that the MLRA model was satisfactory. The regression equation was listed as:
The equation above describes the degree to which the 18 peaks or components in S. miltiorrhizae samples contributed to the anti-P. aeruginosa activity. x 4, x 8, x 13, x 17 and x 18 had the greatest important influence on I, with correlation coefficients >0.4, indicating that peak 4 (protocatechualdehyde), peak 8 (unidentified compound), peak 13 (salvianolic acid B), peak 17 (unidentified compound), and peak 18 (unidentified compound) might be the main bioactive components that conferred the anti-P. aeruginosa activity of S. miltiorrhizae samples. In contrast, other peaks, including peak 2 (danshensu), peak 6 (protocatechuic acid), and peak 10 (rosmarinic acid) contributed little to the anti-P. aeruginosa activity. Among the five components with major contributions to activity, peaks 4, 8, 13, and 17 were positively correlated to I, which meant that concentrations of these four compounds were associated with greater anti-P. aeruginosa activity. Peak 18, however, was negatively correlated with antibacterial activity; therefore increased concentration of this compound would decrease antibacterial activity. Interestingly, Fig. 1B and Table S1 show that the areas of peak 4 (protocatechualdehyde), peak 8 (unidentified compound), peak 17 (unidentified compound) and peak 18 (unidentified compound) are often quite small, in spite of these components’ major influence on anti-P. aeruginosa activity. In contrast, peak 13 (salvianolic acid B), together with other peaks or components that have bigger peak areas (therefore higher contents) exhibited minor influences on anti-P. aeruginosa activity. In other words, the most abundant compounds in S. miltiorrhizae might not be the major contributors to antibacterial activity, and some compounds (including unidentified compounds) should be the focus of future studies. The chemical structures of compounds 8, 17, and 18 need be identified.
Conclusion
To explore the main antibacterial components of Salvia miltiorrhizae that inhibit the growth of P. aeruginosa, this study established the chemical fingerprints of 32 batches of S. miltiorrhizae samples from different sources using HPLC-DAD method. Further, the biothermal activities of these samples on P. aeruginosa growth were determined using microcalorimetry. With the help of chemometric approaches such as HCA, PCA and MLRA, the chemical fingerprints and biothermal activity data were evaluated together to establish the fingerprint-activity relationships. These approaches gave similar results. The 32 S. miltiorrhizae samples were grouped into three clusters according to their chemical fingerprints and anti-P. aeruginosa activities. All the samples from various sources expressed strong anti-P. aeruginosa activities. Furthermore, protocatechualdehyde, salvianolic acid B and three unidentified compounds might be the major components that primarily contribute to antibacterial activity, and could be important factors for quality control of S. miltiorrhizae.
To the best of our knowledge, this is the first report to establish fingerprint-activity relationship for exploring the possible antibacterial components of S. miltiorrhizae. This study’s approach could act as a model for future studies. We believe that our method will provide a powerful way to combine chemical fingerprints and biothermal activity evaluation to identify the bioactive components in medicinal plants and other valuable materials, which could be crucial for quality control.
Materials and Methods
Materials
Six top-geoherbs of S. miltiorrhizae samples (labeled as S1–S6) and the rest of 3 batches (S7–S9), 4 batches (S10–S13), 2 batches (S14 and S15) were purchased from Sichuan province, Henan province, Shandong province, Tongrentang and Hongshengtang Co. Ltd., respectively, the other 17 batches of S. miltiorrhizae samples (labeled as S16–S32) were offered by Guizhou Xinbang Pharmaceutical Co. Ltd., which were authenticated by Prof. Yulin Lin (Institute of Medicinal Plant Development, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China). All samples were collected in sterilized polyethylene bags and stored at −20 °C before analysis.
The standards of salvianolic acid B, danshensu, protocatechuate, protocatechualdehyde and rosmarinic acid (purity >99%) were provided by Chengdu Must Bio-Technology Co. Ltd, Chengdu, China.
Pseudomonas aeruginosa (ATCC27853) was provided by Clinical Examination Center of 302 Hospital of People’s Liberation Army, Beijing, China. The Luria-Bertani (LB) culture medium was prepared by dissolving 10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl in 1000 mL of distilled water at pH7.0–7.2. Then, the LB culture medium was sterilized by autoclaving at 0.1 MPa and 121 °C for 30 min. Initially, P. aeruginosa was inoculated in a 30-mL sterilized flask and incubated in the shaker at rotation rate of 110 rpm for 6 h at 37 °C, then was stored in a refrigerator at 4 °C.
Methanol and acetonitrile (HPLC grade) were obtained from Fisher Scientific (Fair Lawn, NJ, USA). Formic acid was bought from Xilong Chemical Co., Ltd. (Guangdong, China). Purified water was used (Wahaha, Hangzhou, China). A KQ-500 ultra-sonic cleaning bath (50 × 30 × 35 cm) manufactured by Kunshan Ultrasonic Instrument Co., Ltd. was used (Jiangsu, China).
Instrumentation
HPLC fingerprints were measured with a Shimadzu Prominence system equipped with LC-20AT quaternary gradient pump, a SPD-M20A diode array detector (DAD), CBM-20A communication bus module, CTO-20A thermostatic column compartment, SIL-20A autosampler and Shimadzu LC solution software (Version 1.21 SP1).
A 3114/3236 TAM air microcalorimeter (Thermometric AB, Sweden) was used to determine the metabolic heat-flow power-time curves of P. aeruginosa growing in the LB culture medium. As an isothermal heat conduction calorimeter operating in the microwatt range, the microcalorimeter has eight calorimetric channels to keep the temperature in the range of 20–80 °C. All channels were mounted together to form a single heat-sink block housed in a temperature-controlled air thermostat. Each calorimetric channel was constructed in twin configuration with one side for the sample and the other side for a static reference. For more details about the instrument, refer to ref. 27.
HPLC fingerprints
HPLC conditions
For chromatographic separation, a COSMOSIL Packed 5C18-PAQ column (5 μm, 250 mm × 4.6 mm, COSMOSIL, Japan) was used at 30 °C. The mobile phase consisted of 1% formic acid in water (eluent A) and acetonitrile (eluent B) by ultrasounding for deaeration prior to use. The flow rate was 1.0 mL/min at the following gradient program: 0.01–15 min, 7–11% B; 15–27 min, 11–26% B; 27–55 min, 26–27% B; 55–75 min, 27–40% B; 75–105 min, 40–100% B; 105–113 min, 100-7% B. The detection wavelength was set at 284 nm and an aliquot of 20 μL of sample solution was injected into the HPLC-DAD system. All samples and standards were filtered through a 0.22-μm Millipore membrane before use.
Standards preparation
The mixed standards solution was prepared by adding an accurately weighed amount of salvianolic acid B, danshensu, protocatechuate, protocatechualdehyde and rosmarinic acid to a volumetric flask and dissolved with 5 mL of methanol to make a final concentration of 500 µg/mL.
Sample preparation
An aliquot of 0.2 g of dried S. miltiorrhizae powder (filtered through a 50-mesh sieve) was accurately weighed and transferred into a 50-mL flask. After addition 20 mL of 5% methanol, the flask was weighed and transferred into an ultrasonic cleaning bath for extraction for 30 min at room temperature. Then, any lost weight was made up and the mixture was centrifuged at 10000 rpm for 10 minutes. Afterward, the supernatant was transferred into a centrifuge tube and filtered through a 0.22-μm Millipore membrane and the filtrate was collected as sample solution. All samples were stored at 4 °C in the dark until analysis.
Method validation
The methodology of chromatographic fingerprinting was validated for its precision, repeatability and stability. Intra- and inter-day variations were determined to assess the precision of the established HPLC-DAD method. Intra-day precision was evaluated by six successive injections of the randomly selected sample 27 (S27) solution on the same day, while inter-day precision was determined on five consecutive days. To confirm the repeatability, six different solutions prepared from S27 were analyzed. Meanwhile, the sample stability was examined by analysis of S27 solution stored at 4 °C for different time periods (0, 2, 4, 8, 24, 36 and 48 h). The variation for all the above assessment was expressed as relative standard deviation (RSD).
Similarity analysis
Under the optimized chromatographic conditions, each sample solution of 5% methanol extract from S. miltiorrhizae and the reference standard solution were injected into the HPLC system to create a HPLC-DAD chromatogram. Relevant information including peak area and retention time of all peaks was recorded and entered into the professional software named Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine developed by Chinese Pharmacopoeia Committee (Version 2004A) (Beijing, China) to reconstruct the chemical fingerprints for automatic match. Similar chromatogram shapes generated from S. miltiorrhizae samples from different sources were difficult to identify or sort using only observation with the naked eye or microscopic observation. Therefore, hierarchical clustering analysis (HCA), a powerful chemometric method, was introduced.
Hierarchical clustering analysis
As a multivariate analysis technique, HCA can be used to sort samples into groups according to their internal characteristics. A dendrogram of all samples was obtained to represent the similarity or dissimilarity between samples. Each sample within the same group is similar to the others but different from those in other groups, with respect to a predetermined selection criterion. In this study, 32 batches of S. miltiorrhizae samples were analyzed using HCA according to the peak area of each of the 18 common peaks by HemI software (Heatmap Illustrator, version 1.0). Between-groups linkage clustering was chosen and the Euclidean distance was selected to assess the resemblance of the 32 samples, and then classify them into groups. Results were presented as a hierarchical cluster heat-map and two-way clustering was represented as a rectangular tiling of a data matrix, with clustering trees appended to the margins between characteristic peak areas. Within a relatively compact display area, this visual representation facilitates inspection of row, column, and joint cluster structure. This flexible architecture underscores the fact that a heat-map is a visual reaction of a statistical model. The hierarchical cluster heat-map is commonly used in the natural sciences and one of the most widely used graphs in the biological sciences.
Microcalorimetric measurement
Sample preparation
An aliquot of 0.2 g dried S. miltiorrhizae powder (through a 50-mesh sieve) was accurately weighed and transferred into a 50-mL flask. After being added with 20-mL of 70% methanol, the flask was weighed and transferred into the ultrasonic cleaning bath for extraction for 30 min at room temperature. Then, the lost weight was made up and the mixture was centrifuged at 4000 rpm for 10 min. Afterward, the supernatant was transferred into a 125-mL crucible to dry on the water bath at 90 °C. All samples were stored at 4 °C in the dark. For the bioactive evaluation, the dried extract was re-dissolved in the LB culture medium to yield the final concentration of 1000 µg/mL.
Experimental procedure
First, all the 10 mL ampoules were washed with an acid detergent, rinsed with deionized water, and dried at 60 °C for 60 min in a drying cabinet, followed by autoclaving at 121 °C for 25 min. Initially, the concentration of P. aeruginosa inoculated into the LB culture medium was controlled at 2 × 106 cells/mL. For the experiment, the first ampoule with no sample extract was regarded as the control, the remaining ampoules were aseptically filled with 6 mL of LB culture medium, 3 mL of P. aeruginosa suspension, and 1 mL of re-dissolved sample solution of S. miltiorrhizae from different sources. All experimental procedures were performed in a super-clean worktable. The ampoules were sealed with a crimped metal lid and silicon rubber seal. Ampules were then sequentially introduced into the instrument previously stabilized at 37 °C. Due to the temperature differences between the ampoules and the microcalorimeter, the ampoules will absorb heat. After about 45 min (when the temperature of ampoules reached 37 °C), the heat-flow power-time (p-t) curves were recorded until the recorder returned to baseline. All measurements were performed at 37 °C and all data were collected continuously using the dedicated software package.
Principal component analysis
Principal component analysis (PCA) is a simple, non-parametric method for extracting relevant information from a complex data set. It is a standard modern tool for data analysis used in various fields from neuroscience to computer graphics28. PCA was applied to reduce the dimensionality of the original data set and to explain the correlation of a large number of independent variables in terms of a smaller number of underlying factors (principal components or PCs) without losing much information29. From the power-time curve of P. aeruginosa growth in the presence of S. miltiorrhizae, many important parameters could be obtained for quantitative analysis. However, the different changing trends and occasional overlap of these parameters usually impedes quick and accurate explanation of the bioactivities of these samples on P. aeruginosa. To solve this problem, the main parameter(s) could be selected. Therefore, in this study, the quantitative thermo-kinetic parameters were analyzed by PCA using SIMCA-P 11.5 software (Umetrics AB, Umea, Sweden).
Multiple linear regression analysis
Multiple linear regression analysis (MLRA) is usually used to model the best combination of two or more independent variables (x i) to predict or estimate the dependent variable (Y) by fitting a linear equation. It shows the contribution of each independent variable to the dependent variable in the following form:
where Y is the estimated value and represents the dependent variable and x i are the uncorrelated variables; b 0 represents the estimated constant, and b i is called the regression coefficients. In this study, MLRA was introduced to combine data from the chemical fingerprints (peak area of each component) and biothermal activity (main quantitative thermo-kinetic parameters) of S. miltiorrhizae samples on P. aeruginosa. This allowed us to establish the fingerprint-activity relationships using SPSS statistical software (SPSS for Windows 13.0, SPSS Inc., USA), to further explore the active components of S. miltiorrhizae that primarily contributed largely to the anti-P. aeruginosa activity.
References
Chen, X. P., Guo, J. J., Bao, J. L., Lu, J. J. & Wang, Y. T. The anticancer properties of salvia miltiorrhiza Bunge (Danshen): a systematic review. Med Res Rev. 34, 768–794 (2014).
Cheng, T. O. Cardiovascular effects of Danshen. Int J Cardiol. 121, 9–22 (2007).
Zhou, L. M., Zuo, Z. & Chow, M. S. S. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharm. 45, 1345–1359 (2005).
Jiang, R. W. et al. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem. 12, 237–246 (2005).
Cheng, X. M. et al. HPLC fingerprints combined with principal component analysis, hierarchical cluster analysis and linear discriminant analysis for the classification and differentiation of Peganum sp. indigenous to China. Phytochem Analysis 21, 279–289 (2010).
Xie, P. S. et al. Chromatographic fingerprint analysis-a rational approach for quality assessment of traditional Chinese herbal medicine. J Chromatogr A 1112, 171–180 (2006).
Lu, G. H. et al. Development of high-performance liquid chromatographic fingerprints for distinguishing Chinese Angelica from related umbelliferae herbs. J Chromatogr A 1073, 383–392 (2005).
Ma, C. F., Wang, H. H., Lu, X., Xu, G. W. & Liu, B. Y. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatography-mass spectrometry. J Chromatogr A 1186, 412–419 (2008).
Gu, M., Zhang, S. F., Su, Z. G., Chen, Y. & Ouyang, F. Fingerprinting of Salvia miltiorrhiza Bunge by non-aqueous capillary electrophoresis compared with high-speed counter-current chromatography. J Chromatogr A 1057, 133–140 (2004).
Chen, S. B. et al. High-performance thin-layer chromatographic fingerprints of isoflavonoids for distinguishing between Radix Puerariae Lobate and Radix Puerariae Thomsonii. J Chromatogr A 1121, 114–119 (2006).
Proestos, C., Loukatos, P. & Komaitis, M. Determination of biogenic amines in wines by HPLC with precolumn dansylation and fluorimetric detection. Food Chem. 106, 1218–1224 (2008).
Zhou, X., Peng, J. Y., Fan, G. R. & Wu, Y. T. Isolation and purification of flavonoid glycosides from Trollius ledebouri using high-speed counter-current chromatography by stepwise increasing the flow-rate of the mobile phase. J Chromatogr A 1092, 216–221 (2005).
Orzel, J. et al. Modeling of the total antioxidant capacity of rooibos (Aspalathus linearis) tea infusions from chromatographic fingerprints and identification of potential antioxidant markers. J Chromatogr A 1366, 101–109 (2014).
Kong, W. J. et al. Effect of emodin on Candida albicans growth investigated by microcalorimetry combined with chemometric analysis. Appl Microbiol Biot. 83, 1183–1190 (2009).
Kwon, H. A., Kwon, Y. J., Kwon, D. Y. & Lee, J. H. Evaluation of antibacterial effects of a combination of Coptidis Rhizoma, Mume Fructus, and Schizandrae Fructus against Salmonella. Int J Food Microbiol. 127, 180–183 (2008).
Liu, S. X. et al. Anti-bacterial effect of four extracts from leaves of Dracontomelon dao on Escherichia coli growth using microcalorimetry coupled with principal component analysis. J Therm Anal Calorim. 116, 491–497 (2014).
Braissant, O., Bachmann, A. & Bonkat, G. Microcalorimetric assays for measuring cell growth and metabolic activity: Methodology and applications. Methods 76, 27–34 (2015).
Wang, J. B. et al. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 17, 684–689 (2010).
Kong, W. J. et al. Antibacterial evaluation of flavonoid compounds against E. coli by microcalorimetry and chemometrics. Appl Microbiol Biotechnol. 99, 6049–6058 (2015).
Zhao, Y. L. et al. Microcalorimetry coupled with principal component analysis for investigating the anti-Staphylococcus aureus effects of different extracted fractions from Dracontomelon dao. J Therm Anal Calorim. 120, 913–920 (2015).
Hardalo, C. & Edberg, S. C. Pseudomonas aeruginosa: assessment of risk from drinking water. Crit rev in microbiol. 23, 47–75 (1997).
Lyczak, J. B., Cannon, C. L. & Pier, G. B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2, 1051–1060 (2000).
Stover, C. K. et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406, 959–964 (2000).
Gasink, L. B. et al. Fluoroquinolone-resistant Pseudomonas aeruginosa: assessment of risk factors and clinical impact. Am J Med. 119(526), e19–526.e25 (2006).
Wu, Y. W., Ouyang, J., Xiao, X. H., Gao, W. Y. & Liu, Y. Antimicrobial properties and toxicity of anthraquinones by microcalorimetric bioassay. Chinese J Chem. 24, 45–50 (2006).
Eriksson, L., Andersson, P. L., Johansson, E. & Tysklind, M. Megavariate analysis of environmental QSAR data. Part I-a basic framework founded on principal component analysis (PCA), partial least squares (PLS), and statistical molecular design (SMD). Mol Divers. 10, 169–186 (2006).
Wadsö, I. Isothermal microcalorimetry in applied biology. Thermochim Acta 394, 305–311 (2002).
Shlens, J. A tutorial on principal component analysis. arXiv preprint arXiv 1404, 1100 (2014).
Liu, J., Li, Q., Dong, J. J., Chen, J. & Gu, G. X. Multivariate modeling of aging in bottled lager beer by principal component analysis and multiple regression methods. J Agr Food Chem. 56, 7106–7112 (2008).
Acknowledgements
The authors are grateful for the support from the National Natural Science Foundation of China (81473346, 81573595 and 81673593) and CAMS Innovation Fund for Medical Sciences (2016-I2M-3-010).
Author information
Authors and Affiliations
Contributions
W.J.K. and Y.L.Z. conceived the project. S.S.Z. designed the structure and carried out the calculations. S.S.Z., Y.L.Z., M.Q.W., P.C., X.R.W., X.P.M., W.Y.G. and M.H.Y. discussed the results and the experimental implementations. W.J.K. wrote the paper and all authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kong, WJ., Zhang, SS., Zhao, YL. et al. Combination of chemical fingerprint and bioactivity evaluation to explore the antibacterial components of Salvia miltiorrhizae . Sci Rep 7, 8112 (2017). https://doi.org/10.1038/s41598-017-08377-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08377-0
This article is cited by
-
Nanopore analysis of salvianolic acids in herbal medicines
Nature Communications (2024)
-
Biological studies and chromatograms aided by chemometric analysis in evaluation of seasonality and extraction method of Croton grewioides extracts
Brazilian Journal of Botany (2022)
-
A strategy for quality evaluation of salt-treated Apocyni Veneti Folium and discovery of efficacy-associated markers by fingerprint-activity relationship modeling
Scientific Reports (2019)
-
Screening for the anti-inflammation quality markers of Xiaojin Pills based on HPLC-MS/MS method, COX-2 inhibition test and protein interaction network
Scientific Reports (2018)
-
Discovery of Hepatotoxic Equivalent Combinatorial Markers from Dioscorea bulbifera tuber by Fingerprint-Toxicity Relationship Modeling
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.