Abstract
Previous clinical studies have found that the levels of tumor-infiltrating lymphocytes (TILs) significantly correlated with prognosis in hepatocellular carcinoma (HCC). However, these conclusions and data remain controversial. We performed a systematic review and meta-analysis to assess the prognostic value and clinical utilization of TILs in patients with HCC. A total of 23 relevant studies of 3173 patients were included into our meta-analysis. The results demonstrated that high levels of CD8+ and CD3+ TILs had a better prognostic value on overall survival (OS), with HRs of 0.71 (P = 0.04) and 0.63 (P = 0.03), respectively, compared to low levels, as did high levels of CD8+, CD3+ and CD4+ TILs on disease/recurrence-free survival (DFS/RFS), with HRs of 0.66 (P = 0.01), 0.60 (P = 0.01) and 0.79 (P = 0.04), respectively. In contrast, high levels of FoxP3+ TILs had a worse prognostic value on OS and DFS/RFS, with HRs of 2.06 (P < 0.00001) and 1.77 (P < 0.00001), respectively. The FoxP3+/CD4+ and FoxP3+/CD8+ ratios negatively correlated with OS and DFS/RFS. These findings suggest that TILs may serve as a prognostic biomarker in HCC. However, further research should be performed to clarify the clinical value of TILs in HCC.
Similar content being viewed by others
Introduction
The oncogenesis and development of malignant tumors, including initiation, progression, malignant conversion, invasion and metastasis, are dynamic processes that involve multiple links, stages and genes. Traditional cancer research exclusively focuses on the internal changes of tumor cells themselves, including genetic and phenotypic changes. However, the continuous progress of gene and molecular biology technology has revealed the complicated functions of the tumor microenvironment in tumor evolution1,2,3. The tumor microenvironment plays a vital role in tumor epigenetics, tumor differentiation, immune escape, and infiltration metastasis. The host immune response and immune cells are crucial factors of the tumor microenvironment that are consistently involved throughout tumor development4. The immune response has a vital function via regulation of carcinogenesis and cancer progression, including promotion and suppression5, 6. Related research has demonstrated that immune factors are accurate independent prognostic factors that are superior to the TNM stage7, 8. Cancer immunologists and cancer biologists achieved a consensus that cancer is a disease of the microenvironment and immunity9. Current research has also demonstrated that immunotherapy plays a valuable role in anti-tumor treatments, such as active vaccination, adoptive cell transfer therapy and immune checkpoint blockade. Various therapies are being assessed in clinical trials, and the results have demonstrated a definite clinical application value10. Therefore, tumor-infiltrating lymphocytes (TILs), as the most important monitor of the immune response, are a focus of cancer research.
TILs are a group of lymphocytes located around tumor cells that exhibit diverse functions in various subsets. TILs have been identified in primary tumors, lymph nodes, and metastases. CD3+, CD4+, CD8+ and FoxP3+ T lymphocytes are the most common subsets of TILs. CD8+ T lymphocytes primarily belong to cytotoxic T lymphocytes (CTLs), which are primarily responsible for the removal of target cells, including tumor cells. CD4+ T lymphocytes, which are also known as the “auxiliary hand” of the immune system, are referred to as T helper lymphocytes (Ths). Mosmann et al.11 first divided CD4+ T lymphocytes into Th1 and Th2 cells in the early 1980s based on different cell functions and cytokines secreted. Th1 cells enhance the toxic effects of killer cells, such as activating CTLs, or stimulate a delayed-type hypersensitivity to mediate the cell immune response. Th2 cells promote antibody production and mediate the humoral immune response. Researchers also confirmed that other subsets exist in CD4+ T lymphocytes, such as CD4+ regulatory T lymphocytes (Tregs), which characteristically express Forkhead box P3 (Foxp3). Tregs are the most important immunosuppressive cells in the body12, 13. The ratios of the different subsets also have important implications in carcinogenesis. The value of TILs in oncology is not difficult to imagine based on the important position of these cells in tumor immunity, and immune cells, especially TILs, have been a hotspot in cancer research. TILs may present a key breakthrough for anti-tumor therapy.
HCC is one of the most common cancers worldwide, and it has attracted widespread attention because of its high incidence and mortality rate14. The prognosis of HCC patients remains dismal despite the enormous achievements made in clinical treatments during recent decades. There is an urgent need for related targeted molecules to predict outcomes and for use as oncotherapy in HCC. Extensive research has assessed the relationship between TIL levels and HCC, particularly tumor characteristics and prognostic outcome. Some conclusions have been mentioned previously, but the results remain inconsistent and debatable in HCC. We performed a meta-analysis based on data acquired from published studies using specific inclusion and exclusion criteria to clarify the prognostic value of TILs and the ratios of different subsets in HCC. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used as effect measures.
Results
Study selection and characteristics
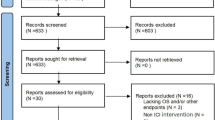
The full texts of 91 articles were scrutinized. Twenty-nine of these articles did not report adequate data to calculate HRs and 95% CIs, and 16 articles were studies of peri-tumoral tissues or peripheral blood. Nine articles were not related to survival analyses, and 7 articles were categorized as meta-analyses, review articles, or case reports. Seven articles were non-English reports. All of these articles were excluded. We identified 23 articles for inclusion in this meta-analysis15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37. Our search and selection processes were performed in strict adherence with the inclusion and exclusion criteria.
These observational retrospective studies evaluated TIL levels and prognostic parameters in HCC from 2004 to 2016. Six of the eligible studies assessed CD3+ T lymphocytes, and 4 studies investigated CD4+ T lymphocytes. Fourteen studies examined CD8+ T lymphocytes, and 13 studies reported FoxP3+ T lymphocytes. Only 2 studies reported FoxP3+/CD8+ ratios, and 3 studies reported FoxP3+/CD4+ ratios. Two studies assessed CD8+/CD3+ ratios. Overall survival (OS) and disease-free survival (DFS) were commonly assessed. However, recurrence-free survival (RFS) and cancer-related/specific survival (CRS/CSS) were not generally reported. CRS/CSS was only reported for FoxP3+ T lymphocyte levels. The median number of patients evaluated per study was 138, and 4 studies included more than 200 patients. Most studies were from Asia, especially China. The clinicopathological characteristics of the patients involved generally provided information on HBV infection, liver cirrhosis, TNM-stage, Child-Pugh score, tumor number, and vascular invasion. Table 1 summarizes some of the characteristics of the eligible studies in the present systematic review and meta-analysis. And Newcastle-Ottawa Scale (NOS) scores of the studies ranged from 5 to 8.
Overall meta-analysis
CD8+ T lymphocytes
Fourteen studies of 2015 patients assessed the effects of CD8+ T lymphocytes on survival and were included in this meta-analysis. We concluded that a positive relationship existed between high levels of CD8+ T lymphocytes and OS (HR = 0.71; 95% CI, 0.51–0.99; P = 0.04; Fig. 1A). The effect on DFS/RFS was also significant (HR = 0.66; 95% CI, 0.47–0.92; P = 0.01; Fig. 1B).
We performed subgroup analyses to assess whether various clinical variables affected survival results (Supplementary Table S1). Patients with high levels of CD8+ T lymphocytes exhibited good OS based on the number of patients (<200; HR = 0.77; 95% CI, 0.62-0.94), percentage of HBsAg(+) patients (≥90%; HR = 0.26; 95% CI, 0.11–0.64), and percentage of patients with multiple tumors (<30%; HR = 0.39; 95% CI, 0.16–0.98). Patients with high levels of CD8+ T lymphocytes benefited from increased DFS/RFS based on the percentage of males (≥80%; HR = 0.64; 95% CI, 0.45–0.91), percentage of patients with liver cirrhosis (≥80%; HR = 0.77; 95% CI, 0.61–0.88), percentage of patients with multiple tumors (<30%; HR = 0.43; 95% CI, 0.19–0.93), and the percentage of vascular invasion (<50%; HR = 0.70; 95% CI, 0.57–0.86). Subgroup analyses indicated that CD8+ T lymphocytes were a positive predictor of OS or DFS/RFS in studies with these clinical variables.
CD3+ T lymphocytes
Six studies involving 1421 patients detected the relationship between the infiltration of CD3+ T lymphocytes and HCC patient survival. The results indicated that high levels of CD3+ T lymphocytes were associated with good OS (HR = 0.63; 95% CI, 0.41–0.94; P = 0.03; Fig. 1C) and DFS/RFS (HR = 0.60; 95% CI, 0.40–0.89; P = 0.01; Fig. 1D).
Subgroup analyses were used to assess whether different clinical characteristics affected survival results. Supplementary Table S2 shows that patients with high levels of CD3+ T lymphocytes benefitted from increased OS based on the percentage of HBsAg(+) patients (≥90%; HR = 0.35; 95% CI, 0.23–0.53), percentage of patients with liver cirrhosis (≥80%; HR = 0.76; 95% CI, 0.59–0.98), percentage of Child-Pugh score A patients (<80%; HR = 0.58; 95% CI, 0.35–0.94), and percentage of vascular invasion (<50%; HR = 0.78; 95% CI, 0.61–0.99). The same results were noted for DFS/RFS based on the number of patients included in the study (<200; HR = 0.67; 95% CI, 0.47–0.95), the percentage of males (≥80%; HR = 0.66; 95% CI, 0.44–0.98; <80%; HR = 0.26; 95% CI, 0.10–0.71), the percentage of HBsAg(+) patients (≥90%; HR = 0.38; 95% CI, 0.27–0.53; <90%; HR = 0.76; 95% CI, 0.60–0.96), the percentage of TNM stage I-II patients (<70%; HR = 0.60; 95% CI, 0.38–0.97), and the percentage of vascular invasion (<50%; HR = 0.73; 95% CI, 0.56–0.93). We concluded that high levels of CD3+ T lymphocytes were a positive prognostic marker of OS or DFS/RFS in studies with these clinical variables.
CD4+ T lymphocytes
The prognostic value of CD4+ T lymphocytes was reported in four studies including 700 patients. High levels of CD4+ T lymphocytes exhibited no significant effect on OS (HR = 0.83; 95% CI, 0.66–1.05; P = 0.12; Fig. 2A), but improved DFS/RFS was observed (HR = 0.79; 95% CI, 0.63–0.99; P = 0.04; Fig. 2B).
Forest plots of relationships between levels of CD4+ and Foxp3+ T lymphocytes and survival. (A) The effect of CD3+ T lymphocytes on OS. (B) The effect of CD3+ T lymphocytes on DFS/RFS. (C) The effect of Foxp3+ T lymphocytes on OS. (D) The effect of Foxp3+ T lymphocytes on DFS/RFS. (E) The effect of Foxp3+ T lymphocytes on CSS/CRS.
Subgroup analyses were also used to assess the consistency of conclusions between different clinical characteristics of patients. Supplementary Table S3 shows that patients with high levels of CD4+ T lymphocytes exhibited improved DFS/RFS based on the number of patients included in the study (<200; HR = 0.69; 95% CI, 0.49–0.96), percentage of males (≥80%; HR = 0.79; 95% CI, 0.63–0.99), percentage of HBsAg(+) patients (<90%; HR = 0.79; 95% CI, 0.63–0.99), percentage of Child-Pugh score A patients (<80%; HR = 0.66; 95% CI, 0.44–0.98), percentage of TNM stage I-II patients (<70%; HR = 0.69; 95% CI, 0.49–0.96), and percentage of patients with multiple tumors (≥30%; HR = 0.69; 95% CI, 0.49–0.96). Subgroup analyses revealed that CD4+ T lymphocytes were a positive predictor of DFS/RFS in studies with these clinical variables.
Foxp3+ Treg lymphocytes
Thirteen studies including 1961 patients reported the relationship between Foxp3+ T lymphocytes infiltration and HCC patient survival. Low levels of Foxp3+ lymphocytes were associated with improved OS (HR = 2.06; 95% CI, 1.74–2.43; P < 0.00001; Fig. 2C) and DFS/RFS (HR = 1.77; 95% CI, 1.53–2.04; P < 0.00001; Fig. 2D). However, the effect on CSS/CRS was not significant (HR = 1.55; 95% CI, 0.83–2.90; P = 0.17; Fig. 2E).
We also used subgroup analyses to assess the consistency of conclusions between different clinical variables. Notably, patients with low Foxp3+ T lymphocytes levels exhibit good OS and DFS/RFS based on all of the clinical variables and characteristics listed in Supplementary Table S4. Subgroup analyses demonstrated that Foxp3+ T lymphocytes were a poor predictor of OS and DFS/RFS in studies regardless of the number of patients (≥200 vs. <200), percentage of HBsAg(+) patients (≥90% vs. <90%), percentage of patients with liver cirrhosis (≥80% vs. <80%), percentage of Child-Pugh score A patients (≥80% vs. <80%), percentage of TNM stage I-II patients (≥70% vs. <70%), percentage of patients with multiple tumors (≥30% vs. <30%), and percentage of vascular invasion (≥50% vs. <50%). These results suggest that Foxp3+ T lymphocytes may be a validated risk biomarker of survival.
Ratios between different subsets
Different TIL subsets exhibited significant prognostic values on survival. Therefore, we continued to use a meta-analysis to evaluate the effect of ratios between different subsets on survival. There were 533 and 392 patients in the study of the effect of Foxp3+/CD4+ ratio on OS and RFS/DFS, respectively. Figure 3 shows that a low Foxp3+/CD4+ ratio correlated with improved OS (HR = 2.11; 95% CI, 1.11–3.98; P = 0.02; Fig. 3A) and DFS/RFS (HR = 2.11; 95% CI, 1.49–2.99; P < 0.0001; Fig. 3B). The effect of Foxp3+/CD8+ ratio on OS and DFS/RFS was analysed on 145 patients. A low Foxp3+/CD8+ ratio correlated with OS (HR = 1.16; 95% CI, 1.03–1.30; P = 0.01; Fig. 3C) and DFS/RFS (HR = 1.15; 95% CI, 1.03–1.30; P = 0.02; Fig. 3D). A total of 386 patients were included in a study on the effect of CD8+/CD3+ ratio on OS, and DFS/RFS was assessed in 245 patients. In contrast, the CD8+/CD3+ ratio exhibited no significant effect on OS (HR = 1.00; 95% CI, 0.68–1.47; P = 1.00; Fig. 3E) or DFS/RFS (HR = 0.88; 95% CI, 0.58–1.34; P = 0.55; Fig. 3F). These results indicate that the Foxp3+/CD4+ and Foxp3+/CD8+ ratios may be risk factors for survival.
Forest plots of relationships between the Foxp3+/CD4+ ratio, Foxp3+/CD8+ ratio, CD8+/CD3+ ratio and survival. (A) The effect of the Foxp3+/CD4+ ratio on OS. (B) The effect of the Foxp3+/CD4+ ratio on DFS/RFS. (C) The effect of the Foxp3+/CD8+ ratio on OS. (D) The effect of the Foxp3+/CD8+ ratio on DFS/RFS. (E) The effect of the CD8+/CD3+ ratio on OS. (F) The effect of the CD8+/CD3+ ratio on DFS/RFS.
Publication bias
Potential publication biases were evaluated by constructing a funnel plot and applying Begg’s test and Egger’s test. The results revealed that no publication bias existed in studies on CD8+ T lymphocytes in association with OS (Begg’s test, P = 0.951; Egger’s test, P = 0.974; Fig. 4A) and DFS/RFS (Begg’s test, P = 0.436; Egger’s test, P = 0.417; Fig. 4B). Similar results were noted for the effect of Foxp3+ TILs on OS (Begg’s test, P = 0.580; Egger’s test, P = 0.650; Fig. 4C) and DFS/RFS (Begg’s test, P = 0.328; Egger’s test, P = 0.396; Fig. 4D).
Funnel plots, Begg’s and Egger’s tests of the meta-analyses assessing the associations between TILs cells and survival. (A) Studies on the effect of CD8+ T cells on OS. (B) Studies on the effect of CD8+ T cells on DFS/RFS. (C) Studies on the effect of Foxp3+ T cells on OS. (D) Studies on the effect of Foxp3+ T cells on DFS/RFS.
Regarding the sample sizes and studies about the the effect of CD3+ and CD4+ TILs on OS and DFS/RFS, Foxp3+ TILs on CRS/CSS were too little, we only conducted the Begg’s test and Egger’s test. The results provided no evidence of publication bias for CD3+ TILs on OS (Begg’s test, P = 0.548; Egger’s test, P = 0.995) and DFS/RFS (Begg’s test, P = 0.339; Egger’s test, P = 0.892), CD4+ TILs on OS (Begg’s test, P = 1.000; Egger’s test, P = 0.746) and DFS/RFS (Begg’s test, P = 0.308; Egger’s test, P = 0.110). We also found no publication bias for the Foxp3+ TILs on CRS/CSS (Begg’s test, P = 0.540; Egger’s test, P = 0.137).
Discussion
Our increasing knowledge of the immune response and immune cells, especially tumor-infiltrating lymphocytes, support the significant value of these cells in multiple malignant tumors. Some studies assessed the prognostic value of tumor-infiltrating lymphocytes in various types of tumors, such as breast cancer, gastric cancer, non-small cell lung cancer, and ovarian cancer38,39,40,41. Many results indicated that TILs may be clinically significant prognostic biomarkers. A meta-analysis from MJM Gooden et al.42 reported the prognostic value of TILs in solid tumors. However, there were only 5 studies of HCC in this meta-analysis, and the prognostic data were analysed with various solid tumors that were not independently related to HCC. Some studies43, 44 individually reported the prognostic value of Foxp3+ T lymphocytes without reference to other subsets of TILs in HCC or were performed using the odds ratio (OR) rather than HR. It is almost impossible to perform perfect research on entire TILs subsets, and research to exclusively assess the prognostic value of Foxp3+ T lymphocytes may not represent the complete effect of TILs on survival. Further research should be performed to investigate the prognostic value of TILs in HCC. Our study strictly followed evidence-based medicine in this meta-analysis.
Our team performed a meta-analysis of 23 studies and 3173 patients using several authoritative databases. From these 23 articles involved in this meta-analysis, we could find some data show a strong relationship exist between TILs and survival, but others did not. These previous results made the effect of TILs on survival remain so controversial. However, through our systematic review and meta-analysis, we got a more unified conclusion that CD3+, CD4+, CD8+, and Foxp3+ could serve as prognostic biomarkers in hepatocellular carcinoma. We calculated HRs and 95% CIs associated with high versus low marker counts and demonstrated that high levels of CD8+ and CD3+ TILs improved OS, and high levels of CD8+, CD3+ and CD4+ TILs were associated with improved DFS/RFS. Therefore, these immune cells may be beneficial for survival. In contrast, Foxp3+ TILs levels and the Foxp3+/CD4+ and Foxp3+/CD8+ ratios were negatively associated with OS and DFS/RFS. Therefore, these factors may be prognostic risk factors for survival. Unfortunately, CD4+ TILs exhibited no statistical prognostic value on OS, and the CD8+/CD3+ ratio was not significantly related to OS or DFS/RFS. Sample size was too small to establish significant impacts of FoxP3+ TILs on CRS/CSS, we only found three studies and the heterogeneity was high (I2 = 76%). The current data are not comprehensive, and CRS/CSS was not generally reported. Therefore, further exploration is needed to obtain more credible data to analyse the effect of Foxp3+ TILs on CRS/CSS.
The mechanisms by which immune cells predict prognosis are not clear. Various types of immune cells play different roles in the tumor microenvironment, primarily via immunosuppressive and immunological effects. Some cells exert immunosuppressive effects, such as Foxp3+ Treg cells and mastocytes, and other cells exert immunological effects, such as cytotoxic T lymphocytes (CTLs), memory T lymphocytes, macrophages, and T helper lymphocytes. These effects are indispensable and influence each other. The determining factor of overall immune status depends on the sum of their effector functions or secretion of immuno-active substances. Immunological effector cells can be inhibited via the secretion of immunosuppressive factors, such as IL-10 and TGF-β1, granzyme and perforin expression, or competitive binding with IL-2 by immunosuppressive cells when the immunosuppressive role was strong, similar to the high levels of Foxp3+ TILs in this meta-analysis. These conditions promote the generation of immune tolerance and escape in tumor cells45, 46. These immune conditions hamper the anti-tumor immune response, which is more favourable for tumor growth and metastasis. Our results on the prognostic value of Foxp3+ TILs in this meta-analysis are consistent with this hypothesis and suggest that Foxp3+ TILs play pro-tumor roles. CD4+ and CD8+ TILs promote an immunoreaction against these extraneous agents in a manner similar to tumor cells and enhance anti-tumor immunity. This interaction may explain our conclusion of the prognostic value of CD8+ and CD4+ TILs. However, we did not completely reveal the complicated network connections, and the mechanisms of immune responses in oncology require further exploration.
Previous researchers investigated the effect of clinical characteristics on tumor prognosis. Information on patients’ clinical features is more visible and accessible for clinicians. Many researchers did not take this aspect into consideration. Our meta-analysis performed subgroup analyses of several clinical characteristics that were especially targeted to these HCC patients, such as hepatitis B virus (HBV) infection, liver cirrhosis, TNM stage, Child-Pugh score, and vascular invasion. Subgroup analyses were also crucial to discuss the sources of heterogeneity. The positive effect of CD8+ TILs on OS was associated with sample size, HBV infection, and tumor number. The positive effect on DFS/RFS was associated with sex, liver cirrhosis, tumor number, and vascular invasion. The positive effect of CD3+ TILs on OS was associated with HBV infection, liver cirrhosis, Child-Pugh score, and vascular invasion. The positive effect of CD3+ TILs on DFS/RFS was associated with sample size, sex, HBV infection, TNM stage, and vascular invasion. The effect of CD4+ TILs on OS was related to sample size, sex, HBV infection, Child-Pugh score, TNM-stage, and tumor number. The prognostic value of Foxp3+ TILs on OS or DFS/RFS was associated with all of the listed clinical characteristics, which demonstrated that Foxp3+ TILs was a valuable and impressive poor predictor of survival. These results suggest that clinicians should pay more attention to these clinical features on survival.
Several limitations of this meta-analysis exist despite the rigorous design. First, we could not create a unified standard to identify high or low levels of TILs or the ratios. Different standards were used in various studies, and concrete data of expression levels were not accessible. These defects prevented us from advancing more reliable results. Second, publication bias was not assessed for studies on CD3+ and CD4+ TILs because of the limited number of published studies, which may influence the applicability of the results. High heterogeneity was frequently noted, especially in studies on CD8+ and CD3+ TILs, despite our use of several subgroup analyses. Therefore, we urge researchers to perform studies derived from more homogeneous populations. Then, after scrutinizing these 23 articles, we found only one study was conducted with training cohort and testing cohort. So we should conduct further research with training cohort and testing cohort to set up more predictive value of TIL data. Finally, this meta-analysis was based on retrospective studies with some unavoidable deficiencies, such as insufficient information on alcoholism and smoking history, surgical methods and therapeutic approaches, and lymph nodes. These confounding variables may affect the prognostic results.
This meta-analysis demonstrated the prognostic value of TILs in HCC using a comprehensive literature search, data extraction, and outcomes measured despite these limitations. This study provides significant information on TIL subsets, such as CD3+, CD4+, CD8+, and Foxp3+, and indicates that they can be used as prognostic biomarkers for HCC or as targeted molecules for anti-tumor treatment. Our research advanced current knowledge of the functions of the immune responses in oncology. Future rigorous studies of the effect of TILs in cancer are encouraged to promote human health.
Methods
Literature search
Two independent reviewers identified relevant articles via an electronic search of PubMed, Embase, and Web of Science. The electronic database was searched from study inception to April 2017. The following terms were used: “Lymphocytes, Tumor-Infiltrating”, “TILs” “T Lymphocytes”, “FoxP3-positive T lymphocytes”, “CD8-Positive T Lymphocytes”, “CD3-Positive T Lymphocytes”, “CD4-Positive T Lymphocytes”, “prognosis”, “survival”, “recurrence free survival”, “disease free survival”, “Carcinomas, Hepatocellular”, “Hepatocellular Carcinoma” and “HCC”. Additional pertinent studies were incorporated from a review of the reference lists of selected papers and related articles.
Inclusion and exclusion criteria
Eligible studies were assessed using the following criteria: (1) the prognostic value of CD3+, CD4+, CD8+, and FoxP3+ T lymphocytes as subsets of TILs were examined, including their ratios; (2) these lymphocyte markers were detected using immunohistochemistry from human tissues; (3) the related research should originate from original articles; (4) prognostic indicators were calculated as OS, DFS/RFS, or CSS/CRS; and (5) hazard ratios (HRs) and 95% confidence intervals (95% CIs) were used as effect measures or adequate data for calculating HRs and 95% CIs were provided, such as Kaplan–Meier curves.
The following exclusion criteria were used: (1) reviews, case reports, conference abstracts, editorials, and expert opinion; (2) non-English articles; (3) lymphocytes markers were detected in peri-tumoral tissues or peripheral blood; and (4) non-primary HCC, such as colorectal liver metastases. We used updated and proximate articles if similar data were repeated in several articles.
Data extraction and outcome measure
Two independent reviewers extracted data based on the criteria mentioned above. Disagreements were resolved by consensus or a re-review of the article. The following data were extracted from articles: first author, year of publication, ethnicity, mean or median age_(year), number of patients and sex, TILs subsets, and outcomes measured. We also recorded relative information on HBV infection, liver cirrhosis, TNM-stage, Child-Pugh score, tumor number, and vascular invasion, especially for the percentage of patients with a certain kind of clinical characteristic in a independent study. In subgroup analyses, the research is divided into two groups of studies with different demographics for each clinical characteristic.
HRs and 95% CIs were used as effect measures. Univariate and multivariate analyses were performed, and we selected the latter analysis for more accurate HRs and 95% CIs. If Kaplan–Meier curves were available rather than HRs, then we calculated HRs using the tabulation from Tierney et al.47, which is based on the method reported by Parmar et al.48. HRs and 95% CIs for survival were associated with high versus low levels of TILs. Therefore, when the data were associated with low levels versus high levels of TILs, the reciprocals of HRs and 95% CIs were calculated to indicate the effect on survival. The weighting was used to mean the proportion of each study’s result in the overall results. The weighting depends on the samples size and estimated value of effects in this study. The larger the sample size is, or more accurate the estimates value of effect is, the bigger the weighting is.
We have used the Newcastle-Ottawa Scale (NOS) to assess the quality of studies with its design, content and ease of use directed to the task of incorporating the quality assessments in the interpretation of meta-analytic results49. NOS scores of the studies ranged from 5 to 8, which were considered high quality.
Statistical analysis
We used HRs and their 95% CIs to demonstrate the relationship between TILs and patients prognosis, including their ratios. Furthermore, HRs less than 1 represented a better survival result for patients with high levels of TILs based on the data estimated with high versus low levels of TILs. P < 0.05 indicated statistically significant results. The χ2 test and I 2 index were used to measure the heterogeneity50, which may represent the degree of heterogeneity resulting from variables between studies (25% low heterogeneity, 50% medium, 75% high). A fixed-effect model was used only when I 2 < 50% and P > 0.1. Otherwise, we used a random-effects model. Subgroup analyses were performed when the overall results had statistical significance to investigate potential sources of heterogeneity and assess whether various clinical variables or study characteristics affected survival results. We also used funnel plots and Begg’s and Egger’s tests51, 52 to detect publication bias. P < 0.05 indicated publication bias, and P > 0.05 indicated no bias. All statistical analyses were performed with Revman software (version 5.3; Cochrane Collaboration, Oxford, United Kingdom), with the exception of the Begg’s and Egger’s tests, which were assessed using STATA12.0.
References
Hanahan, D. & Weinberg, R. A. Hallmarks of Cancer: The Next Generation. Cell 144, 646–674, doi:10.1016/j.cell.2011.02.013 (2011).
Joyce, J. A. & Pollard, J. W. Microenvironmental regulation of metastasis. Nature reviews. Cancer 9, 239–252, doi:10.1038/nrc2618 (2009).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nature medicine 19, 1423–1437, doi:10.1038/nm.3394 (2013).
Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nature reviews. Immunology 6, 715–727, doi:10.1038/nri1936 (2006).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 331, 1565–1570, doi:10.1126/science.1203486 (2011).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nature Reviews Cancer 12, 298–306, doi:10.1038/nrc3245 (2012).
Pages, F. et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med 353, 2654–2666, doi:10.1056/Nejmoa051424 (2005).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964, doi:10.1126/science.1129139 (2006).
Prendergast, G. C. & Jaffee, E. M. Cancer immunologists and cancer biologists: Why we didn’t talk then but need to now. Cancer Res 67, 3500–3504, doi:10.1158/0008-5472.CAN-06-4626 (2007).
Radvanyi, L. G. Tumor-Infiltrating Lymphocyte Therapy: Addressing Prevailing Questions. Cancer journal 21, 450–464, doi:10.1097/PPO.0000000000000162 (2015).
Mosmann, T. R., Cherwinski, H., Bond, M. W., Giedlin, M. A. & Coffman, R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. Journal of immunology 136, 2348–2357 (1986).
Liston, A. & Gray, D. H. Homeostatic control of regulatory T cell diversity. Nature reviews. Immunology 14, 154–165, doi:10.1038/nri3605 (2014).
Sakaguchi, S., Miyara, M., Costantino, C. M. & Hafler, D. A. FOXP3(+) regulatory T cells in the human immune system. Nature Reviews Immunology 10, 490–500, doi:10.1038/nri2785 (2010).
Forner, A., Llovet, J. M. & Bruix, J. Hepatocellular carcinoma. Lancet 379, 1245–1255, doi:10.1016/S0140-6736(11)61347-0 (2012).
Wang, F. et al. Prognostic Role of Immune Cells in Hepatitis B-associated Hepatocellular Carcinoma Following Surgical Resection Depends on Their Localization and Tumor Size. Journal of Immunotherapy 39, 36–44 (2016).
Gabrielson, A. et al. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunology Research 4, 419–430 (2016).
Sun, C. et al. The predictive value of centre tumour CD8(+) T cells in patients with hepatocellular carcinoma: comparison with Immunoscore. Oncotarget 6, 35602–35615, doi:10.18632/oncotarget.5801 (2015).
Brunner, S. M. et al. Tumor-infiltrating, interleukin-33-producing effector-memory CD8(+) T cells in resected hepatocellular carcinoma prolong patient survival. Hepatology 61, 1957–1967, doi:10.1002/hep.27728 (2015).
Garnelo, M. et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. doi:10.1136/gutjnl-2015-310814 (2015).
Lin, S. Z. et al. Prediction of Recurrence and Survival in Hepatocellular Carcinoma Based on Two Cox Models Mainly Determined by FoxP3(+) Regulatory T Cells. Cancer Prev Res 6, 594–602 (2013).
Chen, K. J. et al. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Medical oncology 29, 1817–1826, doi:10.1007/s12032-011-0006-x (2012).
Mathai, A. M. et al. Role of Foxp3-positive tumor-infiltrating lymphocytes in the histologic features and clinical outcomes of hepatocellular carcinoma. The American journal of surgical pathology 36, 980–986, doi:10.1097/PAS.0b013e31824e9b7c (2012).
Huang, Y. et al. Tumor-infiltrating FoxP3+ Tregs and CD8+ T cells affect the prognosis of hepatocellular carcinoma patients. Digestion 86, 329–337, doi:10.1159/000342801 (2012).
Gao, Q. et al. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Annals of surgical oncology 19, 455–466, doi:10.1245/s10434-011-1864-3 (2012).
Wang, F. et al. Foxp3+ regulatory T cells are associated with the natural history of chronic hepatitis B and poor prognosis of hepatocellular carcinoma. Liver international: official journal of the International Association for the Study of the Liver 32, 644–655, doi:10.1111/j.1478-3231.2011.02675.x (2012).
Li, Y. W. et al. Intratumoral neutrophils: a poor prognostic factor for hepatocellular carcinoma following resection. Journal of hepatology 54, 497–505, doi:10.1016/j.jhep.2010.07.044 (2011).
Chen, K. J. et al. Selective Recruitment of Regulatory T Cell through CCR6-CCL20 in Hepatocellular Carcinoma Fosters Tumor Progression and Predicts Poor Prognosis. Plos One 6 (2011).
Shen, S. L. et al. Foxp3+ regulatory T cells and the formation of portal vein tumour thrombus in patients with hepatocellular carcinoma. Canadian journal of surgery. Journal canadien de chirurgie 54, 89–94, doi:10.1503/cjs.028009 (2011).
Lin, G. H. et al. Relationship and clinical significance of TGF-beta1 expression with Treg cell infiltration in hepatocellular carcinoma. Chinese journal of cancer 29, 403–407 (2010).
Zhou, J. et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. International journal of cancer 125, 1640–1648, doi:10.1002/ijc.24556 (2009).
Gao, Q. et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research 15, 971–979, doi:10.1158/1078-0432.CCR-08-1608 (2009).
Pang, Y. L. et al. The immunosuppressive tumor microenvironment in hepatocellular carcinoma. Cancer Immunol Immun 58, 877–886, doi:10.1007/s00262-008-0603-5 (2009).
Sasaki, A. et al. Prognostic value of tumor-infiltrating FOXP3(+) regulatory T cells in patients with hepatocellular carcinoma. Ejso-Eur J Surg Onc 34, 173–179, doi:10.1016/j.ejso.2007.08.008 (2008).
Gao, Q. et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 25, 2586–2593, doi:10.1200/Jco.2006.09.4565 (2007).
Kobayashi, N. et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clinical cancer research: an official journal of the American Association for Cancer Research 13, 902–911, doi:10.1158/1078-0432.CCR-06-2363 (2007).
Ikeguchi, M. & Hirooka, Y. Interleukin-2 gene expression is a new biological prognostic marker in hepatocellular carcinomas. Onkologie 28, 255–259, doi:10.1159/000084695 (2005).
Ikeguchi, M., Oi, K., Hirooka, Y. & Kaibara, N. CD8+ lymphocyte infiltration and apoptosis in hepatocellular carcinoma. Ejso-Eur J Surg Onc 30, 53–57 (2004).
Dushyanthen, S. et al. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC medicine 13, 202, doi:10.1186/s12916-015-0431-3 (2015).
Kang, B. W. et al. Prognostic value of tumor-infiltrating lymphocytes in Epstein-Barr virus-associated gastric cancer. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO 27, 494–501, doi:10.1093/annonc/mdv610 (2016).
Schalper, K. A. et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. Journal of the National Cancer Institute 107, doi:10.1093/jnci/dju435 (2015).
Santoiemma, P. P. & Powell, D. J. Tumor infiltrating lymphocytes in ovarian cancer. Cancer Biol Ther 16, 807–820 (2015).
Gooden, M. J., de Bock, G. H., Leffers, N., Daemen, T. & Nijman, H. W. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. British journal of cancer 105, 93–103, doi:10.1038/bjc.2011.189 (2011).
Huang, Y. et al. Prognostic Value of Tumor-Infiltrating FoxP3(+) T Cells in Gastrointestinal Cancers: A Meta Analysis. Plos One 9 (2014).
Shang, B., Liu, Y., Jiang, S. J. & Liu, Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Scientific reports 5, 15179, doi:10.1038/srep15179 (2015).
Assudani, D. P., Horton, R. B. V., Mathieu, M. G., McArdle, S. E. B. & Rees, R. C. The role of CD4(+) T cell help in cancer immunity and the formulation of novel cancer vaccines. Cancer Immunol Immun 56, 70–80, doi:10.1007/s00262-006-0154-6 (2007).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nature reviews. Immunology 6, 295–307, doi:10.1038/nri1806 (2006).
Tierney, J. F., Stewart, L. A., Ghersi, D., Burdett, S. & Sydes, M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8, doi:10.1186/1745-6215-8-16 (2007).
Parmar, M. K., Torri, V. & Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in medicine 17, 2815–2834 (1998).
Andreas, S. Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiologh 25, 603–605 (2010).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Statistics in medicine 21, 1539–1558, doi:10.1002/sim.1186 (2002).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. Bmj 315, 629–634 (1997).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 81572417 to Professor Jian-ming Wang).
Author information
Authors and Affiliations
Contributions
Wei Yao, Jun-chuang He and Jian-ming Wang designed the study. Wei Yao, Jun-chuang He and Yan Yang analysed the data. Wei Yao, Ya-wei Qian, Tao Yang and Lei Ji wrote the manuscript and were responsible for language revisions. Jian-ming Wang supervised the project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, W., He, Jc., Yang, Y. et al. The Prognostic Value of Tumor-infiltrating Lymphocytes in Hepatocellular Carcinoma: a Systematic Review and Meta-analysis. Sci Rep 7, 7525 (2017). https://doi.org/10.1038/s41598-017-08128-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08128-1
This article is cited by
-
Identification of a Novel CD8+ T cell exhaustion-related gene signature for predicting survival in hepatocellular carcinoma
BMC Cancer (2023)
-
Tumour-infiltrating lymphocytes: from prognosis to treatment selection
British Journal of Cancer (2023)
-
Tumor infiltrating lymphocytes and radiological picture of the tumor
Medical Oncology (2023)
-
Analysis of the expression and prognosis for leukocyte immunoglobulin-like receptor subfamily B in human liver cancer
World Journal of Surgical Oncology (2022)
-
Anti-neoplastic sulfonamides alter the metabolic homeostasis and disrupt the suppressor activity of regulatory T cells
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.