Abstract
Sweet potato, Ipomoea batatas, is a widely cultivated vegetable worldwide. The leaves contain polyphenolic natural products called caffeoylquinic acids (CQAs), which possess biological activities including inhibition of aggregation of amyloid peptides. The present study describes an efficient extraction and isolation procedure for CQAs from sweet potato leaves using a cellulose-dissolving ionic liquid. The results showed that, compared to methanol, use of 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) allowed the extraction of a 6.5-fold greater amount of CQAs. This protocol will enable the efficient extraction of other organic compounds and biopolymers from natural materials.
Similar content being viewed by others
Introduction
Sweet potatoes, Ipomoea batatas, are cultivated as a vegetable in more than 100 countries, including China and African countries1. In general, after cultivation and harvest, portions of the roots are collected for food, while the leaves and stalks are discarded. However, the leaves are a good source of proteins, metals (including calcium, zinc, and iron), and natural products1, 2. In 2002, caffeoylquinic acids (CQAs, Fig. 1) were isolated from sweet potato leaves, and their structures were determined to be 3,4-dicaffeoylquinic acid (3,4-diCQA), 3,5-dicaffeoylquinic acid (3,5-diCQA), 4,5-dicaffeoylquinic acid (4,5-diCQA), and 3,4,5-tricaffeoylquinic acid (3,4,5-triCQA)3. CQAs have been shown to have anti-oxidant effects4, exhibit cytotoxicity5, and so on. Recently, it was also found that CQAs exert the potent inhibitions of the aggregation of amyloid beta peptides, which is known as one of the causes of Alzheimer’s disease6. Thus, sweet potato leaves are important resources for pharmacological, biomedical, and food chemical applications.
Ionic liquids are salts with melting points of below 100 °C and have unique physicochemical properties, such as high thermal stability, negligible vapor pressure, and excellent capacity for solubilizing organic compounds and cellulose7. Therefore, ionic liquids have potential for the efficient extraction of natural products from plant leaves8. Among the ionic liquids that dissolve cellulose, 1-butyl-3-methylimidazolium chloride ([C4mim]Cl, Fig. 2) has an excellent cellulose-dissolving capacity that is greater at high temperature of above 100 °C9.
Recently, the efficient extraction and isolation of shikimic acid, the starting material in the commercial synthesis of oseltamivir phosphate, and bilobalide, a unique terpene trilactone isolated from Ginkgo biloba leaves, were reported using cellulose-dissolving ionic liquids10, 11. Meanwhile, extraction of essential oils from lemon grass and lemon myrtle using ionic liquids was also succeeded12, 13. Since a plant cell wall consists mainly of cellulose and hemicellulose, cellulose-dissoluble ionic liquids are able to extract plant natural products efficiently14 because most natural products in leaves are stored in vacuoles mainly as secondary metabolites. Thus, the goal of the present study was to develop a new method for the efficient extraction and isolation of CQAs from sweet potato leaves using a cellulose-dissoluble ionic liquid [C4mim]Cl15.
Results and Discussion
First, extraction of CQAs from sweet potato leaves was conducted using methanol (MeOH) and water (H2O) for reference. The leaves used in this study were “Sui-Oh”, which is commercially available leaves used to make tea from the farm Sui-En (Fukuoka, Japan)16. The leaves of sweet potato were added to MeOH or H2O, respectively, and the solution was stirred under reflux for 1 hour. The resulting solution was then filtered to remove the residue of leaves. The filtrate was evaporated, and then the resulting extract was dissolved in H2O/MeOH (7:3 v/v). The solution was washed with n-hexane three times to remove undesired hydrophobic compounds. Removal of the organic solvent afforded crude extracts as a brownish solid.
Next, the extraction procedure using the cellulose-dissolving ionic liquid [C4mim]Cl was carried out as follows. The crushed sweet potato leaves were added to extraction solvents, and the solution was stirred for 1 hour: [C4mim]Cl at 150 °C, [C4mim]Cl/MeOH (1:1 w/w, 3:1 w/w) at 100 °C, [C4mim]Cl/H2O (1:1 w/w, 3:1 w/w) at 120 °C, and [C4mim]Cl/MeOH/H2O (2:1:1 w/w/w) at 100 °C. Then, MeOH was added to the solution, and the mixture was stirred at room temperature for 30 seconds. The solution was filtered, evaporated, and the extract was dissolved in H2O/MeOH (7:3 v/v). The solution was washed with n-hexane three times. Removal of organic solvents afforded the crude extract as a [C4mim]Cl solution.
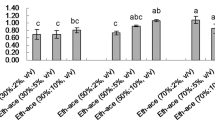
Quantitative analysis of the obtained extracts was performed using reversed-phase high performance liquid chromatography (RP-HPLC). For the analysis, the quantity of CQAs in individual extracts was calculated using a calibration curve. To prepare the calibration curves, 3,4-, 3,5-, and 4,5-diCQAs were purchased from a commercial supplier, whereas 3,4,5-triCQA was prepared by organic synthesis according to literature procedure starting from quinic acid17. Extraction yields were calculated as [amount of CQAs] per [original quantity of leaves], and the values for each CQA extracted using MeOH, [C4mim]Cl, [C4mim]Cl/MeOH, [C4mim]Cl/H2O, and [C4mim]Cl/MeOH/H2O are summarized in Fig. 3.
Extraction yields of (a) 3,4-diCQA, (b) 3,5-diCQA, (c) 4,5-diCQA, and (d) 3,4,5-triCQA. Experiments for MeOH (reflux), H2O (reflux), [C4mim]Cl (150 °C), and [C4mim]Cl/H2O (1:1 w/w, 120 °C) were performed three times and the average values are reported. Experiments for [C4mim]Cl/MeOH (1:1 w/w, 3:1 w/w, 100 °C), [C4mim]Cl/H2O (3:1 w/w, 120 °C), and [C4mim]Cl/MeOH/H2O (2:1:1 w/w/w, 100 °C) were carried out one time.
The extraction yields for 3,4-diCQA using H2O and [C4mim]Cl/H2O (1:1 w/w) were the same (0.43%), while the yield (0.40%) with [C4mim]Cl/MeOH (3:1 w/w) was 2.4 times greater than that obtained using MeOH (0.17%). The extraction yields for 3,5-diCQA using [C4mim]Cl/MeOH (3:1 w/w) and [C4mim]Cl/H2O (3:1 w/w) were 1.40% and 0.95%, respectively, which are 2.0 and 2.6 times greater than the yields achieved using MeOH (0.71%) and H2O (0.36%). The extraction yields for 4,5-diCQA using [C4mim]Cl/MeOH (3:1 w/w) and [C4mim]Cl/H2O (1:1 w/w) were 0.41% and 0.76%, respectively, which are 3.4 and 1.4 times higher than the yields achieved using MeOH (0.12%) and H2O (0.53%). Extraction of 4,5-diCQA with [C4mim]Cl afforded the best yield (0.78%), while yields for 3,4,5-triCQA using [C4mim]Cl/MeOH (1:1 w/w or 3:1 w/w) and [C4mim]Cl/H2O (1:1 w/w) were 0.26% and 0.30%, respectively, 2.9 and 1.9 times greater than those achieved using MeOH (0.09%) and H2O (0.16%). As the best comparison among the obtained results, the extraction yield of 4,5-diCQA using [C4mim]Cl (0.78%) was 6.5 times better than that using MeOH (0.12%).
Factors affecting the extraction results include the solubility of the cellulose making up the plant cell wall, the solubility of associated compounds in the extraction solvent, and the efficiency of stirring process. Because MeOH and H2O are poor solvents for cellulose, cellulose solubility decreases in a mixed solvent such as [C4mim]Cl/MeOH or [C4mim]Cl/H2O. However, this tendency did not affect overall extraction yields. The extraction yield for 3,5-diCQA using MeOH was much better than that using H2O, while the yields for other CQAs using H2O were much better than those using MeOH. This difference was probably due to the formation of micelle-like aggregation by CQAs except for 3,5-diCQA in H2O derived from the orientation of caffeoyl groups. The relatively long distance between the two caffeoyl groups in 3,5-diCQA may prevent the aggregation.
To investigate the stirring efficiency, the viscosity of the extraction solvents was measured (Fig. 4). The ionic liquid [C4mim]Cl possessed extremely high viscosity as expected (Fig. 4a). A comparison between [C4mim]Cl/MeOH (1:1 w/w) and [C4mim]Cl/MeOH (3:1 w/w) indicated that greater amounts of [C4mim]Cl promoted greater viscosity (Fig. 4b), and a comparison between [C4mim]/H2O (1:1 w/w) and [C4mim]/H2O (3:1 w/w) also indicated the same tendency (Fig. 4c). Thus, the viscosity of a mixed solvent of [C4mim]Cl/MeOH or [C4mim]Cl/H2O dramatically decreased compared to that of [C4mim]Cl, which indicates that the stirring efficiency promoted an increase in the extraction yield using [C4mim]Cl/MeOH or [C4mim]Cl/H2O.
Furthermore, isolation of CQAs from the [C4mim]Cl layer was investigated using a salting-out and decantation technique. The [C4mim]Cl extract was added to a saturated sodium chloride solution, followed by addition of an extractipm solvent such as EtOAc/MeOH (10:1 v/v), EtOAc/MeOH (5:1 v/v), EtOAc/MeOH/HCO2H (100:10:1 v/v/v), or EtOAc/MeOH/HCO2H (100:20:1 v/v/v). The organic layer was collected from the mixture by decantation. This procedure was repeated four times, and the combined organic layers were removed to obtain the raw CQAs. The CQAs were subjected to HPLC analysis, and the amounts of CQAs obtained were compared to the original amounts of CQAs in [C4mim]Cl layer (Table 1).
Extraction of CQAs from the [C4mim]Cl layer was attempted using an EtOAc/MeOH (10:1 v/v) extraction solvent (Table 1, entry 1), assuming a salting-out effect by saturated sodium chloride. However, the method afforded low yields with slight contamination of the [C4mim]Cl. An increase in MeOH promoted the solubility of the target compounds, resulting in an increase in the extraction yield (entry 2). To prevent the electrolytic dissociation of CQAs and increase the extraction efficiency, EtOAc/MeOH/HCO2H (100:10:1 v/v/v) was used as the extraction solvent. The results showed that most of the CQAs could be extracted from the [C4mim]Cl layer in 85 to 94% yields (entry 3). A change in the EtOAc/MeOH/HCO2H ratio to 100:20:1 v/v/v produced much better CQA extraction yields (entry 4). Because small amount of [C4mim]Cl was found in the resultant CQAs under the condition of entry 4, the condition of entry 3 would be the best for the extraction of CQAs from [C4mim]Cl layer. Thus, dissociation of a proton from formic acid probably prevented dissociation of protons from the CQAs, increasing the solubility in organic solvents (EtOAc).
Conclusions
In summary, a method for the efficient extraction and isolation of CQAs from sweet potato leaves was developed using the cellulose-dissolving ionic liquid [C4mim]Cl. The use of [C4mim]Cl allowed the CQAs to be extracted more efficiently than using conventional MeOH or H2O solvents. The results demonstrated that the extraction yield of 4,5-diCQA using [C4mim]Cl (0.78%) was 6.5 times better than that using MeOH (0.12%). This method is expected to be applicable to the extraction and isolation of other organic compounds and biopolymers from natural materials.
Experimental
General methods
All reagents were obtained from commercial suppliers and used without further purification unless otherwise stated. The [C4mim]Cl and HPLC-grade acetonitrile were purchased from Sigma Aldrich (St. Louis, USA). Analytical thin layer chromatography (TLC) was performed on silica gel 60 F254 plates produced by Merck KGaA (Darmstadt, Germany). The HPLC-grade MeOH and HPLC-grade distilled H2O were purchased from Kanto Chemicals, Co. Ltd (Tokyo, Japan). Column chromatography was performed with acidic silica gel 60 (spherical, 40–50 μm) or neutral silica gel 60 N (spherical, 40–50 μm) produced by Kanto Chemicals, Co. Ltd (Tokyo, Japan). The HPLC standards, 3,4-dicaffeoylquinic acid (3,4-diCQA), 3,5-dicaffeoylquinic acid (3,5-diCQA), and 4,5-dicaffeoylquinic acid (4,5-diCQA), were purchased from ChromaDex® Inc. (Irvine, USA). Sweet potato leaves (Sui-oh) were purchased from Shijoukai, Midorien (Fukuoka, Japan)16. The HPLC analyses were performed using a JASCO instrument equipped with a multiwavelength detector (MD-2010), semi-micro HPLC pump (PU-2085), autosampler (AS-2057), and column thermostat (CO-2060). Optical rotation was measured using a JASCO P-2200 digital polarimeter at the sodium D line (λ = 589 nm) and is reported as follows: [α]D T (c g/100 mL, solvent). 1H nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-EXC 300 spectrometer. 1H NMR data are reported as follows: chemical shift (δ, ppm), integration, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet), coupling constants (J) in Hz, assignments. Densities were measured using a DMA38 instrument (Anton Paar K. G.) from 15 °C to 40 °C. Viscosities were measured using an automated micro viscometer (Anton Paar Gmbh) from 40 °C to 100 °C.
Extraction of CQAs from sweet potato leaves
The CQAs were extracted from sweet potato leaves using MeOH or H2O as a reference. The sweet potato leaves (1 g) were treated with liquid nitrogen and then crushed into approximately 0.5 × 0.5 cm2 pieces in a mortar. Then, the crushed leaves were added to MeOH (16 g) or H2O (16 g), and the solution was stirred under reflux at around 80 °C or 120 °C, respectively, for 1 hour. The resulting solution was filtered with Celite 545 to remove the leaf residue. The filtrate was evaporated, and the extract was dissolved in H2O/MeOH (7:3 v/v). The solution was washed with n-hexane three times to remove undesired hydrophobic materials. Removal of the organic solvent afforded a crude extract as a brownish solid. Analyses of the extracts obtained were performed using RP-HPLC.
Extraction using the cellulose-dissolvable ionic liquid [C4mim]Cl was carried out as follows. Crushed sweet potato leaves (1 g) were added to the extraction solvents (16 g), such as [C4mim]Cl, [C4mim]Cl/MeOH (1:1 w/w, 3:1 w/w), [C4mim]Cl/H2O (1:1 w/w, 3:1 w/w), or [C4mim]Cl/MeOH/H2O (2:1:1 w/w/w), and the solution stirred under reflux for 1 hour at specific temperature: [C4mim]Cl at 150 °C, [C4mim]Cl/MeOH (1:1 w/w, 3:1 w/w) at 100 °C, [C4mim]Cl/H2O (1:1 w/w, 3:1 w/w) at 120 °C, and [C4mim]Cl/MeOH/H2O (2:1:1 w/w/w) at 100 °C. Then, MeOH (50–80 mL) was added to the solution, and the mixture was stirred at room temperature for 30 seconds. The solution was filtered with Celite 545, the filtrate was evaporated using a rotary evaporator, and then the extract was dissolved in H2O/MeOH (7:3 v/v). The solution was washed with n-hexane three times. After evaporation, the crude extract derived from an ionic liquid method was obtained as a [C4mim]Cl solution. Analyses of the extracts obtained were performed on RP-HPLC.
HPLC analysis of CQAs
Quantitative analyses of the extracts obtained were performed using an RP-HPLC system [column: YMC-Pack ODS-AM (150 × 4.6 mm); mobile phase: 0.2% formic acid aqueous solution/acetonitrile (gradient), flow rate: 1.0 mL/min; detection: 326 nm; injection amount: 10 μL; temperature: 40 °C]3. The quantity of CQA from individual extraction experiments was calculated using a calibration curve. The equations 1–4 for each CQA were obtained using the formulas below, where x represents peak area and y represents the amount of CQA (mg):
The extraction yields were calculated as the [quantity of CQAs] per [original quantity of leaves].
Synthesis of 3,4,5-triCQA
The 3,4,5-triCQA was synthesized starting from quinic acid17. 3,4,5-Triacety-lcaffeoyl-1,7-isopropyridenequinide was synthesized using a previously reported method17. To a solution of 3,4,5-triacetylcaffeoyl-1,7-isopropyridenequinide (12 mg, 0.012 mmol, 1.0 eq) in THF (1 mL) was added 2 M HCl (1 mL, 1.1 mmol, 94 eq). After stirring at room temperature for 11 days, the reaction mixture was concentrated in vacuo. Purification by HPLC afforded 1 as a colorless solid (2.2 mg, 0.0032 mmol, 26%); [α]D 25 −343 (c 0.13, MeOH) [ref. 15: [α]D 25 −314 (c 1.0, MeOH)]; 1H NMR (300 MHz, CD3OD) δ7.61 (1H, d, J = 15.6 Hz), 7.54 (1H, d, J = 15.9 Hz), 7.51 (1H, d, J = 15.9 Hz), 7.08–7.03 (1H, m), 7.02–6.97 (2H, m), 6.94–6.90 (2H, m), 6.85–6.79 (1H, m), 6.78–6.68 (2H, m), 6.35 (1H, d, J = 15.9 Hz), 6.21 (1H, d, J = 15.9 Hz), 6.19 (1H, d, J = 15.6 Hz), 5.68 (2H, m), 5.31 (1H, dd, J = 8.7, 3.0 Hz), 2.34–2.11 (4H, m).
Extraction of CQAs from [C4mim]Cl layer
A total of 10 g of [C4mim]Cl extract was added to 100 mL of saturated sodium chloride solution, and the solution was stirred for less than 1 min. The extraction solvent (150 mL), EtOAc/MeOH (10:1 v/v), EtOAc/MeOH (5:1 v/v), EtOAc/MeOH/HCO2H (100:10:1 v/v/v), or EtOAc/MeOH/HCO2H (100:20:1 v/v/v), then was added to the mixture, and the resulting mixture was stirred for 5 min. The organic layer was collected from the mixture by decantation. This procedure was repeated four times, and the combined organic layers were evaporated to obtain the crude CQA. The resultant CQAs were subjected to HPLC analysis, and the amount of CQA obtained was compared to the original amounts in the [C4mim]Cl layer.
Densimetry of [C4mim]Cl and its co-solvents
Densities of [C4mim]Cl, [C4mim]Cl/MeOH (1:1, 3:1, w/w), [C4mim]Cl/H2O (1:1, 3:1, w/w), and [C4mim]Cl/MeOH/H2O (2:1:1 w/w/w) were measured using a DMA 38 instrument (Anton Paar K. G.) at temperatures from 15 °C to 40 °C. Theoretical densities at temperatures above 50 °C were calculated using individual calibration curves.
Viscometry of [C4mim]Cl and its co-solvents
Viscosities of [C4mim]Cl, [C4mim]Cl/MeOH (1:1, 3:1, w/w), and [C4mim]Cl/H2O (1:1, 3:1, w/w) were measured using an automated micro viscometer (Anton Paar K. G.). Theoretical viscosities for rest temperature were calculated using the VFT equation18.
References
Woolfe J. A. Sweet Potato. An Untrapped Food Resource (Cambridge University Press: Cambridge, U.K., 1992).
Pace, R. D., Sibiya, T. E., Phills, B. R. & Dull, G. G. Ca, Fe and Zn content of ‘Jewel’ sweet potato greens as affected by harvesting practices. J. Food Sci. 50, 940–941 (1985).
Islam, S. et al. Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomoea batatas L.) genotypes. J. Agric. Food. Chem. 50, 3718–3722 (2002).
Yen, W. J., Wang, B. S., Chang, L. W. & Duh, P. D. Antioxidant properties of roasted coffee residues. J. Agric. Food Chem. 53, 2658–2663 (2005).
Kurata, R., Adachi, M., Yamakawa, O. & Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 55, 185–190 (2007).
Miyamae, Y. et al. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Bioorg. Med. Chem. 20, 5844–5849 (2012).
Pinkert, A., Marsh, K. N., Pang, S. & Staiger, M. P. Ionic liquids and their interaction with cellulose. Chem. Rev. 109, 6712–6728 (2009).
(a) Dai, Y., van Spronsen, J., Witkamp, G.-J., Verpoorte, R. & Choi, H. Y. Ionic liquids and deep eutectic solvents in natural products research: mixtures of solids as extraction solvents. J. Nat. Prod. 76, 2162–2173 (2013); (b) Ventura, S. P. M. et al. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 117, 6984–7052 (2017).
Swatloski, R. P., Spear, S. K., Holbrey, J. D. & Rogers, R. D. Dissolution of cellulose with ionic liquids. J. Am. Chem. Soc. 124, 4974–4975 (2002).
Usuki, T., Yasuda, N., Yoshizawa-Fujita, M. & Rikukawa, M. Extraction and isolation of shikimic acid from Ginkgo biloba leaves utilizing an ionic liquid that dissolves cellulose. Chem. Comm. 47, 10560–10562 (2011).
Onda, S., Usuki, T., Yoshizawa-Fujita, M. & Rikukawa, M. Ionic liquid-mediated extraction of bilobalide from Ginkgo biloba leaves. Chem. Lett. 44, 1461–1463 (2015).
Murata, C., Yoshizawa-Fujita, M., Rikukawa, M. & Usuki, T. Extraction of essential oils from lemongrass using ionic liquid. Asian J. Chem. 29, 309–312 (2017).
Munakata, K., Yoshizawa-Fujita, M., Rikukawa, M. & Usuki, T. Improved extraction yield of citral from lemon myrtle using a cellulose-dissolving ionic liquid. Aust. J. Chem. 70, 69–704 (2017).
Sun, N., Rodríguez, H., Rahman, M. & Rogers, R. D. Where are ionic liquid strategies most suited in the pursuit of chemicals and energy from lignocellulosic biomass? Chem. Commum. 47, 1405–1421 (2011).
Tan, T., Lai, C.-J.-S., OuYang, H., He, M.-Z. & Feng, Y. Recently, ultrasound-assisted extraction using ionic liquid of caffeoylquinic acids was reported Ionic liquid-based ultrasound-assisted extraction and aqueous two-phase system for analysis of caffeoylquinic acids from Flos Lonicerae Japonicae. J. Pharm. Biomed. Anal. 120, 134–141 (2016).
Ministry of Agriculture, Forestry and Fisheries of Japan, http://www.maff.go.jp/j/pr/aff/0907/mf_news_04.html (in Japanese, accessed on November 25, 2016).
Miyamae, Y., Kurisu, M., Han, J., Isoda, H. & Shigemori, H. Structure-activity relationship of caffeoylquinic acids on the accelerating activity on ATP production. Chem. Pharm. Bull. 59, 502–507 (2011).
(a) Vogel, H. The law of the relationship between viscosity of liquids and the temperature. Phys. Z. 22, 645–646 (1921); (b) Fulcher, G. S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 8, 339–355 (1925); (c) Tamman, G. & Hesse, W. Die Abhängigkeit der Viscosität von der Temperatur bie unterkühlten Flüssigkeiten. Z. Anorg. Allg. Chem. 156, 245–257 (1926).
Acknowledgements
We thank Mr. T. Kaneko (Sophia University) for providing the preliminary information, Ms. N. Yasuda (Sophia University) for measuring the viscosity of [C4mim]Cl, and Prof. M. Fujiwara (Sophia University) for fruitful discussions. This work was supported by a Sophia University Special Grant for Academic Research.
Author information
Authors and Affiliations
Contributions
T.U., S.O., and M.F.-Y. wrote the main manuscript text. S.O. conducted the all experiments. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usuki, T., Onda, S., Yoshizawa-Fujita, M. et al. Use of [C4mim]Cl for efficient extraction of caffeoylquinic acids from sweet potato leaves. Sci Rep 7, 6890 (2017). https://doi.org/10.1038/s41598-017-07291-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07291-9
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.