Abstract
Altered mean platelet volume (MPV) is found in several malignancies. Remarkably, there is little consensus on using the value of MPV in the prognostic evaluations of renal cell carcinoma (RCC). The aim of this study is to examine the feasibility of MPV value as a prognostic indicator of RCC. The retrospective study recruited 306 consecutive RCC patients between January 2009 and December 2009. The relationships between MPV and clinicopathological characteristics were analyzed. Kaplan-Meier method and Cox regression were used to evaluate the prognostic impact of MPV. Of the 306 RCC patients, low MPV levels were detected in 61 (19.9%) patients. Reduced MPV was associated with histology types, T classification, UCLA Integrated Scoring System (UISS) category, and Mayo clinic stage, size, grade, and necrosis score (SSIGN) category (P < 0.05). Patients with decreased MPV had significantly shorter survival time than patients with normal MPV (P < 0.001). Cox regression analysis revealed that reduced MPV was an independent prognostic factor for overall survival (hazard ratio, 1.758; 95% confidence interval [CI], 1.083–2.855, P = 0.023). Moreover, the prognostic accuracy of TNM stage, UISS, and SSIGN prognostic models were improved when MPV was added. In conclusion, reduced MPV is identified as an independent predictor of adverse clinical outcome in RCC.
Similar content being viewed by others
Introduction
Renal cell carcinoma (RCC) is the most common type of malignant tumor in the adult kidney and represents 2–3% of all adult cancers1. Despite increasing insights into the biology of RCC and advances in therapeutic techniques to RCC, one-third of RCC patients present with metastasis at the time of diagnosis. Therefore, searching for biomarkers to predict the prognosis in RCC is of critical importance.
Platelets are critical for cancer progression and metastasis2. The interplay between platelets and tumor cells lead to tumor growth, angiogenesis, and dissemination3. Elevated platelets are associated with a reduction in overall survival and poorer prognosis in various types of cancer, such as pancreatic, gastric, colorectal, endometrial, and ovarian cancers4,5,6,7,8. However, platelet count is not only determined by the rate of production but also determined by how much platelets are used in the body. A normal platelet count could conceal the presence of highly hypercoagulative and pro-inflammatory cancer phenotypes in the presence of efficient compensatory mechanisms9.
Mean platelet volume (MPV), the most commonly used index of platelet size, is a surrogate marker of platelet activation10. Altered MPV levels were observed in gastric cancer, ovarian cancer, lung cancer, colon cancer, and breast cancer11,12,13,14. However, its clinical implications in RCC have not been reported.
The purpose of this study was to assess whether MPV holds a prognostic role in RCC patients.
Results
306 RCC patients were enrolled in this study between Jan 2009 and Dec 2009. The median age was 57.8 ± 8.5 years (range 37–80) with 196 men and 110 women. Of the 306 patients, 286 presented with locally confined disease (T1–2), while 20 presented with locally advanced disease (T3–4). Of the 306 patients, 290 had no metastasis (M0), and 16 presented with metastasis (M1). 40 of the 306 cases were categorized as stage I and stage II, 266 as stage III and stage IV. With a median follow up of 60 months, 100 (32.7%) patients had death events.
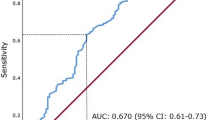
A ROC curve for OS prediction was plotted to verify the optimal cut-off value for MPV, which was 7.5 fL (Fig. 1). It demonstrated that MPV predicts cancer prognosis with a sensitivity of 35.0% and a specificity of 87.4% (AUC = 0.610, 95% CI: 0.553–0.665, p = 0.002). Patients then were sub-divided into 2 groups: patients with MPV ≤ 7.5 fL and patients with MPV > 7.5 fL. There were 61 (19.9%) patients with MPV ≤ 7.5 fL and 245 (80.1%) patients with MPV > 7.5 fL.
The relationship between MPV and clinical characteristics is shown in Table 1 and Table 2. Our study revealed that MPV was associated with histology types, T classification, UISS category, and SSIGN category. However, no significant differences were found between the groups with regard to age, gender, tumor size, Fuhrman grade, microvascular invasion, lymph node metastasis, distant metastasis, ECOG PS, and tumor stage.
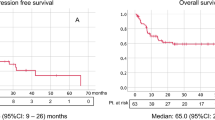
Kaplan-Meier survival analysis was performed to compare OS according to MPV levels. Patients with low MPV levels had worse 5-year OS (50.8% vs. 75.9%) than those with high MPV levels as is shown in Fig. 2 (P < 0.001).
To further determine the prognostic value of MPV, we applied univariate and multivariate Cox proportional hazard models to evaluate the hazard ratio (HR) and 95% confidence interval (CI). In the univariate analyses, MPV together with age (categorical variable), hemoglobin, platelet count, tumor size, T classification, lymph node metastasis, distant metastasis, microvascular invasion, and TNM stage were associated with OS (Table 3). Variables with p value lower than 0.10 in univariate analysis were included in the multivariate analysis. In the multivariate analyses, MPV (categorical variable), hemoglobin, platelet count, tumor size, lymph node metastasis, and TNM stage were independent factors for OS (Table 4). Patients with MPV ≤ 7.5 fL had a HR of 1.758 [95% CI: 1.083–2.855, P = 0.023] for OS.
To further confirm the predictive ability of MPV, we compared MPV with classic prognostic models, such as TNM staging system, the University of Los Angeles integrated staging system (UISS) scoring systems, and the Mayo clinic stage, size, grade, and necrosis score (SSIGN), respectively. Concordance index (C-index) and Akaike information criteria (AIC) analysis were used for prognostic power evaluation. As shown in Table 5, the C-indexes for OS were 0.618, 0.631, and 0.699, respectively, when assessed with the TNM, UISS, and SSIGN models alone. The C-indexes were improved to 0.700, 0.681, and 0.734, respectively, when MPV was added into models. Moreover, the AIC value of each model combined with MPV was lower than each model alone.
Discussion
This study found that MPV correlates with patient’s survival and is an independent predictor of adverse clinical outcome in RCC.
Platelets act as a crucial modulator in tumor development, tumor cell growth, angiogenesis, and metastasis. In RCC, platelet-derived growth factor-alpha alpha receptor over-expression is associated with adverse outcomes15. High platelet-derived endothelial cell growth factor activity is linked to the malignant potential of RCC16. Low expression of platelet-derived growth factor-BB (PDGF-BB) predicts distant metastasis and poor prognosis17. On the contrary, blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits the progression of metastatic RCC18. However, these parameters reflecting activated platelets are expensive and are not commonly used in clinical practice. MPV is available in routine practice and can be easily evaluated prior to treatment.
The mechanisms underlying the association between MPV and survival in RCC have yet to be fully defined. There are several mechanisms that are potentially responsible for decreased MPV in RCC. Firstly, chronic inflammation plays a pivotal role. There is a firm linkage between inflammation and cancer19. MPV was an early marker of activated platelets. Decreased MPV could be regarded as an enhanced consumption of large platelets in inflammatory states10. Recent studies confirmed that low levels of MPV are associated with high-grade inflammatory diseases and are reversed in the course of anti-inflammatory therapy10. Secondly, platelets can induce tumor growth via increasing angiogenesis. Platelets coordinate complex angiogenic responses through a number of different mechanisms, including direct cellular contact, local release of pro-angiogenic proteins into the tumor microenvironment, and recruitment of distant cells into the tumor mircroenvironment20. Thirdly, platelets promote tumor cell migration and invasion. A platelet cloak protects tumor cells in circulation from extreme shear forces encountered in the vascular system, preventing mechanical damage to the cells21. Moreover, platelets release transforming growth factor-β1 (TGF-β1) that induces phenotypic changes of epithelial to mesenchymal-like transition of tumor cells and facilitates their extravasation to distant sites during metastasis. Activated platelets release secretory factors that promote chemokines, proteolytic enzymes and microparticles within the microenvironment to promote tumor cell invasion22.
In line with our results, Aksoy et al. showed that solid tumors with bone marrow metastasis were more likely to have low MPV levels23. Inagaki N and Kumagai S et al. revealed that low MPV level was associated with poor prognosis in non-small-cell lung cancer13, 24. These data are also consistent with the current knowledge that anti-platelet treatment is considered to be a part of cancer adjuvant therapy3. Therefore, our results may help to inform treatment decisions and predict treatment outcomes.
The present study has some limitations. First, this was a single-center retrospective study and additional larger validation studies with multiethnic groups are needed to confirm our results. Second, we were unable to explore the exact mechanism of MPV in RCC. Third, the patients were composed of Chinese. The application to other ethnic groups still needs further investigation.
In conclusion, MPV is easily available with routine blood counts. Reduced MPV may serve as a marker of adverse prognosis in RCC. Further studies are warranted to clarify the exact role of MPV in RCC.
Patients and Methods
Study population
This study consisted of 306 consecutive RCC cases (mean age 56.3 ± 11.1 years, range 19–80 years). Cases were admitted to Harbin Medical University Cancer Hospital, Harbin Medical University between January 2009 and December 2009. All patients underwent complete surgical resection. The pathologic diagnoses of RCC were evaluated by pathologists from biopsy reports. None of the patients received preoperative chemotherapy or radiation therapy. Patients were excluded if they had hematological disorders, coronary artery disease, hypertension, diabetes mellitus, and medical treatment with anticoagulant, statins, and acetylic salicylic acid.
Standard demographic and clinicopathological data were collected from the patients’ records in hospital. Survival data were obtained through follow-up. Overall survival (OS) was defined as the interval from the date of diagnosis to death or last follow-up. The median follow-up time was 60 months. White blood cell (WBC), haemoglobin, and platelet indices were measured by an autoanalyzer (Sysmex XE-2100, Kobe, Japan). The whole blood samples were collected in EDTA-containing tubes, and all samples were processed within 30 minutes after blood collection. The method was validated in a previous report25.
The Institutional Ethics Review Board of Harbin Medical University Cancer Hospital approved this study prior to commencement of data collection and waived the informed consent requirement because it was a retrospective study. All studies were conducted according to guidelines (Declaration of Helsinki) for biomedical research.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). The descriptive statistics were presented as means ± SD or medians (interquartile range) for continuous variables and percentages of the number for categorical variables. Inter-group differences in categorical variables were assessed for significance using the Chi-square test; differences in continuous variables were assessed using the Mann-Whitney U test or t-test. OS was analyzed using the Kaplan-Meier method, and differences between curves were assessed for significance using the log-rank test. Variables that showed a p value < 0.1 in univariate analysis were included in a multivariate Cox proportional hazards regression model. Multivariate Cox proportional hazards modeling was used to identify independent prognostic factors. Receiver-operating characteristics (ROC) curve analysis was performed to identify cut-off value of MPV. A value of p < 0.05 was regarded as a significant difference between groups.
References
Bhatt, J. R. & Finelli, A. Landmarks in the diagnosis and treatment of renal cell carcinoma[J]. Nat Rev Urol 11(9), 517–525, doi:10.1038/nrurol.2014.194 (2014).
Kubes, P. The versatile platelet contributes to inflammation, infection, hemostasis, coagulation and cancer[J]. Semin Immunol 28(6), 535, doi:10.1016/j.smim.2016.11.002 (2016).
Mezouar, S., Frere, C. & Darbousset, R. et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res. 139, 65–76 (2016).
Suzuki, K., Aiura, K. & Kitagou, M. et al. Platelets counts closely correlate with the disease-free survival interval of pancreatic cancer patients. Hepatogastroenterology. 51(57), 847–53 (2004).
Long, Y., Wang, T., Gao, Q., Zhou, C. Prognostic significance of pretreatment elevated platelet count in patients with colorectal cancer: a meta-analysis. Oncotarget (2016).
Pietrzyk, L., Plewa, Z., Denisow-Pietrzyk, M., Zebrowski, R. & Torres, K. Diagnostic Power of Blood Parameters as Screening Markers in Gastric Cancer Patients. Asian Pac J Cancer Prev. 17(9), 4433–4437 (2016).
Ekici, H. et al. Do Leukocyte and Platelet Counts Have Benefit for \Preoperative Evaluation of Endometrial Cancer. Asian Pac J Cancer Prev. 16(13), 5305–10 (2015).
Qiu, J. et al. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 38(4), 651–7 (2012).
Seretis, C., Youssef, H. & Chapman, M. Hypercoagulation in colorectal cancer: what can platelet indices tell us. Platelets. 26(2), 114–8 (2015).
Gasparyan, A. Y., Ayvazyan, L., Mikhailidis, D. P. & Kitas, G. D. Mean platelet volume: a link between thrombosis and inflammation. Curr Pharm Des 17, 47–58 (2011).
Shen, X. M., Xia, Y. Y. & Lian, L. et al. Mean platelet volume provides beneficial diagnostic and prognostic information for patients with resectable gastric cancer[J]. Oncol Lett 12(4), 2501–2506, doi:10.3892/ol.2016.4913 (2016).
Tanriverdi, O., Menekse, S. & Teker, F. et al. The mean platelet volume may predict the development of isolated bone metastases in patients with breast cancer: a retrospective study of the Young Researchers Committee of the Turkish Oncology Group (TOG)[J]. J BUON 21(4), 840–850 (2016).
Kumagai, S., Tokuno, J. & Ueda, Y. et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer[J]. Mol Clin Oncol 3(1), 197–201, doi:10.3892/mco.2014.436 (2015).
Kemal, Y., Demirağ, G. & Ekiz, K. et al. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer[J]. J Obstet Gynaecol 34(6), 515–518, doi:10.3109/01443615.2014.912620 (2014).
Sulzbacher, I., Birner, P., Träxler, M., Marberger, M. & Haitel, A. Expression of platelet-derived growth factor-alpha alpha receptor is associated with tumor progression in clear cell renal cell carcinoma. Am J Clin Pathol 120, 107–12 (2003).
Mizutani, Y., Wada, H. & Yoshida, O. et al. The significance of thymidine phosphorylase/platelet-derived endothelial cell growth factor activity in renal cell carcinoma. Cancer 98, 730–6 (2003).
Qi, L. et al. Low differentiated microvascular density and low expression of platelet-derived growth factor-BB (PDGF-BB) predict distant metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int 112, E415–23 (2013).
Xu, L., Tong, R., Cochran, D. M. & Jain, R. K. Blocking platelet-derived growth factor-D/platelet-derived growth factor receptor beta signaling inhibits human renal cell carcinoma progression in an orthotopic mouse model. Cancer Res 65, 5711–9 (2005).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–44 (2008).
Sharma, D., Brummel-Ziedins, K. E., Bouchard, B. A. & Holmes, C. E. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol 229, 1005–1015 (2014).
Franco, A. T., Corken, A. & Ware, J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood 126, 582–588 (2015).
Tesfamariam, B. Involvement of platelets in tumor cell metastasis. Pharmacol Ther 157, 112–119 (2016).
Aksoy, S., Kilickap, S. & Hayran, M. et al. Platelet size has diagnostic predictive value for bone marrow metastasis in patients with solid tumors[J]. Int J Lab Hematol 30(3), 214–219, doi:10.1111/j.1751-553X.2007.00947.x (2008).
Inagaki, N., Kibata, K. & Tamaki, T. et al. Prognostic impact of the mean platelet volume/platelet count ratio in terms of survival in advanced non-small cell lung cancer[J]. Lung Cancer 83(1), 97–101, doi:10.1016/j.lungcan.2013.08.020 (2014).
Yun, Z.Y. et al. Mean platelet volume, platelet distribution width and carcinoembryonic antigen to discriminate gastric cancer from gastric ulcer. Oncotarget (2017).
Acknowledgements
This work was supported financially by grants from the Harbin special fund for scientific and technological innovation talents (RC2016XK004068).
Author information
Authors and Affiliations
Contributions
Z.Y.Y. and X.Z. participated in study design, data analysis and manuscript preparation. Y.S.L. participated in data collection and data analysis. T.M.L. and Z.P.L. R.T.W. and K.J.Y. participated in study design, data analysis, manuscript preparation and editing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yun, Zy., Zhang, X., Liu, Ys. et al. Lower mean platelet volume predicts poor prognosis in renal cell carcinoma. Sci Rep 7, 6700 (2017). https://doi.org/10.1038/s41598-017-07168-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-07168-x
This article is cited by
-
Blood platelet volume predicts treatment-specific outcomes of metastatic castration-resistant prostate cancer
International Journal of Clinical Oncology (2020)
-
Mean platelet volume-to-lymphocyte ratio: a novel biomarker associated with overall survival in patients with nonmetastatic clear cell renal cell carcinoma treated with nephrectomy
International Urology and Nephrology (2020)
-
Decreased mean platelet volume predicts poor prognosis in metastatic colorectal cancer patients treated with first-line chemotherapy: results from mCRC biomarker study
BMC Cancer (2019)
-
Preoperative mean platelet volume predicts survival in breast cancer patients with type 2 diabetes
Breast Cancer (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.