Abstract
Hippolyte is a genus of small bodied marine shrimps, with a global distribution. Here, we studied the phylogenetic and biogeographic relationships amongst the species of this genus with two mitochondrial and two nuclear markers, using Bayesian Inference, Maximum Likelihood, genetic divergence, molecular clock and S-DIVA. In addition, the Indo-West Pacific genus Alcyonohippolyte was included. Based on sequences from 57 specimens of 27 species, we recovered a robust biogeographic scenario that shows the Indo-West Pacific as the probable ancestral area of the genus Hippolyte, which emerged in the Paleocene, followed by dispersal in three general directions: (1) South Pacific, (2) eastern Atlantic and Mediterranean Sea and (3) Americas, the latter with a primary colonization in the eastern Pacific followed by a radiation into the western Atlantic. Our analysis reveals that the species of the H. ventricosa group do not constitute a monophyletic group and Alcyonohippolyte does not constitute a reciprocally monophyletic group to Hippolyte, with both genera herein synonimised. The relationships and systematic status of several transisthmian and Atlantic species are clarified.
Similar content being viewed by others
Introduction
The genus Hippolyte Leach, 1814 currently includes 32 species with a distribution spanning across the globe: 13 species are known from the Mediterranean Sea and the adjacent eastern Atlantic, nine species occur in the Indo-West Pacific region, four species in the western Atlantic, three species in the eastern Pacific, two species in the southern Pacific area, whilst one species is amphi-Atlantic1,2,3. The genus is considered taxonomically challenging due to the small morphological differences between species coupled with significant intra-specific variation1. The species from the Mediterranean Sea and adjacent eastern Atlantic were revised in 19961, with further information as well as an identification key for Atlantic species in 20072. However, the Indo-West Pacific species have not been thoroughly reviewed and remain challenging to identify4. For example, many species were, in the past, confused under the name Hippolyte ventricosa H. Milne Edwards, 1837, often considered as occurring across the entire Indo-West Pacific. However, the distribution of H. ventricosa was restricted to the coasts of India and Pakistan4 and it was postulated that records from elsewhere in the Indo-West Pacific could be undescribed species of the H. ventricosa group2, 4, with recently a further species in this group described3.
Until quite recently, the genus had an additional species, Hippolyte commensalis Kemp, 1925. However, this species was transferred to a new genus Alcyonohippolyte Marin, Chan & Okuno, 2011, together with two newly described species5, with three more species added to this genus later on6, 7. This latter genus is restricted to the Indo-West Pacific, with all species considered to be obligatory symbionts of alcyonacean soft corals (Octocorallia, Alcyonacea)7. Both genera are clearly very closely related, sharing some important characters, e.g. the long slender rostrum, the presence of supraorbital, hepatic and antennal teeth on the carapace and the similar shape of the mouthparts and ambulatory pereiopods5. Nevertheless, these genera are currently thought to be distinguished by a suite of rather minor morphological differences5.
Most species of the genus Hippolyte are small bodied, between 7 to 20 mm in length with a few European species reaching 30 to 50 mm1, 2, 8. Perhaps not all, but certainly in most species of this genus females reach larger sizes than males1, 9, 10 and are often more abundant, with the taxonomy of the genus largely based on adult female morphology1, 2.
No studies on the phylogenetic relationships of the closely related genera Hippolyte and Alcyonohippolyte have been published, nor has their relationship to other hippolytid genera been fully resolved. Indeed, few species of the genus Hippolyte have been included in the broader phylogenetic studies of Decapoda11 nor across caridean families12 or the in-depth study of the family Hippolytidae sensu lato13. Thus, the aim of this study was to analyze the phylogenetic and biogeographic relationships amongst the species of the genera Hippolyte and Alcyonohippolyte using two mitochondrial and two nuclear markers, based on a broad representation of species.
Results
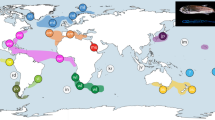
For this study, specimens from 31 of the 32 species currently assigned to the genus Hippolyte, and four of the six species currently in the genus Alcyonohippolyte were available. No material was available for Hippolyte multicolorata Yaldwyn, 1971, Alcyonohippolyte brachycarpus Marin & Chan, 2012 and Alcyonohippolyte maculata Marin, Okuno & Chan, 2010. However, not all species were successfully amplified during the present work as some specimens were from old museum samples, possibly fixed in formaldehyde, whilst others simply did not work. This was the case for: Hippolyte caradina Holthuis, 1947, Hippolyte coerulescens (Fabricius, 1775), Hippolyte lagarderei d’Udekem d’Acoz, 1995, Hippolyte leptometrae Ledoyer, 1969, Hippolyte palliola Kensley, 1970, Hippolyte proteus (Paulson, 1875), Hippolyte ventricosa H. Milne Edwards, 1837 sensu stricto and Alcyonohippolyte tenuicarpus Marin, 2011. Thus, in the final analyses sequences from 24 Hippolyte species (i.e 75% of total known species diversity) and three Alcyonohippolyte species (i.e 50% of total known species diversity) were included with global coverage (Supplementary Table S1, Fig. 1).
Sampling locations of specimens included in the analyses; see Supplementary Table S1 for details. The map was built with a template of the software Diva-Gis 7.574 (http://www.diva-gis.org/).
Phylogenetic analyses
All genes showed little saturation (Iss < Iss.c, p < 0.001), indicating that the data are robust enough for phylogenetic analyses. The best-fitted substitution models, selected with a corrected Bayesian information criterion were: 16S - TrN + G14 assumed nucleotide frequencies A = 0.3875, C = 0.0741, G = 0.1897, T = 0.3486; rate of substitution AC = 1, AG = 4.8024, AT = 1, CG = 1, CT = 14.1455, GT = 1; substitution model variable sites followed a gamma distribution with shape parameter = 0.2860; COI - TIM2 + I + G15 assumed nucleotide frequencies A = 0.3528, C = 0.1787, G = 0.1166, T = 0.3520; rate of substitution AC = 0.2273, AG = 4.7358, AT = 0.2273, CG = 1, CT = 7.8761, GT = 1; substitution model variable sites followed a gamma distribution with shape parameter = 0.3850; invariable sites = 0.3850; 18S - TPM2 + G16 assumed rate of substitution AC = 3.1084, AG = 4.8975, AT = 3.1084, CG = 1, CT = 4.8975, GT = 1; substitution model variable sites followed a gamma distribution with shape parameter = 0.1530; H3 - TrNef + I + G15 assumed rate of substitution AC = 1, AG = 2.6420, AT = 1, CG = 1, CT = 12.1326, GT = 1; substitution model variable sites followed a gamma distribution with shape parameter = 0.6010; invariable sites = 0.5940. We used these parameters in order to obtain the BAY tree (all markers) and the molecular clock (16S and COI).
We obtained almost the same topology for the phylogeny using the BAY and ML analyses. Thus, we selected the BAY tree as the basis for discussions, showing posterior probabilities (expressed as percentage), but also including bootstrap supporting values for ML analyses (Fig. 2A). The only difference between the two analyses was the relative positions of H. californiensis, H. obliquimanus and H. williamsi. In the BAY analysis, H. williamsi was the sister group of H. californiensis, and H. obliquimanus was sister group of H. williamsi/H. californiensis (Fig. 2A); whilst in the ML analysis, H. obliquimanus was the sister group of H. californiensis, and H. williamsi was the sister group of H. obliquimanus/H. californiensis (Fig. 2B). However, in both analyses, these relations (H. williamsi/H. californiensis, H. obliquimanus/H. californiensis and H. williamsi/H. californiensis/H. obliquimanus) had low support values.
(A) Bayesian evolutionary tree based on combined 16S, COI, 18S and H3 DNA sequence data. Numbers at nodes represent posterior probabilities/bootstraps expressed as a percentage (BAY/ML). Numbers <80% are not shown. Fully supported (100/100) branches are marked with solid circles. The numbers between parentheses correspond to the Figure 1. Data about the specimens can be obtained in the Supplementary Table S1. (B) Maximum Likelihood tree showing the only topological difference found between both analyses in the position of H. californiensis, H. obliquimanus and H. williamsi. Abbreviations for seas and oceans are A: Atlantic Ocean, C: Caribbean Sea, GMX: Gulf of Mexico, M: Mediterranean Sea, P: Pacific Ocean.
The resulting trees clearly demonstrate the monophyly of the genus Hippolyte, and a separation into four geographically delineated clades (Fig. 2A), as follows. A basal separation splits off a clade composed of almost all Indo-West Pacific (IWP) Hippolyte, as well as all included Alcyonohippolyte species. The New Zealand species, H. bifidirostris is sister to all remaining taxa, with these further splits into two big clades, one composed almost exclusively of species from the Americas (eastern Pacific - EP and western Atlantic - WA), but with the inclusion of the Malagasy species, H. kraussiana and another comprised entirely of species from the eastern Atlantic (EA) and Mediterranean Sea (MS).
In the Indo-West Pacific clade, two results with significant systematic importance can be found (Fig. 2A): (1) the specimens of the H. ventricosa group do not constitute a monophyletic group;(2) A. commensalis and A. tubiporae constitute a single taxon.
Genetic divergence
As expected, the mitochondrial markers were more variable than the nuclear markers (Table 1). For each of the four markers, the divergence among specimens of the genera Alcyonohippolyte and Hippolyte were of the interspecific type (Table 1). The highest divergence amongst Alcyonohippolyte and Hippolyte was lower than the highest interspecific divergence amongst Hippolyte species, whilst the lowest divergence amongst Alcyonohippolyte and Hippolyte was lower than the intergeneric divergence clearly placing Alcyonohippolyte within Hippolyte.
For the 16S marker, the sequences of H. sapphica forms A and B showed 0% of genetic divergence, which was also the case for the specimens of H. clarki (n = 2), H. edmondsoni (n = 2), H. jarvinensis (n = 3), H. nicholsoni (n = 2), H. obliquimanus (n = 2) and H. williamsi (n = 2) analysed. The intraspecific divergence of A. dossena (n = 2), H. bifidirostris (n = 2), H. leptocerus (n = 4), H. pleuracanthus (n = 3), H. varians (n = 6) and H. ngi (n = 2) ranged from 0.3 to 0.8%, with the highest intraspecific divergence being among specimens of H. inermis (n = 5) (0–1.1%).
For the COI marker, the sequences of H. sapphica forms A and B showed 0% of genetic divergence, which was equally the case for the specimens of H. clarki (n = 2), H. edmondsoni (n = 2), H. nicholsoni (n = 2), H. pleuracanthus (n = 3) and H. williamsi (n = 2) included in the analysis. The intraspecific divergence of H. obliquimanus (n = 2) and H. leptocerus (n = 4) ranged from 0.2 to 0.4%, whilst the divergence amongst specimens of H. varians (n = 6) was 0.8%, but again the highest intraspecific divergence was amongst specimens of H. inermis (n = 5) (0.2–4.4%). The divergence between the specimen of A. commensalis and A. tubiporae respectively was 0.6%. For comparison, the divergences were 15 and 15.5% between A. dossena and A. commensalis or A. tubiporae, respectively.
For the 18S and H3 markers, the divergence was nil between the two specimens of A. dossena, and between A. commensalis and A. tubiporae, respectively. For comparison the divergences were 0.6% (18S) and 1.3% (H3) between A. dossena and both A. commensalis/A. tubiporae.
Biogeographical analyses and molecular dating
The S-DIVA results (Fig. 3) postulate that the ancestors of Hippolyte originated in the present day IWP (optimal area reconstruction at basal node 64) dated at 57 ± 8 Myr (Fig. 4). Node 64 indicates an early dispersion of the genus across the IWP and into the EA (probability 0.90), whilst node 63 indicates vicariance between IWP and southern Pacific (SP; probability 1.00) (Fig. 3). The separation of the clade SP dates to 56 ± 8 Myr (Fig. 4). Node 62 also indicates a further dispersion event across the IWP (probability 1.00; Fig. 3).
Biogeographic history of Hippolyte, highlighting vicariant and dispersal events. (A) Graphical results of ancestral distributions at each node of the phylogeny of the genus Hippolyte obtained by S-DIVA based on the combined 16S, COI, 18S and H3 dataset; colour key to possible ancestral ranges at different nodes; black with an asterisk represents other ancestral ranges. The numbers between parentheses correspond to the numbers of specimens. Outgroups have been excluded from the phylogeny. (B) Maps showing the possible geographic history of the genus. Biogeographical regions as follows: IWP: Indo-West Pacific, SP: southern Pacific, EP: eastern Pacific; WA: western Atlantic; EA: eastern Atlantic; MS: Mediterranean Sea. The maps were built with a template of the software Diva-Gis 7.574 (http://www.diva-gis.org/).
Divergence time chronogram for Hippolyte species estimated using a Bayesian analysis for concatenated dataset 16S/COI. Mean divergence time estimates (million years ago = MYR) are noted adjacent to their respective nodes, the bars represent the highest posterior density (95%). Geological periods of Cenozoic Era are superimposed onto the phylogeny, abbreviated as follows: Hol, Holocene; Ple, Pleistocene; Pli, Pliocene; Mio, Miocene; Oli, Oligocene; Eoc, Eocene; Pal, Paleocene. Outgroups have been excluded from the phylogeny. Biogeographical regions as in Fig. 3.
Possible ancestral ranges at node 52 are IWP + EA, EA + EP and EA + WA, the frequency of occurrence of these ranges being 70.23, 17.06 and 12.71%, respectively (Fig. 3), thus, the most favored ancestral range at this node being IWP + EA. This node resolves a separation, dated at 48 ± 6 Myr (Fig. 4) between a EA + MS clade with origin in EA versus a EP + WA (plus one IWP species) clade with an origin in the IWP (Fig. 3).
Node 51, dated at 47 ± 7 Myr (Fig. 4), highlights a dispersal event into the EA (probability 1.00; Fig. 3), whilst nodes 43, 45, 47, 48 and 50 (Fig. 3) suggest repeated dispersal events from EA to MS (probabilities 0.82, 0.82, 0.50, 0.38 and 0.75, respectively). Further, nodes 41 and 46 (Fig. 3) suggest vicariance events between EA and MS (probabilities 1.00 for both).
Possible ancestral ranges at node 40 are IWP + EP and IWP + WA, the frequency of occurrence of these ranges being 58.50 and 41.50% respectively (Fig. 3), thus, the most favored ancestral range at node 40 is IWP + EP (Indo-West Pacific + eastern Pacific). Also, node 40 reveals a dispersal event from IWP to EP (probability 0.90), dated 44 ± 9 Myr (Fig. 4). As node 39 (Fig. 3) is constituted by 58.5% EP and 41.5% WA + EP, possible origin is indicated for American species in the Pacific, dated at 36 ± 7 Myr (Fig. 4). Also, nodes 35, 38 and 39 reveal repeated dispersal from EP to WA (probabilities 1.00, 0.75 and 0.80, respectively) and node 37 indicates a dispersion event across the WA (probability 1.00).
Discussion
The present analysis allows for the first time a phylogenetically based scenario for the relationships of the species of the genus Hippolyte and the morphologically similar Alcyonohippolyte, and an in depth discussion about the biogeography of this genus. Our results unambiguously demonstrate that Alcyonohippolyte is nested within a clade of IWP Hippolyte species (Fig. 2A). As our analysis included the type species of the genus, A. dossena Marin, Chan & Okuno, 2011, we herein formally consider the genus Alcyonohippolyte Marin, Chan & Ouno, 2011 to be a junior synonym of the genus Hippolyte Leach, 1814. However, it is clear that A. dossena is a distinct, valid species (Fig. 2A), within Hippolyte (i.e. Hippolyte dossena (Marin, Chan & Okuno, 2011)). A further result of the present analysis is that Hippolyte commensalis Kemp, 1925 (as A. commensalis in Fig. 2A) and Alcyonohippolyte tubiporae Marin, 2011 are clearly conspecific taxa, given that the genetic divergence between these two nominal taxa is far lower than intraspecific variation rates with the genus.
Evolutionary biogeography
The phylogenetic tree abundantly shows the monophyly of the herein reconstituted genus Hippolyte and its separation into three major clades, as well as a monospecific minor clade (Fig. 2A). A complex biogeographic history is suggested by the S-DIVA analysis, which many dispersal and vicariant events shaping the current distribution pattern in the genus.
In the phylogeny, the outermost clade was formed exclusively by IWP species. The ancestral area reconstruction recovered this region as the probable ancestral area of the genus, which emerged in the Paleocene (about 57 Myr). The IWP is known as a vast tropical marine species rich hotspot17, with many taxa originating and diversifying in the early Cenozoic Era (65 Myr)18,19,20. This radiation has been linked to various geological events, such as the closure of the Tethys Sea, collision of Australia and proto-New Guinea with southeastern Asia, with continual changes in the availability of new tropical shallow-water habitats causing a postulated rise in the number of reefal associated taxa, such as Hippolyte species.
For some taxa, the IWP can be separated into three broad biogeographically distinct regions, the Indian Ocean, the Indo-Australian Archipelago and the Central West Pacific Islands21. For Hippolyte, this pattern was not observed, instead, considerable mixing of regional lineages is evident (Fig. 2A), linked to the absence of physical barriers across the region to planktonic dispersal of wide spread taxa such as H. jarvinensis and H. dossena. Clearly the presence of such taxa in peripheral areas, such as H. dossena in the Red Sea is linked to the opening up of those areas in the past22.
Besides the radiation across the entire IWP region, the spread of the genus appears to have occurred in three general directions
-
(1)
South Pacific, indicated by the clade formed by H. bifidirostris [the co-distributed H. multicolorata was not included in our analysis]. Our analysis suggest an early vicariant event separating H. bifidirostris from the IWP species (Fig. 3), dated at 56 ± 8 Myr, close to the postulated origin of the genus (Fig. 4). It is worth to mention that the position of H. bifidirostris differs in the phylogenetic tree/molecular clock (outside of the EA + MS/EP + WA clades, Figs. 2A and 4) versus the S-DIVA results (outside of IWP clade, Fig. 3). As the origin of this species is very close to the herein resolved origin of the genus, it becomes difficult determine its true position, as nodes with rapid and ancient radiations are more complex to solve. In this way, although the S-DIVA suggests an early vicariant event, we are more inclined towards a dispersal scenario, as it is unclear precisely which geological event would underlie such a vicariant event.
-
(2)
Eastern Atlantic and Mediterranean Sea, indicated by a mixed geographical clade dated at 47 ± 7 Myr (Fig. 4). Our analysis suggests dispersal from the IWP into the eastern Atlantic, which such a migration around Cape of Good Hope demonstrated for fishes22. Our analysis also indicates repeated dispersal and vicariant events between the EA and the MS, in both directions linked to the evolution of the Tethys seaway. According to the molecular clock, the endemic Mediterranean species (H. holthuisi, H. niezabitowskii and H. sapphica) arose in the Oligocene/Miocene, thus, before the Messinian Salinity Crisis (MSC) which occurred in the Pliocene23. Could these species have survived the MSC? The eastern Mediterranean probably showed less drastic changes following the connection closure between the Atlantic Ocean and the Mediterranean Sea compared to the western Mediterranean24. A significant water body could have remained in parts of the eastern basin24 acting as refugia for some species. It is indeed believed that some species actually survived the MSC in situ 25, 26. Perhaps this is also the case for Hippolyte, especially for H. sapphica, which is endemic to the eastern Mediterranean1, although it cannot be ruled out that these species originated in the nearby Atlantic Ocean and migrated into the Mediterranean after the MSC.
-
(3)
Americas, indicated by the clade formed by species of the EP and WA, but with the Malagasy H. kraussiana included. The presence of H. kraussiana outside this clade is anomalous. The position of this species in the analysis is best interpreted as somewhat doubtful, as only the 16S marker could be sequenced herein, potentially influencing the analysis. According to the present analysis, the colonization of the Americas by Hippolyte species likely resulted from dispersal from the IWP into the EP in the Eocene, followed by a radiation into the WA. The eastern Pacific is separated from the Indo-West Pacific by a 5.000–8.000 km expanse of open ocean which has been suggested to be the world’s most effective barrier to larval dispersal21, 27 for the past 65 Myr28. However, there are examples of species and genera which have crossed this barrier, most often from west to east21, 22, 29, including some caridean shrimps30. After the colonization of the Pacific side of the Americas, it appears that the genus radiated into the western Atlantic in the Oligocene (Figs. 3 and 4).
Somewhat unexpectedly the separation of the clades of EP + WA versus EA + MS is also supported by sperm morphology. The overall sperm cell morphology of EP + WA species (H. obliquimanus, H. williamsi and H. zostericola) is very different from the EA + MS species (H. inermis and H. niezabitowskii). In the first group the sperm shows numerous posterior nuclear arms, perhaps unique within Decapoda31, 32, whilst in the latter clade no posterior nuclear arms are known, this being as the pattern in the majority of caridean shrimps33, 34.
Cryptic or pseudocryptic species
Four specimens morphologically identified as H. ventricosa from Fiji, Indonesia, and Taiwan were included in the present analyses. These specimens all have the first article of the peduncle of the antennula with a single tooth on the outer distal corner and are different (morphologically as well as genetically) from the related taxa, H. australiensis, H. edmondsoni, H. jarvinensis and H. ngi.
Among these specimens, four genetically distinct taxa can be recognized (Fig. 2A): H. ventricosa group – sp. 1 (morphologically closest to the type specimens redescribed by d’Udekem d’Acoz in 19994) and sp. 2 from Indonesia; H. ventricosa group – sp. 3 from Fiji; and H. ventricosa group – sp. 4 from Taiwan. Despite morphological similarities, these four species did not form a clade in our phylogenetic analysis (Fig. 2A), as sp. 1 was more external; sp. 2 is positioned close to H. jarvinensis, whilst sp. 3 and sp. 4 were positioned close to H. australiensis. Although we tissue plucked the syntypes and other material of H. ventricosa from India used in the redescription by d’Udekem d’Acoz4 these did not successfully amplify and no fresh material from the type locality is presently available. Hence at the moment, it is not clear which of these corresponds to the true H. ventricosa. Solving this taxonomic puzzle is beyond the present work, but it evident that several potentially new species await formal description.
Transisthmian relationships
Three species of Hippolyte inhabit the eastern Pacific, H. californiensis and H. clarki are restricted to the northeastern Pacific35 whilst H. williamsi occurs in tropical and subtropical waters36. Previous studies based on morphology and molecules37, 38 considered the western Atlantic H. obliquimanus more closely related to H. williamsi, than to the other western Atlantic Hippolytespecies. However up to now, H. californiensis has not been included in these comparisons. A clear morphological feature which unites these three species is the presence of up to three teeth on the outer distal corner of the first article of antennular peduncle (H. californiensis with 1–3, H. obliquimanus with 2–3, H. williamsi with 335, 37,38,39).
Here, we analyzed specimens of H. obliquimanus from Brazil and Caribbean Panama, specimens of H. williamsi from Chile and Costa Rica and specimens of H. californiensis from the USA (Supplementary Table S1). The genetic divergence amongst these three species is very low (16S: 2.7%; COI: 14.3%; 18S: 0.2%; H3: 0.8–1.3%) compared to interspecific divergence across the genus (Table 1). In the present analyses, the only tree topology difference in the BAY and ML analyses was the positioning of these species (Fig. 2A vs. B), but support values in either analysis were low. However, the exact relationships of these taxa remain unclear as only H3 was successfully amplified for H. californiensis (Supplementary Table S1), effectively impeding fully resolved topologies. As concerns morphological data, except by two rows of subdorsal spines on pereiopods 3–5 dactylus found in H. williamsi, the variations among these species show clear overlap. Based on molecular data it is clear that all three species are valid and very closely related, but they can be distinguished by the combination of geographic distribution and some morphological characteristics. According the S-DIVA and the molecular clock analysis, there was a dispersion event between specimens from the western Atlantic (H. obliquimanus) and the eastern Pacific (H. williamsi/H. californiensis) in the Miocene (dated 11 ± 3 Myr), evidently before the final closure of Isthmus of Panama, dated 2.8 Myr40.
Systematic issues in Atlantic taxa
Hippolyte zostericola was considered to be closely allied to the sympatric H. pleuracanthus by its describer41, with the main differentiating character being rostrum length (longer in H. zostericola than H. pleuracanthus). Although there is a considerable doubt that these species are distinct8, some differences among larvae of H. pleuracanthus from North Carolina (USA) and larvae of H. zostericola from Bermuda were found42, and there are clear colour pattern differences between both species (SDG, pers. obs.). In the present analyses, were included H. zostericola from the British Virgin Islands with long rostrum and H. pleuracanthus specimens from Florida (Atlantic Coast and Gulf of Mexico) with rostra of variable sizes. Furthermore, we expand the distribution of H. pleuracanthus now registered on both sides of the Florida peninsula (see Supplementary Table S1).
Hippolyte sapphica was described43 with two distinct morphs (A and B), separated on the basis of rostral morphology, with no known intermediate forms1. This species inhabits shallow seagrass meadows in the central and eastern Mediterranean2, 44, although forma B has only been reported from the Amvrakikos Gulf (Greece) and Venice Lagoon (Italy), always sympatric with forma A45. Larval development studies of both forms concluded that females of both forms can generate larvae ultimately developing in adults of either form46. The present phylogenetic analysis indeed corroborates that these forms belong to the same species with negligible differences in their genetic makeup.
In the present analysis (Fig. 2A), H. sapphica was closely related to H. garciarasoi and H. leptocerus, with low genetic divergence among these three species, compared to other species pairs (e.g. 18S: 0.6–0.8%; H3: 1.2–1.5%). In contrast, morphology clearly separates H. sapphica from H. garciarasoi and H. leptocerus 2, a difference also reflected in larval morphology47.
Hippolyte garciarasoi and H. leptocerus however share many morphological characters, with older studies having considered them to be the same species48, this situation was, however, resolved and both species can be clearly distinguished49, 50. Nevertheless, some specimens show considerable morphological variation, confounding definite assignment to either species on the basis of morphology alone1. However, our molecular data do indeed confirm that H. garciarasoi and H. leptocerus are two distinct species.
The specimens of H. inermis showed the highest intraspecific genetic divergence in the present dataset for the mitochondrial markers (16S and COI), with both the BAY and ML analyses showing a clear geographical division;(1) France (Atlantic Coast), Greece and Tunisia and (2) Italy and Portugal, although no morphological differences could be discerned between both groups.
Hippolyte catagrapha was described from False Bay (South Africa)2, with presumed affinities, based on morphology, to the only two other crinoid-associated species, H. leptometrae and H. prideauxiana (more northern Atlantic taxa), with within the genus only H. catagrapha and H. leptometrae having whorls of setae on the tip of their third maxilliped2. The present analysis (Fig. 2A) indeed considers H. catagrapha and H. prideauxiana to be sister groups. Unfortunately, the phylogenetic position of H. leptometrae remains unresolved.
Hippolyte longiallex was relatively recently described based on samples from São Tomé and Príncipe2, and considered as a probable sister species to the western Atlantic H. nicholsoni, due to shared morphological characters2, but perhaps more strongly suggested by the fact that both species are gorgonian commensals. The present analysis demonstrates no such phylogenetic relationship between these taxa exists, and their morphological similarity is best interpreted as an adaptation to similar hosts in these symbiotic species.
Hippolyte niezabitowskii from the Mediterranean Sea, is morphologically close to both H. inermis and the Mediterranean Sea H. holthuisi 1. Previously H. holthuisi was considered to be but a junior synonym of the Atlantic H. varians. However, morphological differences between specimens from the eastern Atlantic and the Mediterranean Sea were highlighted1, with specimens from Madeira being morphologically intermediate. In 1998, specimens from either side of the Strait of Gibraltar were studied51, an Atlantic taxon H. varians as well as the exclusively Mediterranean H. holthuisi were recognized, which was subsequently accepted in the key of the genus to species from Atlantic2. In the present analysis, samples of H. holthuisi were included from Mediterranean Spain, as well as a widespread sampling of Atlantic localities for H. varians. Genetics clearly demonstrates two taxa should be recognized, with H. holthuisi being phylogenetically closer to H. niezabitowskii and H. longiallex than H. varians. As for the mitochondrial markers, the divergences among specimens of H. varians were low, between specimens from Madeira versus the other localities, and it is also confirmed that specimens from this archipelago are true H. varians.
Methods
Specimens of Hippolyte and Alcyonohippolyte were obtained from the following collections: Crustacean Collection of the Department of Biology of FFCLRP, University of São Paulo, Brazil (CCDB); Florida Museum of Natural History, USA (FLMNH); Muséum National d’Histoire Naturelle, France (MNHN); Museo de Zoología de la Universidad de Costa Rica, Costa Rica (MZ-UCR); National Museum of Natural History, Smithsonian Institution, USA (USNM); Oxford University Museum of Natural History, United Kingdom (OUMNH); Naturalis Biodiversity Centre, Leiden, the Netherlands (RMNH); University of Louisiana-Lafayette Zoological Collection, USA (ULLZ). Additional specimens were donated by Cédric d’Udekem d’Acoz (Belgium), José Cuesta and José Enrique García-Raso (Spain), Mary Wicksten (USA) and Valério Zupo (Italy); these samples were incorporated in the CCDB. All specimens were identified with the identification keys1, 2, 8 with reference to type and other descriptions as needed. Specimens from all regions of occurrence were examined, with global coverage (Fig. 1).
Tissue extraction, PCR amplification, product cleanup, and sequencing were conducted following previously described protocols52 with some modifications, mainly in product cleanup53. The primers and melting temperatures used were: mitochondrial 16S ribosomal gene [1472 (5′-AGATAGAAACCAACCTGG-3′)54, 16SL2 (5′-TGCCTGTTTATCAAAAACAT-3′)55, 46–48 °C]; mitochondrial Cytocrome Oxidase subunit I (COI) [COL6b (5′-ACAAATCATAAAGATATYGG-3′) and COH6 (5′-TADACTTCDGGRTGDCCAAARAAYCA-3′)56, COIAL2o (5′-ACGCAACGATGATTATTTTCTAC-3′) and COIAH2m (5′- GACCRAAAAATCARAATAAATGTTG-3´)57, 48–50 °C]; nuclear small subunit 18S ribosomal gene [18S-A (5′-AACCTGGTTGATCCTGCCAGT-3′) and 18S-L (5′-CCAACTACGAGCTTTTTAACTG-3′)58, 59 °C]; nuclear Histone 3 (H3) gene [H3AF (5′-ATGGCTCGTACCAAGCAGACVGC-3′) and H3AR (5′-ATATCCTTRGGCATRATRGTGAC-3′)59, 46–48 °C].
All sequences were confirmed by sequencing both strands (forward and reverse directions). Non-readable parts at the beginning of the sequences were omitted. All newly generated sequences were submitted to GenBank (Supplementary Table S1). Eight species were used as outgroup species, based on the phylogeny proposed in 201413, some of these were retrieved from Genbank, whilst some were newly generated (Supplementary Table S1). Sequences were aligned with MUSCLE60, with default settings, using the online version at the Cyberinfrastructure for Phylogenetic Research (CIPRES) website61.
For each marker, we separately tested substitution saturation62, 63 in the software DAMBE 564. A matrix of genetic distances was calculated under the Kimura 2-parameter (K2P) model65 in MEGA v566 for each gene dataset (16S: 505 base pairs (bp), 59 sequences; COI: 557 bp, 46 sequences; 18S: 622 pb, 54 sequences; H3: 369 bp, 54 sequences).
A combined 16S/COI/18S/H3 dataset with 2053 bp was used for the phylogenetic analyses, with any unavailable data coded as missing and partioned by gene. The Maximum Likelihood analysis (ML) was conducted with RAxML 8.2.867 using the online version at CIPRES, ML was conducted with the default parameters for RAxML for the GTR model of evolution, using the option to automatically determine the number of bootstraps to be run in RAxML68, thus, 900 bootstrap pseudo-replicates were run, and only confidence values >80% are reported. Prior to conducting the Inference Bayesian analysis (BAY), the model of evolution that best fitted the data was determined with the software jModelTest using BIC69. BAY was conducted in MrBayes v3.2.670, implemented in CIPRES, with the parameters obtained from jModelTest (nucleotide frequencies, transition/transversion ratio and shape of the gamma distribution). This analysis was conducted by sampling one tree every 1,000 generations for 20,000,000 generations, starting with a random tree. Four independent BAY runs were performed and the convergence of the runs was assessed using Tracer 1.671. The first 10% of parameters and trees was discarded (burn-in) and a final tree was generated in software Tree Annotator 1.8.4 (implemented in the BEAST package72) and visualized with FigTree 1.4.373. Confidence values of posterior probabilities > 80% only are reported. The map was built with a template of the software Diva-Gis 7.574.
In order to reconstruct the possible ancestral ranges of Hippolyte species, we used the S-DIVA75 analysis implemented in RASP software76. We used the trees generated (18,000) by our BAY run as input and defined the geographic areas as follows, based on the presence of the species in the obtained clades: IWP: Indo-West Pacific, SP: southern Pacific, EP: eastern Pacific; WA: western Atlantic; EA: eastern Atlantic; MS: Mediterranean Sea. The number of maximum ancestral areas was kept as 2. The possible ancestral ranges at each node on a selected tree were obtained. The state was sampled every 200 generations.
Divergence times within the Hippolyte clade were estimated using a strict clock in MrBayes implemented in CIPRES, as described above, with the same parameters obtained from jModelTest. For this analysis, only the mitochondrial markers were used and the substitution rate was fixed to 0.007 (COI) and 0.004 (16S) based on the divergence rate per one million years (Myr) reported in literature for these markers54, 77.
References
d’Udekem d’Acoz, C. The genus Hippolyte Leach, 1814 (Crustacea: Decapoda: Caridea: Hippolytidae) in the East Atlantic Ocean and the Mediterranean Sea, with a checklist of all species in the genus. Zool. Verh. 303(1), 1–133 (1996).
d’Udekem d’Acoz, C. New records of Atlantic Hippolyte, with the description of two new species, and a key to all Atlantic and Mediterranean species (Crustacea, Decapoda, Caridea). Zoosystema. 29(1), 183–207 (2007).
Gan, Z. & Li, X. A new species of the genus Hippolyte (Decapoda: Caridea: Hippolytidae) from South China Sea and Singapore. Zootaxa. 4258, 34–42 (2017).
d’Udekem d’Acoz, C. Redescription of Hippolyte ventricosa H. Milne Edwards, 1837 based on syntypes, with remarks on Hippolyte orientalis Heller, 1862 (Crustacea, Decapoda, Caridea). Zoosystema. 21(1), 65–76 (1999).
Marin, I., Okuno, J. & Chan, T. Y. On the “Hippolyte commensalis Kemp, 1925” species complex (Decapoda, Caridea, Hippolytidae), with the designation of a new genus and description of two new species from the Indo-West Pacific. Zootaxa. 2768, 32–54 (2011).
Marin, I. Two new species of the genus Alcyonohippolyte Marin, Okuno & Chan, 2010 (Crustacea: Decapoda: Hippolytidae) from the Great Barrier Reef of Australia. Zootaxa. 3123, 49–59 (2011).
Marin, I. & Chan, T. Y. Description of Alcyonohippolyte brachycarpus sp. nov., a further new hippolytid shrimp (Decapoda, Caridea, Hippolytidae) associated with soft coral from Taiwan, with the key to the species of the genus Alcyonohippolyte Marin, Okuno & Chan, 2011. Zootaxa. 3534, 53–60 (2012).
Chace, F. A. Jr. The shrimps of the Smithsonian-Bredin Caribbean Expeditions, with a summary of the West Indian shallow-water species (Crustacea: Decapoda: Natantia). Smithson. Contrib. Zool. 98(1), 1–179 (1972).
Espinoza-Fuenzalida, N. L., Thiel, M., Dupre, E. & Baeza, J. A. Is Hippolyte williamsi gonochoric or hermaphroditic? A multi-approach study and a review of sexual systems in Hippolyte shrimps. Mar. Biol. 155, 623–635 (2008).
Terossi, M., López Greco, L. S. & Mantelatto, F. L. Hippolyte obliquimanus (Decapoda: Caridea: Hippolytidae): a gonochoric or hermaphroditic shrimp species? Mar. Biol. 154(1), 127–135 (2008).
Bracken, H. D. et al. The Decapod Tree of Life: Compiling the data and moving toward a consensus of decapod evolution. Arthropod Syst. Phylo. 67(1), 99–116 (2009).
Bracken, H. D., De Grave, S. & Felder, D. L. Phylogeny of the infraorder Caridea based on mitochondrial and nuclear genes (Crustacea: Decapoda). Crustac. Issues. 18, 281–308 (2009).
De Grave, S., Li, C. P., Tsang, L. M., Chu, K. H. & Chan, T. Unweaving hippolytoid systematics (Crustacea, Decapoda, Hippolytidae): resurrection of several families. Zool. Scr. 43(5), 496–507 (2014).
Tamura, K. & Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526 (1993).
Posada, D. Using Modeltest and PAUP* to select a model of nucleotide substitution in Current Protocols in Bioinformatics (eds. Baxevanis, A. D. et al.) 6.5.1-6.5.14 (John Wiley & Sons, 2003).
Kimura, M. Estimation of evolutionary distances between homologous nucleotide sequences. Proc. Natl. Acad. Sci. 78, 454–458 (1981).
Briggs, J. C. Coincident biogeographic patterns: Indo-West Pacific Ocean. Evolution. 53(2), 326–335 (1999).
Signor, P. W. The geologic history of diversity. Annu. Rev. Ecol. Syst. 21, 509–539 (1990).
Crame, J. A. Evolution of taxonomic diversity gradients in the marine realm: evidence from the composition of recent bivalve faunas. Paleobiology. 26, 188–214 (2000).
Crame, J. A. & Rosen, B. R. Cenozoic palaeogeography and the rise of modern biodiversity patterns in Palaeobiogeography and Biodiversity Change: the Ordovician and Mesozoic-Cenozoic Radiations (eds. Crame, J. A. & Owen, A. W.) 153-168 (Geological Society, 2002).
Cowman, P. F. & Bellwood, D. R. Vicariance across major marine biogeographic barriers: temporal concordance and the relative intensity of hard versus soft barriers. Proc. R. Soc. Lond. B. 280, 20131541 (2013).
Briggs, J. C. Global Biogeography. 452 pp. (Elsevier, 1995).
Duggen, S., Hoernle, K., Bogaard, P., Rüpke, L. & Morgan, J. P. Deep roots of the Messinian salinity crisis. Nature. 422, 502–606 (2003).
Meijer, P. M. & Krijgsman, W. A quantitative analysis of the desiccation and re-filling of the Mediterranean during the Messinian Salinity Crisis. Earth Planet Sci. Lett. 240, 510–520 (2005).
Boudouresque, C. F. Marine biodiversity of the Mediterranean: status of species, populations and communities. Sci. Rep. Port-Cros Natl. Park. 20, 97–146 (2004).
Calvo, M., Alda, F., Oliverio, M., Templado, J. & Machordom, A. Surviving the Messinian Salinity Crisis? Divergence patterns in the genus Dendropoma (Gastropoda: Vermetidae) in the Mediterranean Sea. Mol. Phylogenet. Evol. 91, 17–26 (2015).
Ekman, S. Zoogeography of the Sea. 417 pp. (Sidgwick and Jackson, 1953).
Rosen, B. R. & Smith, A. B. Tectonics from fossils? Analysis of reef coral and sea urchin distributions from Late-Cretaceous to recent, using a new method in Gondwana and Tethys (eds. Audley-Charles, M. G. & Hallam, A.) 275–306 (Oxford University Press, 1988).
Lessios, H. A. & Robertson, D. R. Crossing the impassable: genetic connections in 20 reef fishes across the eastern Pacific barrier. Proc. R. Soc. Lond. B. 273, 2201–2208 (2006).
De Grave, S. Biogeography of Indo-Pacific Pontoniinae (Crustacea, Decapoda): a PAE analysis. J. Biogeogr. 28, 1239–1253 (2001).
Felgenhauer, B. E. & Abele L. G. Morphological diversity of decapod spermatozoa in Crustacean Sexual Biology (eds Bauer R. T. & Martin, J.W.) 322–341 (Columbia University Press, 1991).
Terossi, M., Tudge, C., López Greco, L. S. & Mantelatto, F. L. A novel spermatozoal ultrastructure in the shrimp Hippolyte obliquimanus Dana, 1852 (Decapoda: Caridea: Hippolytidae). Invertebr. Reprod. Dev. 56(4), 299–304 (2012).
Cobos, V., Díaz, V., García-Raso, J. E. & Manjón-Cabeza, M. E. The male reproductive system of Hippolyte inermis Leach 1815 (Decapoda, Caridea). Helgol. Mar. Res. 65(1), 17–24 (2011).
Manjón-Cabeza, M. E., Cobos, V. & García-Raso, J. E. The reproductive system of Hippolyte niezabitowskii (Decapoda, Caridea). Zoology. 114, 140–149 (2011).
Chace, F. A. Jr. The grass shrimps of the genus Hippolyte from the west coast of North America. J. Wash. Acad. Sci. 41(1), 35–39 (1951).
Wicksten, M. K. Key to the hippolytid shrimp of the eastern Pacific Ocean. Fish. Bull. 88, 587–598 (1990).
d’Udekem d’Acoz, C. Redescription of Hippolyte obliquimanus Dana, 1852, and comparison with Hippolyte williamsi Schmitt, 1924 (Decapoda, Caridea). Crustaceana. 70(4), 469–479 (1997).
Terossi, M. & Mantelatto, F. L. Morphological and genetic variability in Hippolyte obliquimanus Dana, 1852 (Decapoda: Caridea: Hippolytidae) from Brazil and the Caribbean Sea. Crustaceana. 85(6), 685–712 (2012).
Schmitt, W. L. The Macrura and Anomura collected by the Williams Galapagos Expedition, 1923. Zoologica. 5(15), 161–171 (1924).
O’Dea, A. et al. 2016. Formation of the Isthmus of Panama. Sci. Adv. 2, e1600883 (2016).
Smith, S. I. Crustacea in Report of Commissioner of Fish and Fisheries, on the Condition of the Sea-fisheries of the South Coast of New England in 1871 and 1872 (ed. Baird, S. F.) 545–580 (1873).
Shield, P. D. Larval development in the caridean shrimp Hippolyte pleuracanthus (Stimpson), reared in the laboratory. Estuaries. 1(1), 1–16 (1978).
d’Udekem d’Acoz, C. Description d’une nouvelle crevette de l’île de Lesbos: Hippolyte sapphica sp. nov. (Crustacea, Decapoda, caridea, Hippolytidae). Belg. J. Zool. 123(1), 55–65 (1993).
Koukouras, A. & Anastasiadou, C. The genus Hippolyte Leach (Decapoda,Caridea) in the Aegean and Ionian Seas. Crustaceana. 75(3–4), 443–449 (2002).
Liasko, R., Anastasiadou, C., Ntakis, A. & Leonardos, I. D. How a sharp rostral dimorphism affects the life history, population structure and adaptability of a small shrimp: the case study of Hippolyte sapphica. Mar. Ecol. 36, 400–407 (2015).
Ntakis, A., Anastasiadou, C., Liasko, R. & Leonardos, I. D. Larval development of the shrimp Hippolyte sapphica d’Udekem d’Acoz, 1993 forma A and B (Decapoda: Caridea: Hippolytidae) reared in the laboratory, confirmation of the conspecific status of the two forms. Zootaxa. 2579, 45–58 (2010).
Guerao, G., Hernández, E. & Urzúa, A. Early zoeal development of the shrimp Hippolyte leptocerus (Decapoda, Caridea, Hippolytidae). Zootaxa. 2988, 53–65 (2011).
O’Céidigh, P., Murray, A. & McGrath, D. Hippolyte longirostris (Czerniavsky, 1868) (Decapoda, Caridea) off the West and South East Coasts of Ireland. Crustaceana. 43(1), 110–112 (1982).
García-Raso, J. E. Carideos ibéricos (Crustacea, Decapoda): síntesis. Misc. Zool. 11, 113–120 (1987).
d’Udekem d’Acoz, C. Note sur quelques crevettes littorales du Sud-Ouest de la France: Alpheus dentipes, Hippolyte leptocerus, Hippolyte longirostris, Lysmata seticaudata et. Periclimenes sagittifer. De Strandvlo. 12(2), 35–42 (1992).
García-Raso, J. E., Manjón-Cabeza, M. E. & Martínez, J. C. I. Considerations on some species of Hippolyte (Decapoda, Caridea) from southern European waters, H. niezabitowskii, H. holthuisi, and H. varians. Crustaceana. 71(4), 453–467 (1998).
Mantelatto, F. L., Robles, R., Biagi, R. & Felder, D. L. Molecular analysis of the taxonomic and distributional status for the hermit crab genera Loxopagurus Forest, 1964 and Isocheles Stimpson, 1858 (Decapoda, Anomura, Diogenidae). Zoosystema. 28(2), 495–506 (2006).
Almeida, A. O., Terossi, M., Araújo-Silva, C. L. & Mantelatto, F. L. Description of Alpheus buckupi spec. nov., a new amphi-Atlantic snapping shrimp (Caridea: Alpheidae), based on morphological and molecular data. Zootaxa. 3652(4), 437–452 (2013).
Schubart, C. D., Neigel, J. E. & Felder, D. L. Use of the mitochondrial 16S rRNA gene for phylogenetic and population studies of Crustacea. Crustacean Issues. 12, 817–830 (2000).
Schubart, C. D., Cuesta, J. A. & Felder, D. L. Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea. J. Crust. Biol. 22(1), 28–44 (2002).
Schubart, C. D. & Huber, M. G. J. Genetic comparisons of German populations of the stone crayfish, Austropotamobius torrentium (Crustacea: Astacidae). Bull. Fr. Peche Piscic. 380–381, 1019–1028 (2006).
Mantelatto, F. L. et al. New primers of Cytochrome Oxidase Subunit I barcoding region designed for Decapoda species (Crustacea). Nauplius. 24, e2016030 (2016).
Apakupakul, K., Siddall, M. E. & Burreson, E. M. Higher level relationships of leeches (Annelida: Clitellata: Euhirudinea) based on morphology and gene sequences. Mol. Phylogenet. Evol. 12(3), 350–359 (1999).
Colgan, D. J. et al. Histone H3 and U2 snRNA DNA sequences and arthropod molecular evolution. Aust. J. Zool. 46(5), 419–437 (1998).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32(5), 1792–1797 (2004).
Miller, M. A., Pfeiffer, W. & Schwartz, T. “Creating the CIPRES Science Gateway for inference of large phylogenetic trees” in Proceedings of the Gateway Computing Environments Workshop (GCE) 1–8 (New Orleans, LA, 2010).
Xia, X., Xie, Z., Salemi, M., Chen, L. & Wang, Y. An index of substitution saturation and its application. Mol. Phylogenetic. Evol. 26, 1–7 (2003).
Xia, X. & Lemey, P. Assessing substitution saturation with DAMBE in The Phylogenetic Handbook: A Practical Approach to DNA and Protein Phylogeny (eds. Lemey, P., Salemi, M. & Vandamme, A.) 615–630 (Cambridge University Press, 2009).
Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 30, 1720–1728 (2013).
Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16(2), 111–120 (1980).
Tamura, K. et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28(10), 2731–2739 (2011).
Stamatakis, A. RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics, doi:10.1093/bioinformatics/btu033 (2014).
Stamatakis, A., Hoover, P. & Rougemont, J. A fast bootstrapping algorithm for the RAxML web-servers. Syst. Biol. 57(5), 758–771 (2008).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Met. 9, 772 (2012).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61(3), 539–542 (2012).
Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer v1.6. Available from http://beast.bio.ed.ac.uk/Tracer (2014).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Rambaut, A. FigTree 1.4.3. Available: http://tree.bio.ed.ac.uk/software/figtree/ (2016).
Hijmans, R. J., Guarino, L., Cruz, M. & Rojas, E. Computer tools for spatial analysis of plant genetic resources data: 1. DIVA-GIS. Plant. Genet. Resour. 127, 15–19 (2001).
Yu, Y., Harris, A. J. & He, X. J. S-DIVA (statistical dispersal–vicariance analysis): a tool for inferring biogeographic histories. Mol. Phylogenet. Evol. 56, 848–850 (2010).
Yu, Y., Harris, A. J., Blair, C. & He, X. J. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical Biogeography. Mol. Phylogenet. Evol. 87, 46–49 (2015).
Knowlton, N. & Weigt, L. A. New dates and new rates for divergence across the Isthmus of Panama. Proc. R. Soc. Lond. B. 265, 2257–2263 (1998).
Acknowledgements
This study is part of postdoctoral project of the first author, and is carried out within the multidisciplinary research project Temático BIOTA-FAPESP (São Paulo Research Foundation) under the senior author’s responsibility and coordination. Financial support for this project was provided by research grants from FAPESP (PD 2011/11901-3; Temático Biota 2010/50188-8). Additional support came from CAPES (Ciências do Mar II Proc. 2005/2014 − 23038.004308/2014-14). We are deeply grateful to many colleagues and friends (Andrew Cabrinovic, Cédric d’Udekem d’Acoz, Charles Fransen, Darryl Felder, Fernando Alvarez, Gary Poore, Gustav Paulay, Ingo Wehrtmann, José Cuesta, José Enrique García-Raso, Gary Poore, Jose Luis Villalobos, Karen van Dorp, Laure Corbari, Mariana Negri, Mary Wicksten, the late Michael Türkay, Paula Martin-Lefevre, Peter Dworschak, Rafael Robles, Rafael Lemaitre, Raquel Buranelli, Stephen Keable, Tatiana Magalhães and Valério Zupo) for donation of specimens, loaning material from collections, for facilities at their institutions, and for transporting some material.
Author information
Authors and Affiliations
Contributions
M.T. and F.L.M. idealized and executed the project; F.L.M. provided all institution and laboratory facilities and coordinated the main financial grants that supported the project; M.T. and F.L.M. visited collections; SDG co-wrote the text; M.T. carried out the laboratory work and performed the analyses. All authors read, corrected and approved the final version of the text.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Terossi, M., De Grave, S. & Mantelatto, F. Global biogeography, cryptic species and systematic issues in the shrimp genus Hippolyte Leach, 1814 (Decapoda: Caridea: Hippolytidae) by multimarker analyses. Sci Rep 7, 6697 (2017). https://doi.org/10.1038/s41598-017-06756-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06756-1
This article is cited by
-
Morphotype induced changes in the life history and population dynamics of an hippolytid shrimp
Scientific Reports (2023)
-
From East Asia to Beringia: reconstructed range dynamics of Geranium erianthum (Geraniaceae) during the last glacial period in the northern Pacific region
Plant Systematics and Evolution (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.