Abstract
Hyphae of higher fungi grow at their tips and are compartmentalized by porous septa that enable inter-compartmental cytoplasmic streaming. Woronin bodies discontinue cytoplasmic streaming by plugging the septal pores. Here, it was assessed whether apical compartments of Aspergillus niger sustain their own growth or whether their growth depends on subapical compartments. Hyphae of wildtype and the ΔhexA strain, lacking Woronin bodies, had a similar morphology and growth rate. A total of 58% and 17% of the hyphae continued growing, respectively, after dissecting the 2nd compartment. Extension rate of the apical compartments that continued growing was not affected, even when the carbon or nitrogen source was limiting. Thus, apical compartments are self-sustaining in growth. It was also shown that the first 8 subapical compartments of the wildtype, but not of the ΔhexA strain, function as a backup system for growth by forming new branches when their apical neighbouring compartment has been damaged. This backup system is pivotal in nature because of the life style of fungi to continuously explore their surrounding substrate that may prove hostile.
Similar content being viewed by others
Introduction
Mycelia of filamentous fungi consist of interconnected hyphae that grow at their apices and that branch subapically. Hyphae of fungi belonging to the Ascomycota and the Basidiomycota are compartmentalized by septa. These cross-walls have a central pore of 50–500 nm1,2,3 that allows inter-compartmental and inter-hyphal cytoplasmic mixing. However, it was recently shown that mixing in Aspergillus is constrained by Woronin bodies that plug the septal pores4, 5. These peroxisome-derived organelles plug about 50% of the most apical septa. Plugging incidence increases sub-apically resulting in 100% closure after the 9th compartment. From these results it was concluded that hyphal compartments transform from an unicellular to a multicellular system5. Plugging of septal pores thus abolishes indiscriminate mixing of cytoplasm, but selective transport is possible via transporters in the septal plasma membrane6.
The multicellular nature of the sub-apical part of hyphae explains why zones of a colony are heterogeneous with respect to gene expression, growth, and secretion7,8,9,10,11,12,13,14,15,16,17,18. Even neighboring hyphae can be heterogeneous in cytoplasmic composition4, 7, 8, 14, 15, 18,19,20,21,22,23,24. This heterogeneity is abolished in a ΔhexA strain4. This strain lacks Woronin bodies because it does not produce the main protein component of these peroxisome-like organelles25 and, as a consequence, septal plugging is rarely observed4, 26.
The fact that the apical compartments of Aspergillus hyphae form an unicellular system would agree with the concept of the peripheral growth zone defining the number of compartments that are needed to maintain the maximum hyphal growth rate27. This zone was described to consist of 11, 14, 6, and 13 compartments in the case of Aspergillus niger, Aspergillus wentii, Aspergillus nidulans, and Penicillium chrysogenum, respectively. We here however show that apical compartments of A. niger are self-sustaining in growth thus disproving the concept of the peripheral growth zone. The subapical compartments do have a function by acting as a back-up system for growth when an apical compartment is damaged.
Results
Apical compartments maintain their growth after laser-dissection
Light microscopy showed that the hyphal growth rate of the A. niger wild-type and ΔhexA strains was similar with a mean extension rate of 99 ± 5 and 93 ± 8 µm h−1, respectively. Length (358 ± 62 and 350 ± 64) and diameter (5.8 ± 0.2 and 6.0 ± 0.3) of the apical compartments of wild type and ΔhexA were also similar. Hyphae were laser dissected within the 2nd, 3rd, 4th, and 11th compartment. Mean hyphal growth rate of both strains was not reduced when the filaments had been dissected in the 11th compartment. In contrast, it was reduced by 39, 22, and 23% after dissecting the 2nd, 3rd, and 4th compartment of wild-type hyphae, respectively. These values were higher for ΔhexA with 82, 47, and 49%, respectively.
A total of 42, 20, 22, and 9% of wild-type hyphae stopped growing, respectively, when they had been dissected in the 2nd, 3rd, 4th, or 11th compartment (Table 1). The apical septum had been open in all hyphae that had stopped growing after dissection of the 2nd compartment. Conversely, wild-type hyphae that remained growing had either a closed (20%) or open (80%) apical septum at the moment of dissection. The percentage of ΔhexA hyphae halting growth after dissecting the 4th or 11th compartment was similar to that of wild-type. In contrast, the percentage was higher when the 2nd or 3rd compartment was dissected. These data show that Woronin bodies protect hyphae from stopping growth after a subapical compartment is damaged.
Growth rate of wild-type and ΔhexA hyphae that remained growing after cutting in the 2nd, 3rd, 4th, or 11th compartment was not affected (Table 1). Similar results were obtained when the C- or N-source was excluded from the liquid medium (Data not shown; for growth conditions see Material and Methods). These data show that reduction of the mean growth rate of dissected hyphae is solely due to the fraction of hyphae that stop growing after this damaging event.
Predictors for continued growth of dissected hyphae
Growth rate of wild-type and the ΔhexA hyphae was normally distributed (Fig. S1). The relatively slow and fast growing hyphae within these normal distributions did not show differences in the incidence of continued growth after dissection in the second compartment (Data not shown). Thus, no causality was shown between continued growth and growth rate of the hyphae. The initial volume of wild-type apical compartments that continued or stopped growing was not significantly different (Table 2) and therefore was not a predictor of continued hyphal growth. In contrast, residual cytoplasmic volume in the apical compartment did differ after dissecting the 2nd compartment. Similar results were obtained with ΔhexA. However, the cytoplasmic volume that was lost was 5.6- and 2.6-fold higher in the case ΔhexA hyphae that had stopped or continued growing after laser dissection, respectively, when compared to their wild-type equivalents (Table 2). Binary logistic regression showed that the length of the apical compartment is also a predictor for continued growth after laser ablation (Supplemental Text 1).
Hyphae were selected that had their apical septum at a distance <or> 400 µm from the apex. These hyphae were dissected at 400 µm from the tip. 11% and 43% of the wild-type and ΔhexA hyphae with a septum <400 µm from the apex (i.e. these hyphae were cut in the 2nd comprtment) stopped growing. In contrast, all wild type and ΔhexA hyphae with a septum >400 µm from the apex (i.e. these hyphae were cut in the 1st compartment) stopped growing. Cutting within the first compartment at 400 µm from the apex resulted in a loss of 49% and 68% of the cytoplasm for ΔhexA and wild-type hyphae, respectively, while 46% and 32% was lost when the dissection site at 400 µm from the tip was within the second compartment. These results show that the presence of a septum between the apex and the site of dissection, independent of the state of the septum, is the best predictor whether a hyphae continues its growth after ablation.
Disruption of the Spitzenkörper is linked to the fate of hyphal fragments
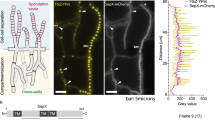
The Spitzenkörper of A. niger FG7 is fluorescently labelled due to a eGFP:: SncA fusion protein28. Surface area, length, and fluorescence intensity of the Spitzenkörper in the apical compartment was determined before and after dissecting the 2nd compartment. Surface area, length, and fluorescence intensity of the Spitzenkörper of hyphae that stopped growing after dissection were smaller when compared to those of hyphae that remained growing (Fig. 1; Supplemental Table 1). These differences were not observed prior to dissection. These data show a relation between continued growth after dissection of the 2nd compartment and integrity of the Spitzenkörper.
Fluorescently labelled Spitzenkörper before and after dissection in the 2nd compartment in a control hypha (A), a hypha continuing (B) and a hypha stopping growth (C) after dissection. Numbers indicate the time after laser dissection. White arrows denote the tip for which fluorescence was recorded. The black arrow indicates the moment of cutting. Bar represents 25 µm.
Subapical compartments reinitiate growth after ablation of their apical neighbouring compartment
Branching incidence was assessed for compartments 2, 5, 8, and 10 of wildtype and ΔhexA hyphae that had either or not been cut in the apical neighbouring compartment. Branching in subapical compartments was hardly (<2% for the 2nd compartment), if at all (for the 5th, 8th, and 10th compartment), observed when ΔhexA hyphae had been dissected in their neigboring apical compartment. In contrast, dissection of compartment 1 of wild-type hyphae increased branching frequency of compartment 2 from 30% to 93% within a 4 h period (p ≤ 0,05). Similarly, dissection of compartment 4 or 7 increased branching incidence from 29% to 71% and from 2% to 31%, respectively. Branching incidence of compartment 10 did not increase significantly (0% versus 4.5% without and with cutting) when hyphae were dissected in compartment 9. Branching incidence in compartments 2 and 5 was statistically higher than branching incidence in compartments 7 and 9 for both cut and uncut wild-type hyphae. Together, these data show that sub-apical compartments act as a back-up for growth when apical compartments are damaged. This back-up is not functional in the absence of Woronin bodies. Moreover, the back-up capacity of hyphal compartments diminishes when compartments are further away from the hyphal tip.
Discussion
Septate fungal hyphae like those of A. niger have been reported to require a minimal length of 1200 µm29 or 11 intact compartments27 to allow the maximum growth rate. We here however showed that apical compartments (mean length 354 µm) sustain their own maximum growth rate. This discrepancy is probably explained by the methodology adopted. Lhoas29 and Trinci27 damaged multiple hyphae with a metal blade and growth rate was expressed as the increase in colony diameter. In contrast, we dissected single hyphae with a laser and mean growth rate was determined taking into account the heterogeneity between the leading hyphae at the edge of the colony. In the case of the wild-type 42% of the hyphae stopped growing after dissecting the second compartment. These hyphae reduced the average growth rate of the whole population of hyphae. At the same time, average growth rate was not affected of those hyphae that remained growing after dissecting the second compartment. This shows that apical compartments do not depend on subapical compartments to sustain their growth.
The autonomy of apical compartments raised the question what the function is of subapical compartments. Subapical compartments may have a feeding function under nutrient deprivation, which was suggested from the finding that glucose is transported from the center to the periphery of the A. niger colony6. Yet, in our experimental set up apical compartments were still autonomous when N- and C-sources were limited. We did show that subapical compartments function as a back-up system. All hyphae stopped growing when apical compartments were dissected. This was accompanied by an increased branching incidence of the second compartment. These branches formed new exploring hyphae. Similarly, branching incidence was increased in compartment 5 and 8 after dissection of compartments 4 and 7, respectively. Branching incidence of compartment 10 was low, if observed at all, after dissecting compartment 9. This implies that this compartment and other more subapical compartments have lost their capacity to reinitiate growth. The ability of subapical compartments 2–8 to restore hyphal growth after their apical neighboring compartment has been damaged is pivotal considering the fungal life style. Mycelia continuously extend by colonizing their surrounding substrate. Apical compartments of exploring hyphae are the first to be confronted with microbes or other organisms within the non-explored substrate that can damage or feed on hyphae. Subapical branching combined with extension at an angle of the axis of the damaged hyphae provides a way to escape these organisms.
Woronin bodies can close septal pores, thus preventing cytoplasmic bleeding after hyphal damage25, 30,31,32. In addition, these organelles maintain inter-hyphal and inter-compartmental heterogeneity of cytoplasmic composition4. We here showed that Woronin bodies are not involved in the growth rate of hyphae before or after ablation but we found two additional functions of these organelles. First, branching in the sub-apical compartment was not observed in ΔhexA hyphae after ablation of the apical neighboring compartment. This shows that Woronin bodies are essential for the sub-apical back-up system of growth. Second, Woronin modies make hyphae more resilient to loss of cytoplasm. Half of wild-type hyphae were calculated to remain growing when 61% of the cytoplasmic volume in the apical compartment is retained, while 76% was needed in the case of ΔhexA hyphae. The resilience of the wild-type to cope with loss of cytoplasm is likely due to its ability to plug the septal pore, thus increasing the chance of the apical compartment to regain turgor. Experimental evidence indicates that not only loss of cytoplasm but also (partial) disintegration of the Spitzenkörper may result in halting of growth. Although in many cases occurrence of these processes will correlate, this is not necessarily the case. For instance, a strong but short outflow of cytoplasm may cause disintegration of the Spitzenkörper but would retain a sufficient amount of cytosol.
The observation that hyphae of A. niger always stop growing when the apical compartment is damaged implies that positioning of a septum close to the tip reduces the chance of abolished growth simply because the length available for a fatal damage would be less. In fact, fungi may increase septal incidence (i.e. reduce compartment length) to cope with stress. Indeed, length of apical compartments of A. niger is reduced at temperatures above 30 °C, coinciding with an increased branching incidence33.
Together, we have for the first time provided evidence that apical hyphal compartments of A. niger are autonomous with respect to growth. The subapical compartments are however metabolically active consuming a major part of resources. This drain of resources could be prevented by controlled autolysis of subapical compartments. This, however, would also result in the absence of the sub-apical back-up system of hyphal growth that is used when apical compartments become damaged.
Materials and Methods
Strains and growth conditions
strains N40234, ΔhexA 26, and FG728 of A. niger were grown at 30 °C in water-saturated air at 700 lux white light (Osram Lumilux L36w/840, Osram, Munich, Germany). Spores were harvested in 10 ml 0.9% NaCl (w/v), 0.05% (v/v) Tween-20 from 7-day-old cultures grown in 9 cm Petri dishes on complete medium (CM) consisting of minimal medium (0.6% NaNO3, 0.15% KH2PO4, 0.05% KCl, 0.05% MgSO4 7H2O, 0.2 ml−1 Vishniac solution35) with 0.2% tryptone, 0.1% casamino acids, 0.1% yeast extract, 0.05% yeast ribonucleic acids, 1.5% agarose, and 25 mM maltose. These spores were used to inoculate glass bottom dishes (MatTek Corporation, Ashland, MA, USA, P35G-1.5-20-C). To this end, the dishes and medium were pre-warmed to 50 °C and 60 °C, respectively. 0.5 µL spore solution (50,000 spores) was placed on a glass coverslip (18 mm in diameter and 0.16–0.19 mm thick) and left to dry. Pre-warmed CDMMA (30 µL) consisting of CD + Met medium (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4.7H2O, 0.002% FeSO4.7H2O, 0.0015% methionine, pH 5.536) with 1% agarose and 25 mM maltose was added on top of the glass bottom dish and immediately covered with the coverslip with the spores facing the medium. After the 118 µm thick layer of medium had solidified, 2 ml liquid CDMM was pipetted on top of the coverslip. CDMMA with 0.2% maltose and CDMM medium without a carbon source or CDMMA and CDMM without methionine were used to expose hyphae to C- or N-limiting conditions.
Microscopy
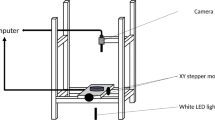
Hyphae were dissected using a PALM Microbeam system linked to an Axiovert 200 inverted microscope (Carl Zeiss AG, Oberkochen, Germany) and a 3CCD color camera (HV-D30,Hitachi Kokusai Electric Inc., Tokyo, Japan). The glass bottom dish cultures were incubated at the microscope stage for 90 min at 25 °C before dissection. Hyphal growth rate within the peripheral zone of the colony was recorded every 5 min during a 45 min period, after which half of the hyphae were dissected in the 2nd, 3rd, 4th, or 11th compartment using the laser pressure catapulting function of the PALM MicroBeam system (laser power 55%, focus 59%). At the moment of dissection it was assessed whether septal pores were open or closed. Septa were classified as open when cytosol was leaking through the septal pore upon dissection4. Hyphal growth was recorded every 5 min during a 45 min period immediately after dissection, non-dissected hyphae serving as control. Width and length of the compartment pre- and post-dissection were also recorded, as well as translocation (µm) of vacuoles after cutting. From these parameters the post-dissection volume of the apical compartment was calculated using V e = V 0 − V 1 , where V 0 = 1/2 π (2/3 \({{\rm{d}}}_{0}^{3}\) + \({{\rm{d}}}_{0}^{2}\) [l − 1/2 d0]) and V 1 = 1/2 π (2/3 \({{\rm{d}}}_{1}^{3}\) + \({{\rm{d}}}_{1}^{2}\) [l − 1/2 d1]) in the case volume was calculated based on reduction of hyphal diameter and where V 0 = 1/2 π (2/3 \({{\rm{d}}}_{0}^{3}\) + \({{\rm{d}}}_{0}^{2}\) [l − 1/2 d0]) and V 1 = 1/2d0 2πtl when volume was calculated based on translocation of vacuoles (Fig. S2).
Fluorescence was recorded using a HV-D30 camera with settings for white balance 5600 K; gain +8 db; contour correction high; shutter speed 1/50; digital gain +6 db; gamma on; contrast off; knee automatic. Total corrected cellular fluorescence was calculated with ImageJ. The surface of the Spitzenkörper was estimated by using the ImageJ threshold function using a brightness of 27.
Statistics
Experiments were performed using biological duplicates (assessment of growth rate) or triplicates (plugging state,loss of volume, and renewed subapical growth). A one-way ANOVA with a Sidak post-hoc test or Kruskall-Wallis test with subsequent Wilcoxson rank sum tests was carried out to determine whether growth rate differed between hyphae with a different number of intact compartments. A χ2 test was performed with post-hoc z-tests and a Bonferroni correction for multiple comparisons to assess differences in plugging incidence between septa. The effect of plugging on hyphal growth was evaluated using one-way ANOVA and a Sidak post-hoc test, differences in hyphal growth before and after cutting were determined using paired sample t-tests. Differences in volume loss were statistically analyzed using two-way ANOVA, followed by correlation and regression analyses. Relative values pertaining to volume of apical compartments before and after cutting were analyzed using a paired sample t–test with and without logit transformations. Analysis of bimodality was performed as described20. Renewed growth from compartments located subapically from compartments damaged by laser dissection was analyzed by a χ2 test with post-hoc z-tests and a Bonferroni correction for multiple comparisons.
References
Shatkin, A. J. & Tatum, E. L. Electron microscopy of Neurospora crassa mycelia. J. Biophys. Biochem. Cytol. 6, 423–426 (1959).
Moore, R. T. & McAlear, J. H. Fine structures of mycota. Observations on septa of ascomycetes and basidiomycetes. Am. J. Bot. 49, 86–94 (1962).
Lew, R. R. Mass flow and pressure-driven hyphal extension in Neurospora crassa. Microbiology 151, 2685–2692 (2005).
Bleichrodt, R. J. et al. Hyphal heterogeneity in Aspergillus oryzae is the result of dynamic closure of septa by Woronin bodies. Mol. Microbiol. 86, 1334–1344 (2012).
Bleichrodt, R. J., Hulsman, M., Wösten, H. A. B. & Reinders, M. J. Switching from a unicellular to multicellular organization in an Aspergillus niger hypha. MBio 6, e00111 (2015).
Bleichrodt, R. J., Vinck, A., Read, N. D. & Wösten, H. A. B. Selective transport between heterogeneous hyphal compartments via the plasma membrane lining septal walls of Aspergillus niger. Fungal Genet. Biol. 82, 193–200 (2015).
Wösten, H. A. B., Moukha, S. M., Sietsma, J. H. & Wessels, J. G. H. Localization of growth and secretion of proteins in Aspergillus niger. J. Gen. Microbiol. 137, 2017–2023 (1991).
Moukha, S. M., Wösten, H. A. B., Mylius, E. J., Asther, M. & Wessels, J. G. H. Spatial and temporal accumulation of mRNAs encoding two common lignin peroxidases in Phanerochaete chrysosporium. J. Bacteriol. 175, 3672–3678 (1993).
Moukha, S. M., Wösten, H. A. B., Asther, M. & Wessels, J. G. H. In situ localization of the secretion of lignin peroxidases in colonies of Phanerochaete chrysosporium using a sandwiched mode of culture. J. Gen. Microbiol. 139, 969–978 (1993).
Masai, K. et al. Square-plate culture method allows detection of differential gene expression and screening of novel, region-specific genes in Aspergillus oryzae. Appl. Microbiol. Biotechnol. 71, 881–891 (2006).
Levin, A. M. et al. Spatial differentiation in the vegetative mycelium of Aspergillus niger. Eukaryot. Cell 6, 2311–2322 (2007).
Levin, A. M., de Vries, R. P. & Wösten, H. A. B. Localization of protein secretion in fungal colonies using a novel culturing technique; the ring-plate system. J. Microbiol. Meth. 69, 399–401 (2007).
Kasuga, T. & Glass, N. L. Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot. Cell 7, 1549–1564 (2008).
de Bekker, C., Bruning, O., Jonker, M. J., Breit, T. M. & Wösten, H. A. B. Single cell transcriptomics of neighboring hyphae of Aspergillus niger. Genome Biol. 12, R71 (2011).
de Bekker, C., van Veluw, G. J., Vinck, A., Wiebenga, L. A. & Wösten, H. A. B. Heterogeneity of Aspergillus niger microcolonies in liquid shaken cultures. Appl. Environ. Microbiol. 77, 1263–1267 (2011).
Krijgsheld, P. et al. Spatially resolving the secretome within the mycelium of the cell factory Aspergillus niger. J. Proteome Res. 11, 2807–2818 (2012).
Krijgsheld, P. et al. Deletion of flbA results in increased secretome complexity and reduced secretion heterogeneity in colonies of Aspergillus niger. J. Proteome Res. 12, 1808–1819 (2013).
Wösten, H. A. B., van Veluw, G. J., de Bekker, C. & Krijgsheld, P. Heterogeneity in the mycelium: implications for the use of fungi as cell factories. Biotechnol. Lett. 35, 1155–1164 (2013).
Teertstra, W. R., Lugones, L. G. & Wösten, H. A. B. In situ hybridisation in filamentous fungi using peptide nucleic acid probes. Fungal Genet. Biol. 41, 1099–1103 (2004).
Vinck, A. et al. Hyphal differentiation in the exploring mycelium of Aspergillus niger. Mol. Microbiol. 58, 693–699 (2005).
Etxebeste, O. et al. The bZIP-type transcription factor FlbB regulates distinct morphogenetic stages of colony formation in Aspergillus nidulans. Mol. Microbiol. 73, 775–789 (2009).
Vinck, A. et al. Heterogenic expression of genes encoding secreted proteins at the periphery of Aspergillus niger colonies. Environ. Microbiol. 13, 216–225 (2011).
Knaus, H. et al. Monitoring the metabolic state of fungal hyphae and the presence of melanin by Nonlinear Spectral Imaging. Appl. Environ. Microbiol. 79, 6345–6350 (2013).
van Veluw, G. J. et al. Heterogeneity in liquid shaken cultures of Aspergillus niger inoculated with melanised conidia or conidia of pigmentation mutants. Stud. Mycol. 74, 47–57 (2013).
Jedd, G. & Chua, N. H. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat. Cell Biol. 2, 226–231 (2000).
Bleichrodt, R. Intercompartmental streaming in Aspergillus. PhD thesis, University of Utrecht (2012).
Trinci, A. P. J. Influence of the width of the peripheral growth zone on the radial growth rate of fungal colonies on solid media. J. Gen. Microbiol. 67, 325–344 (1971).
Kwon, M. J. et al. Molecular genetic analysis of vesicular transport in Aspergillus niger reveals partial conservation of the molecular mechanism of exocytosis in fungi. Microbiology 160, 316–329 (2014).
Lhoas, P. Growth rate and haploidization of Aspergillus niger on medium containing p-fluorophenylalanine. Genet. Res. 12, 305–315 (1968).
Collinge, A. J. & Markham, P. Woronin bodies rapidly plug septal pores of severed Penicillium chrysogenum hyphae. Exp. Mycol. 9, 80–85 (1985).
Tenney, K. et al. Hex-1, a gene unique to filamentous fungi, encodes the major protein of the Woronin body and functions as a plug for septal pores. Fungal Genet. Biol. 31, 205–217 (2000).
Maruyama, J., Juvvadi, P. R., Ishi, K. & Kitamoto, K. Three-dimensional image analysis of plugging at the septal pore by Woronin body during hypotonic shock inducing hyphal tip bursting in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Comm. 331, 1081–1088 (2005).
Abrashev et al. Temperature-stress tolerance of the fungal strain Aspergillus niger. World J. Microbiol. Biotechnol. 30, 1661–1668 (2014).
Bos, C. J. et al. Genetic analysis and the construction of master strains for assignment of genes to six linkage groups in Aspergillus niger. Curr. Genet. 14, 437–443 (1988).
Vishniac, W. & Santer, M. The thiobacilli. Bacteriol. Rev. 21, 195–213 (1957).
Maruyama, J., Escaño, C. S. & Kitamoto, K. AoSO protein accumulates at the septal pore in response to various stresses in the filamentous fungus Aspergillus oryzae. Biochem. Biophys. Res. Commun. 391, 868–873 (2010).
Author information
Authors and Affiliations
Contributions
M.T. performed the experiments, H.W. and M.T. designed experiments and wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
This work is part of research project 823.02.015, which is financed by the Netherlands Organisation for Scientific Research (NWO). H.W. and M.T. confirm not to have any competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tegelaar, M., Wösten, H.A.B. Functional distinction of hyphal compartments. Sci Rep 7, 6039 (2017). https://doi.org/10.1038/s41598-017-06422-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06422-6
This article is cited by
-
Inter- and intra-species heterogeneity in germination of Aspergillus conidia
Antonie van Leeuwenhoek (2022)
-
Protein expression and secretion by filamentous fungi
Journal of Biosciences (2021)
-
The PoV mycovirus affects extracellular enzyme expression and fruiting body yield in the oyster mushroom, Pleurotus ostreatus
Scientific Reports (2020)
-
Apical but not sub-apical hyphal compartments are self-sustaining in growth
Antonie van Leeuwenhoek (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.