Abstract
Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP-2) is a cytoplasmic tyrosine phosphatase that is highly expressed in hematopoietic cells and in the CNS and exerts opposite effects on signal transduction by exerting a neuroprotective or proapoptotic effect. Several mutations of SHP-2 have been found in children with myeloproliferative disorders or malignant leukemia, and some of these can affect brain development. In the present study, we aimed to identify and functionally characterize genetic variations in SHP-2 in 72 preterm and 58 full-term infants and to evaluate the effect of the variations on neurodevelopment in preterm infants. Twelve genetic variations were identified. Among them, two variations in the SHP-2 promoter, g.-317C > T and g.-273G > A, were found to significantly increase promoter activity, and the frequency of g.-273G > A was higher in preterm infants than in full-term infants. Two transcription factors, NF-κB and GABPα, were found to be involved in the transcriptional regulation of SHP-2 by the two above-mentioned variations. In particular, we found that g.-273G > A was significantly associated with delayed myelination and poor motor development in preterm infants. Our results suggest that a functional promoter variation in SHP-2 is associated with spontaneous preterm birth itself as well as white matter myelination and neurodevelopment.
Similar content being viewed by others
Introduction
Preterm birth is defined as live birth before 37 gestational weeks1. Although the mortality rates related to preterm birth have decreased in recent years, neurological impairments in preterm infants remain a problem2. Preterm birth is caused due to multiple factors including inflammation, infection, reactive oxygen species (ROS), and genetic factors3, 4. These factors also affect immature neuronal cells and result in poor neurological outcome later5. To date, several genes such as TNF alpha, IL-1 beta, IL-4, IL-6, and IL-10 have been investigated as candidate genes that modify the risk of preterm labor and perinatal complications6,7,8. Genetic variations in metalloproteinase, endothelial nitric oxide synthetase, superoxide dismutase, and catalase also have been suggested as risk factors for preterm birth9,10,11,12. However, the precise mechanism underlying the regulation of these genes remains obscure.
Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP-2) (also known as PTPN11) is a cytoplasmic tyrosine phosphatase that is widely expressed at high levels in hematopoietic cells and in the central nervous system (CNS)13, 14. SHP-2 is involved in neuroprotection in response to ischemic brain injury, and SHP-2 inhibition leads to reduced survival and increased programmed cell death of primary cultured neurons during nitric oxide exposure15,16,17. SHP-2 also functions as an important protein component of the raft-mediated signaling pathway and as a key regulator of a signaling cascade upon ROS-induced oxidative stress18, 19. Recently, several mutations in SHP-2 were found in children with diverse myeloproliferative disorders or malignant leukemia such as juvenile myelomonocytic leukemia (JMML), myelodysplastic syndrome, B cell acute lymphocytic leukemia, and acute myeloid leukemia20,21,22,23. In particular, the two most common mutations associated with JMML are known to cause a gain of function (GOF)23. Noonan syndrome, which is a frequent genetic disease with an estimated prevalence of approximately 1/2,000 living births, is caused by genetic variations in genes involved in Ras-Erk signaling, including SHP-2 24. Interestingly, a nonsynonymous GOF variation in SHP-2, D61G, is associated with aspects of neurodevelopment such as spatial learning and memory deficits in Noonan syndrome25. These results support a disease-associated function for SHP-2 against leukemia and brain development.
Therefore, we hypothesized that genetic variations in SHP-2 affect spontaneous preterm birth by regulating cytokines and a signaling pathway under ROS-induced oxidative stress. Furthermore, we reckoned that SHP-2 variations might also be associated with neurodevelopment in preterm infants, because SHP-2 is highly expressed in the CNS and works as a regulator of Ras-Erk signaling involved in neurodevelopment. To test our hypothesis, we compared genetic variations in SHP-2 between preterm and full-term infants and functionally characterized each variation using various in vitro assays. To evaluate the effect of the genetic variations in SHP-2 on neurodevelopment in preterm infants, we further analyzed the degree of myelination according to the functional genetic variation in SHP-2 using tract-based spatial statistics (TBSS) and assessed neurodevelopment using the Bayley Scale of Infant and Toddler Development, third edition (Bayley-III), in preterm infants at 18–22 months of corrected age.
Results
Study population
The characteristics of the study subjects are shown in Supplementary Fig. 1. We enrolled 72 preterm infants and 58 full-term infants for the genetic analysis of SHP-2. Blood samples from all the infants were obtained in the neonatal period during hospitalization or at infancy when they visited the outpatient clinic. Table 1 shows the demographic data of the study subjects. Among the mothers of the 72 preterm infants, 52 mothers were pregnant for the first time. Among the remaining 20 mothers, only one mother had a medical history of previous preterm birth. Among the 72 preterm infants, nine died during hospitalization, and 17 were lost during follow-up. In the nine mortality cases, there were no congenital malformations such as congenital heart disease or brain or other organ malformation, and none of the cases showed abnormal appearance. The remaining 46 preterm infants were re-visited at 18–22 months of corrected age for assessment of neurological development.
Genetic variations in SHP-2 in the preterm and full-term infants
To investigate the effect of SHP-2 variations on susceptibility to preterm birth, we identified genetic variations in SHP-2 in 130 preterm or full-term infants through direct sequencing or genotyping and compared the frequency of each variation between the preterm and full-term infants. Table 2 shows the frequencies of SHP-2 variations in our study subjects. Twelve genetic variations including two variations in the promoter region and one nonsynonymous variation were identified. Among them, four intron variations and one nonsynonymous variation were first identified in this study. Table 3 lists the comparison of the frequency of each variation between the two groups. The frequency of one of the promoter variations, g.-273G > A, was higher in the preterm infants than in the full-term infants (P = 0.025). The frequency of the other variations were similar between the two groups.
Effect of SHP-2 promoter variations on the promoter activity of the gene
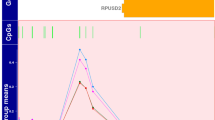
To the best of our knowledge, there has been no report on the functional characterization of genetic variations in the SHP-2 promoter. Therefore, we investigated the effect of SHP-2 promoter variations on the promoter activity of the gene by measuring the luciferase activity of the reporter vectors containing wild-type or mutant SHP-2 promoter. We observed that two variations, g.-317C > T and g.-273G > A, significantly increased the promoter activity of SHP-2 by 36.7% and 34.0%, respectively, compared to that of the wild-type (Fig. 1). We then predicted the potential transcription factors that could bind to the SHP-2 promoter near the two above-mentioned variations using MatInspector (Genomatrix Software GmbH, Munich, Germany) in order to investigate the mechanism underlying the transcriptional regulation of the SHP-2 promoter. Two transcription factors, nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and GA-binding protein alpha (GABPα), were predicted to bind to the two variations, g.-317C > T and g.-273G > A, respectively, and there was a large difference in the binding affinity between the wild-type and variant sequences. To validate our prediction, we conducted electrophoretic mobility shift assays (EMSAs). First, we confirmed the position of DNA-protein complexes consisting of NF-κB consensus oligonucleotides and nuclear extracts through a competition assay and a supershift assay (lanes 1–4, Fig. 2a). Through binding reaction of wild-type (g.-317C) or variant (g.-317T) oligonucleotides with the nuclear protein extracts, we observed that NF-κB could bind to the SHP-2 promoter near g.-317C > T and that this transcription factor bound to the variant more strongly than to the wild-type (lanes 5–8, Fig. 2a). Next, we confirmed the position of DNA-protein complexes consisting of GABPα consensus oligonucleotides and nuclear protein extracts through a competition assay (lanes 1–3, Fig. 2b) and found that GABPα could bind to the SHP-2 promoter near g.-273G > A and that the binding affinity for the SHP-2 promoter was much stronger in the presence of the variant, g.-273A (lanes 4 and 7, Fig. 2b). Figure 2c shows the result of a supershift assay conducted using a GABPα antibody. A supershift in the presence of an antibody confirmed that GABPα was present in the DNA-protein complexes (lanes 2, 4, and 6, Fig. 2c).
Luciferase activities of the vectors containing SHP-2 promoter variations. The luciferase activities were measured 48 h after transfection of the reporter vectors containing the wild-type SHP-2 promoter sequence or its genetic variations into HCT-116 cells. Then, the luciferase activity of each vector was compared with that of the wild-type. The data (mean ± SD) were obtained from triplicate wells. ***P < 0.001 vs. wild-type.
EMSAs for identification of the interaction between SHP-2 promoter variations and transcription factors. (a) 32P-labeled oligonucleotides (lanes 1–3, NF-κB consensus; lanes 4–6, g.-317C wild-type; lanes 7–9, g.-317T variant) were incubated with nuclear protein extracts from HCT-116 cells. A competition assay and a supershift assay were conducted using 100-fold molar excess of unlabeled NF-κB consensus oligonucleotides (lane 2) and antibodies against NF-κB (lanes 3, 4, 7, and 8), respectively. (b) 32P-labeled oligonucleotides (lanes 1–3, GABPα consensus; lanes 4-6, g.-273G wild-type; lanes 7–9, g.-273A variant) were incubated with nuclear protein extracts. A competition assay was performed using 100-fold molar excess of unlabeled GABPα consensus (cons, lanes 2, 5, and 8) or mutant (mut, lanes 3, 6, and 9) oligonucleotides. (c) A supershift assay was performed with the antibody against GABPα (lanes 2, 4, and 6). The arrows indicate DNA-protein complexes.
Effect of a SHP-2 nonsynonymous variation on SHP-2 expression
In general, a nonsynonymous variation, which causes a change in amino acids, could affect the expression or function of a protein. In the current study, one nonsynonymous variation, P559S, was found in a healthy preterm infant. To investigate the effect of this variation on SHP-2 expression, we performed immunoblotting assays and found that SHP-2 expression was increased by 20.7% in the presence of P559S, although the difference was not statistically significant (P = 0.765, Fig. 3).
Effect of a variation, P566S, on SHP-2 expression. SHP-2 expression was investigated using immunoblotting after transfection of wild-type SHP-2 or P566S variation vectors into HCT-116 cells. Data shown represent mean ± SD obtained from three independent experiments and analyzed by Student’s two-tailed t-test. EV; empty vector.
Effect of the functional promoter variation, g.-273G > A, on myelination in preterm infants
To examine the effect of the functional variation in the SHP-2 promoter on myelination in preterm infants, we divided the 46 preterm infants into two groups: a variant group (n = 23) and a control group (n = 23). The variant group consisted of subjects heterozygous or homozygous for g.-273G > A, and the control group consisted of the remaining infants. Because the infant with the other functional SHP-2 promoter variation, g.-317C > T, had passed away during hospitalization, this variation was not considered in our analysis. Tract-based spatial statistics (TBSS) revealed that the fractional anisotropy (FA) value in the corpus callosum, posterior limb of internal capsule (PLIC), and optic radiation was significantly lower in the variant (g.-273A) group than in the control (g.-273G) group (P < 0.05) (Fig. 4). These immature myelinations in the variant group spread around the parietal, frontal, and temporal regions (Supplementary Table S1).
TBSS results. Comparisons of the mean FA maps of preterm infants at 18–22 months of corrected age between the SHP-2 variant and control groups. Mean FA skeleton is shown in green, and areas with significant differences between the two groups are shown in red-yellow (P < 0.05). The FA value in the corpus callosum, posterior limb of internal capsule, and optic radiation was lower in the variant group.
Effect of the functional promoter variation, g.-273G > A, on neurodevelopment in preterm infants
We then examined the effect of the functional variation in the SHP-2 promoter on neurodevelopment in preterm infants. Motor, cognitive, language, and social development were assessed at 18–22 months of corrected age in the 46 preterm infants, and data were analyzed according to the g.-273G > A variation. Grossly, the preterm infants in the variant group showed lower scores than those in the control group (Table 4). In particular, the motor composite score was significantly lower in the preterm infants with the SHP-2 promoter variation (P = 0.027). Table 5 shows the proportion of delayed development in each developmental subscore; in all the subscores, the proportion of delayed development was higher in the variant group. Delayed motor development accounted for approximately 40% in the variant group, which was significantly different compared to that in the control group (P = 0.004).
Discussion
SHP-2 is ubiquitously expressed in mammalian tissues with high levels of expression in hematopoietic cells and in the CNS and has been shown to be essential for organ development and hematopoiesis26. Dysregulation of SHP-2 function or expression has been implicated in the pathogenesis of various human diseases26. In spite of the proven clinical importance of SHP-2, there has been no study examining the association between genetic variations in SHP-2 and preterm births. In the present study, we observed that two genetic variations in the SHP-2 promoter, g.-317C > T and g.-273G > A, resulted in significantly increased promoter activity and that the frequency of g.-273G > A was higher in preterm infants than in full-term infants. In the 1,000 genome project (https://www.ncbi.nlm.nih.gov/variation/tools/1000genomes/), the mean minor allele frequency of g.-273G > A was found to be 0.143. However, the minor allele frequency of this variation was different in different ethnicities; in general, the minor allele frequency was higher in Asians than in other populations such as Africans or Europeans (Supplementary Table S2). In our study population, the minor allele frequency of g.-273G > A was high; in particular, it was higher in the preterm infants (0.292) than in the full-term infants (0.198). In addition, we found that two transcription factors, NF-κB and GABPα, are involved in the transcriptional regulation of SHP-2 by the two above-mentioned variations.
Preterm birth and its associated complications such as brain injury have been known to be caused by multiple pathogenic factors. Maternal or fetal infection and inflammation can induce the release of cytokines such as IL-1, IL-8, IL-10, and TNF, chemokines, and platelet-activating factors27. These responses can induce preterm labor3, 4 and increase ROS production, resulting in the injury of immature oligodendrocytes5. The deficient antioxidant capacity of preterm infants further aggravates the insult caused by ROS. Apoptosis of immature oligodendrocytes by ROS is the main pathology of brain injury in preterm infants5.
While other protein tyrosine phosphatases are widely accepted as negative regulators of signaling events, SHP -2 can act as either a positive or a negative regulator in signaling cascades in a cell type-specific and stimulant-specific manner28. SHP-2 is one of the rare protein tyrosine phosphatases that display dual function in signal transduction, showing both a neuroprotective effect and a proapoptotic effect by promoting caspase activation15, 16, 19. Since previous studies have reported that SHP-2 plays key roles in various cellular processes including proliferation, survival, differentiation, and metabolism26 and because SHP-2 is known to be highly expressed in specific brain regions including the cortex, cerebellum, and hippocampus13, we compared brain diffusion tensor image (DTI) data in the preterm infants according to the SHP-2 promoter variation, g.-273G > A, and found that myelination of the corpus callosum, PLIC, and optic radiation was significantly delayed in the variant group than in the control group. TBSS is an observer-independent tool used for analyzing DTI data29. Using TBSS, brain maturation and tract organization can be detected across all white matter areas in the absence of apparent brain abnormalities in preterm infants30, 31. In general, FA increases with age, which is believed to reflect white matter maturations such as fiber coherence, axonal density, and myelination32. The corpus callosum, PLIC, and optic radiation have been known to be involved in visual motor function33,34,35. Our finding of delayed myelination in these areas and poorer motor function in the same subjects is consistent with the results of the above-mentioned previous studies. Another previous study reported that gain of function (GOF) genetic variations in SHP-2 resulting in increased numbers of oligodendrocyte progenitor cells and MAPK activity negatively influence myelination by inducing abnormal myelination or resulting in fewer myelinated axons in white matter36. This study also supports our results that indicate that GOF variations in SHP-2 can negatively affect myelination.
As mentioned above, SHP-2 is highly expressed in hematopoietic cells and is essential for hematopoiesis26. Therefore, we also investigated whether g.-273G > A can affect hematologic features in the preterm infants. Among the 72 preterm infants, seven had disseminated intravascular coagulation (DIC), which is characterized by systemic activation of pathways leading to and regulating coagulation. DIC may cause organ failure with concomitant consumption of platelets and coagulation factors, which may result in clinical bleeding37. However, we found no statistically significant difference in the occurrence of DIC between the g.-273G > A variant and wild-type groups [5 infants (12.8%) vs. 2 infants (6.1%), P = 0.442]. We also did not find any other abnormal hematological features in our study population. Therefore, we concluded that there was no significant difference in the hematologic features between the preterm infants with g.-273G > A and those without this variation in our study.
Our study has a few limitations. First, the sample size was not large enough to reach sufficient statistical power. However, the strength of our study lies in the fact that we identified genetic variations in our study population and then investigated the effect of each variation on gene function at the molecular level. Moreover, we identified that the functional SHP-2 promoter variation is associated with myelination and neurodevelopment by using advanced MRI and neurodevelopmental assessment tools. The second limitation of our study is that we could not measure SHP-2 expression levels in the infants with functional variations in the SHP-2 promoter. Finally, neurodevelopmental assessment was performed at 18–22 months of corrected age. The evaluation during this period may not be enough to assess cognitive or social development38. Follow-up assessment might provide more information regarding cognitive and social development associated with SHP-2 variations in our population.
In summary, in the present study, we identified functional variations in the promoter region of SHP-2 and found that one GOF variation in the SHP-2 promoter is associated with spontaneous preterm birth itself as well as delayed myelination and poor motor development in preterm infants. To the bets of our knowledge, this is the first study examining the relationship between genetic variations in the SHP-2 promoter and spontaneous preterm birth or brain development. Because SHP-2 is linked with cytokines, ROS production, and apoptosis, which have been known to be the main pathologies of preterm birth and its associated complications such as brain injury, SHP-2 might be an important molecule with regard to preterm births. Further studies with larger sample sizes are necessary to confirm our results.
Methods
Genetic analysis of SHP-2
This study was reviewed and approved by the Institutional Review Board of the Ewha Medical Center, Seoul, Korea. All the experiments were performed in accordance with relevant guidelines and regulations of Institutional Review Board of the Ewha Medical Center. Seventy-two preterm infants (≤35 gestational weeks at birth, preterm group) and 58 healthy full-term infants (≥37 gestational weeks at birth, full-term group) were included in this study. Written informed consent was obtained from the legal representatives of all participants prior to enrollment. To identify the genetic variations in SHP-2, the promoter (up to −2,168 bp from the translation start site) or coding (all exons and exon-intron boundaries) regions of SHP-2 were amplified by PCR using DNA samples obtained from the preterm and full-term infants. The PCR conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55–65 °C for 30 s, initial extension at 72 °C for 30–60 s, and final extension at 72 °C for 10 min. Then, the PCR products were purified using a MultiScreen384-PCR Filter Plate (Milipore, Billerica, MA, USA) and sequenced using a BigDye Terminator Cycle Sequencing Kit and an ABI 3730xl automated sequencer (Applied Biosystems, Foster City, CA, USA). Mutation analyses were performed using Phred, Phrap, Consed, Polyphred 5.04 software (http://droog.mbt.washington.edu/PolyPhred.html).
Construction of vectors containing the SHP-2 promoter or coding regions
To construct a luciferase reporter vector containing the promoter region of the SHP-2 wild-type sequence, a 448-bp region of the SHP-2 promoter was amplified using PCR with the primers listed in Supplementary Table S3 and a genomic DNA sample obtained from an individual with a wild-type sequence. This PCR product was then inserted into the pGL4.11 [luc2P] vector (Promega Corporation, Madison, WI, USA). For a vector containing the wild-type SHP-2 coding region, the pCMV-SHP-2 wild-type vector (Addgene plasmid #8381), a kind gift from Ben Neel, was obtained from Addgene (Addgene, Cambridge, MA, USA). The mutant vectors containing genetic variations in the promoter or coding regions were generated using QuikChange® II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) with primers shown in Supplementary Table S3. The sequences of all the constructs were confirmed by direct sequencing.
Measurement of the promoter activity of wild-type SHP-2 or its variations
Forty-eight hours after the transfection of the reporter vectors into HCT-116 cells using Lipofectamine LTX and Plus reagents (Life Technologies, Carlsbad, CA, USA), the promoter activity of each vector was measured using a Dual-luciferase® reporter assay system and a Glomax 96-well plate luminometer (Promega, Fitchburg, WI, USA) by following the manufacturer’s instructions.
EMSA
EMSAs were conducted as described previously39. First, the binding reaction was performed by incubating 32P-labeled oligonucleotides (1 × 105 counts/min) with 10–20 µg of nuclear protein extracts obtained from HCT-116 cells for 30 min at room temperature. For the competition assay, the unlabeled NF-κB consensus or GABPα consensus or mutant oligonucleotides were added in 100-fold molar excess prior to the binding reaction. The supershift assay was performed using three kinds of antibodies, NF-κB p50 (sc-7178X, Santa Cruz Biotechnology, Santa Cruz, CA, USA), NF-κB p65 (sc-372X, Santa Cruz Biotechnology), and GABPα (sc-22810X, Santa Cruz Biotechnology). Then, each sample was electrophoresed for 90 min at 80 V, and the dried gel was exposed to a CP-BU film (Agfa, Mortsel, Belgium) for 16 h at −80 °C to detect the signal. The intensity of each band was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA). Supplementary Table S3 lists the oligonucleotides used in the EMSAs.
Immunoblotting
Forty-eight hours after the transfection of the wild-type SHP-2 or SHP-2 mutant-containing vectors into HCT-116 cells using Lipofectamine LTX and Plus reagents, immunoblotting was performed using a mouse anti-SHP-2 antibody (BD Biosciences, San Jose, CA, USA) or a goat anti-β-actin antibody (Santa Cruz Biotechnology). The intensity of each band was measured using ImageJ software.
Imaging data acquisition and analysis
DTIs were obtained using 3-tesla MRI (Phillips, Foster City, CA, USA) at 18–22 months of corrected age as described previously31, 40. Before obtaining the DTIs, 3D MPRAGE images and high-resolution T1- and T2-weighted images were obtained. DTI sequence parameters were substituted as follows: b = 0 and 800 s/mm2 and TR/TE = 10,100/76 ms. The scanning times for the DTI sequences were 7 min and 36 s at TEA and 10 min and 18 s. TBSS was performed as described in our previous study31 to compare the degree of myelination between the SHP-2 promoter variant (g.-273A) and control (g.-273G) groups. Briefly, all the FA images were aligned to a target in a common space using an optimized TBSS protocol for neonates41. Voxels with P < 0.05 (corrected for multiple comparisons) were considered significantly different.
Neurodevelopmental assessment
Neurodevelopment was assessed at 18–22 months of corrected age by trained psychologists. Development was assessed using Bayley-III, which provided a motor composite score, a cognitive composite score, a language composite score, and a social composite score. For all the subscores, cut-off points of <85 (1 standard deviation (SD) below normative mean) and <70 (2 SD below normative mean) were used to identify mild to moderate and severe delay, respectively. In the present study, delayed development included mild to moderate and severe delay.
Statistical analysis
Statistical analyses were performed using the SPSS v.23.0 software package (IBM Corporation, Armonk, NY, USA). The data shown in the luciferase assay and immunoblotting represent mean ± SD from more than three separate experiments. P values for comparison of the frequencies of genetic variations between the preterm and full-term infants were calculated using the χ2-test. P values for the luciferase assay and immunoblotting were calculated using one-way analysis of variance, followed by Dunnett’s two-tailed test and Student’s two-tailed t-test, respectively. The comparisons of neurodevelopmental assessment results between the variant and control groups were performed using the χ2-test for categorical variables and Student’s two-tailed t-test for continuous variables. P values < 0.05 were considered to indicate statistical significance.
References
Monangi, N. K., Brockway, H. M., House, M., Zhang, G. & Muglia, L. J. The genetics of preterm birth: Progress and promise. Semin Perinatol 39, 574–583, doi:10.1053/j.semperi.2015.09.005 (2015).
Woodward, L. J. et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed 94, F339–344, doi:10.1136/adc.2008.146282 (2009).
de Andrade Ramos, B. R. & Witkin, S. S. The influence of oxidative stress and autophagy cross regulation on pregnancy outcome. Cell Stress Chaperones 21, 755–762, doi:10.1007/s12192-016-0715-3 (2016).
Varner, M. W. & Esplin, M. S. Current understanding of genetic factors in preterm birth. BJOG 112(Suppl 1), 28–31, doi:10.1111/j.1471-0528.2005.00581.x (2005).
Volpe, J. J. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50, 553–562, doi:10.1203/00006450-200111000-00003 (2001).
Langmia, I. M., Apalasamy, Y. D., Omar, S. Z. & Mohamed, Z. Impact of IL1B gene polymorphisms and interleukin 1B levels on susceptibility to spontaneous preterm birth. Pharmacogenet Genomics 26, 505–509, doi:10.1097/FPC.0000000000000243 (2016).
Huusko, J. M. et al. A study of genes encoding cytokines (IL6, IL10, TNF), cytokine receptors (IL6R, IL6ST), and glucocorticoid receptor (NR3C1) and susceptibility to bronchopulmonary dysplasia. BMC Med Genet 15, 120, doi:10.1186/s12881-014-0120-7 (2014).
Baier, R. J. Genetics of perinatal brain injury in the preterm infant. Front Biosci 11, 1371–1387 (2006).
Frey, H. A. et al. Genetic variation associated with preterm birth in African-American women. Am J Obstet Gynecol 215(235), e231–238, doi:10.1016/j.ajog.2016.03.008 (2016).
Vannemreddy, P., Notarianni, C., Yanamandra, K., Napper, D. & Bocchini, J. Is an endothelial nitric oxide synthase gene mutation a risk factor in the origin of intraventricular hemorrhage? Neurosurg Focus 28, E11, doi:10.3171/2009.10.FOCUS09143 (2010).
Giusti, B. et al. Genetic polymorphisms of antioxidant enzymes as risk factors for oxidative stress-associated complications in preterm infants. Free Radic Res 46, 1130–1139, doi:10.3109/10715762.2012.692787 (2012).
Poggi, C. et al. Genetic Contributions to the Development of Complications in Preterm Newborns. PLoS One 10, e0131741, doi:10.1371/journal.pone.0131741 (2015).
Suzuki, T., Matozaki, T., Mizoguchi, A. & Kasuga, M. Localization and subcellular distribution of SH-PTP2, a protein-tyrosine phosphatase with Src homology-2 domains, in rat brain. Biochem Biophys Res Commun 211, 950–959, doi:10.1006/bbrc.1995.1904 (1995).
Servidei, T. et al. The protein tyrosine phosphatase SHP-2 is expressed in glial and neuronal progenitor cells, postmitotic neurons and reactive astrocytes. Neuroscience 82, 529–543 (1998).
Park, H., Ahn, K. J., Lee Kang, J. & Choi, Y. H. Protein-protein interaction between caveolin-1 and SHP-2 is dependent on the N-SH2 domain of SHP-2. BMB Rep 48, 184–189 (2015).
Morales, L. D. et al. Protein tyrosine phosphatases PTP-1B, SHP-2, and PTEN facilitate Rb/E2F-associated apoptotic signaling. PLoS One 9, e97104, doi:10.1371/journal.pone.0097104 (2014).
Zhang, X. et al. Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB J 26, 2338–2350, doi:10.1096/fj.11-200139 (2012).
Yun, J. H. et al. Caveolin-1 is involved in reactive oxygen species-induced SHP-2 activation in astrocytes. Exp Mol Med 43, 660–668, doi:10.3858/emm.2011.43.12.075 (2011).
Jo, A. et al. SHP-2 binds to caveolin-1 and regulates Src activity via competitive inhibition of CSK in response to H2O2 in astrocytes. PLoS One 9, e91582, doi:10.1371/journal.pone.0091582 (2014).
Loh, M. L. et al. PTPN11 mutations in pediatric patients with acute myeloid leukemia: results from the Children’s Cancer Group. Leukemia 18, 1831–1834, doi:10.1038/sj.leu.2403492 (2004).
Loh, M. L. et al. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 103, 2325–2331, doi:10.1182/blood-2003-09-3287 (2004).
Tartaglia, M. et al. Genetic evidence for lineage-related and differentiation stage-related contribution of somatic PTPN11 mutations to leukemogenesis in childhood acute leukemia. Blood 104, 307–313, doi:10.1182/blood-2003-11-3876 (2004).
Tartaglia, M. et al. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet 34, 148–150, doi:10.1038/ng1156 (2003).
Tartaglia, M. et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet 29, 465–468, doi:10.1038/ng772 (2001).
Lee, Y. S. et al. Mechanism and treatment for learning and memory deficits in mouse models of Noonan syndrome. Nat Neurosci 17, 1736–1743, doi:10.1038/nn.3863 (2014).
Tajan, M., de R Serra, A., Valet, P., Edouard, T. & Yart, A. SHP2 sails from physiology to pathology. Eur J Med Genet 58, 509–525, doi:10.1016/j.ejmg.2015.08.005 (2015).
Manuck, T. A. The genomics of prematurity in an era of more precise clinical phenotyping: A review. Semin Fetal Neonatal Med 21, 89–93, doi:10.1016/j.siny.2016.01.001 (2016).
Dance, M., Montagner, A., Salles, J. P., Yart, A. & Raynal, P. The molecular functions of Shp2 in the Ras/Mitogen-activated protein kinase (ERK1/2) pathway. Cell Signal 20, 453–459, doi:10.1016/j.cellsig.2007.10.002 (2008).
Smith, S. M. et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31, 1487–1505, doi:10.1016/j.neuroimage.2006.02.024 (2006).
Shim, S. Y. et al. Altered microstructure of white matter except the corpus callosum is independent of prematurity. Neonatology 102, 309–315, doi:10.1159/000341867 (2012).
Shim, S. Y. et al. Serial diffusion tensor images during infancy and their relationship to neuromotor outcomes in preterm infants. Neonatology 106, 348–354, doi:10.1159/000363218 (2014).
Dubois, J., Hertz-Pannier, L., Dehaene-Lambertz, G., Cointepas, Y. & Le Bihan, D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 30, 1121–1132, doi:10.1016/j.neuroimage.2005.11.022 (2006).
Thompson, D. K. et al. Corpus callosum alterations in very preterm infants: perinatal correlates and 2 year neurodevelopmental outcomes. Neuroimage 59, 3571–3581, doi:10.1016/j.neuroimage.2011.11.057 (2012).
Bassi, L. et al. Probabilistic diffusion tractography of the optic radiations and visual function in preterm infants at term equivalent age. Brain 131, 573–582, doi:10.1093/brain/awm327 (2008).
Rose, J. et al. Neonatal brain microstructure correlates of neurodevelopment and gait in preterm children 18-22 mo of age: an MRI and DTI study. Pediatr Res 78, 700–708, doi:10.1038/pr.2015.157 (2015).
Ehrman, L. A. et al. The protein tyrosine phosphatase Shp2 is required for the generation of oligodendrocyte progenitor cells and myelination in the mouse telencephalon. J Neurosci 34, 3767–3778, doi:10.1523/JNEUROSCI.3515-13.2014 (2014).
Levi, M., Toh, C. H., Thachil, J. & Watson, H. G. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol 145, 24–33, doi:10.1111/j.1365-2141.2009.07600.x (2009).
Latal, B. Prediction of neurodevelopmental outcome after preterm birth. Pediatr Neurol 40, 413–419, doi:10.1016/j.pediatrneurol.2009.01.008 (2009).
Jang, G. H., Kim, T. H., Choe, Y., Ham, A. & Choi, J. H. Functional characterization of genetic variations in the MDR3 promoter. Biochem Biophys Res Commun 430, 1312–1318, doi:10.1016/j.bbrc.2012.12.041 (2013).
Jeong, H. J. et al. Cerebellar Development in Preterm Infants at Term-Equivalent Age Is Impaired after Low-Grade Intraventricular Hemorrhage. J Pediatr 175(86–92), e82, doi:10.1016/j.jpeds.2016.05.010 (2016).
Ball, G. et al. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage 53, 94–102, doi:10.1016/j.neuroimage.2010.05.055 (2010).
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning [2014R1A1A1004762 and 2010-0027945].
Author information
Authors and Affiliations
Contributions
E.A.P., J.H.C., S.S., Y.C., and S.J.C. designed the study. H.J.J., H.J.P., E.Y.K., B.M.K., and Y.J.C. carried out the experiments and analyzed data. S.S., J.H.C., and E.A.P. wrote the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shim, SY., Jeong, H.J., Park, H.J. et al. Functional variation of SHP-2 promoter is associated with preterm birth and delayed myelination and motor development in preterm infants. Sci Rep 7, 6052 (2017). https://doi.org/10.1038/s41598-017-06401-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-06401-x
This article is cited by
-
Genetic dissection of the BRCA2 promoter and transcriptional impact of DNA variants
Breast Cancer Research and Treatment (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.