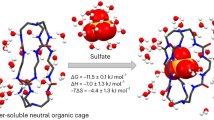
Abstract
The recognition of anions by designed receptors has attracted much attention in recent days. In particular, the selective binding of sulfate with artificial receptors is important because of its relevance to many biological and environmental applications. However, the development of organized molecular receptors with high-efficiency for sulfate binding still remains a significant challenge. We report a novel para-phenylene-bridged hexafunctional tripodal receptor that contains a urea-based inner cleft and a thiourea-based outer cleft, providing perfect sites for step-wise binding of two anions within a single cavity. The new receptor was synthesized in a three-step process, and was investigated for its anion binding properties by 1H NMR titrations, 2D NOESY experiments and computational studies. As indicated by solution binding studies, the receptor selectively binds sulfate over other oxoanions, forming a 1:2 stoichiometric complex that is stabilized via strong H-bonding interactions. High-level DFT calculations reveal that the receptor, owing to the enhanced H-bonding ability of thiourea groups, initially encapsulates one sulfate in its thiourea-based outer cleft, followed by a second encapsulation in its urea-based inner cleft. Such a functionalized receptor with the unique combination of urea-based cleft and thiourea-based cleft in a single receptor has not been reported previously.
Similar content being viewed by others
Introduction
Anion binding and sensing with designed abiotic receptors has become an active area of current research1,2,3,4,5,6. Molecular clefts with organized functional groups in defined geometries have recently gained significant attention in anion coordination chemistry7,8,9,10. Among the various anions, sulfate is particularly important because of its relevance to biological and environmental implications with respect to nuclear waste management11, biosynthesis12, and protein binding13. Thus, there is a growing interest in developing suitable molecular receptors that can strongly and selectively bind sulfate anions14. The use of urea groups appended to the tripodal framework in anion binding is well documented, showing complementary binding with sulfate15,16,17,18,19,20,21,22,23,24,25,26,27,28,29. For instance, tren-based urea receptors substituted with m-cyanophenyl15, p-cyanophenyl16, m-nitrophenyl17, and 3-pyridyl18 were shown to bind sulfate by hydrogen bonding interactions. Although receptors incorporating urea functional groups have been shown as useful binding motifs for anions through NH···O interactions, it has been reported that the incorporation of thiourea groups to synthetic receptors leads to an enhanced acidity of NH25, thereby providing strong affinity for anions30,31,32,33,34,35,36,37,38. Furthermore, recent reports have established the link between the anion binding and certain catalytic reactions, especially for thiourea-based anion receptors39,40,41. For example, Jacobsen et al. have reported a thiourea-based compound that, upon the binding of a fluoride ion, can catalyze the acylation of silyl ketene acetals with acyl fluorides39.
It has been shown that the free energies of association for host-guest interactions are dependent on the number of rotatable bonds formed by the hosts and guests42; therefore, the increased number of binding sites within a single host designed from structural manipulations could lead to the enhancement of its binding ability and selectivity for a specific guest. Recently, Wu et al. have reported tripodal-based hexaurea receptors containing ortho-phenylene-bridged bisurea moieties that encapsulated sulfate through H-bonds, forming 1:1 complexes43, 44. In the pursuit of achieving the higher order of binding sites, we synthesized a pentaflouro-substituted hexaurea receptor that formed an encapsulated complex with a carbonate ion45.
Herein, we report a novel para-phenylene-bridged hexafunctional mixed urea/thiourea receptor L that contains one inner cleft with three urea groups and one outer cleft with three thiourea groups. We hypothesized that such an organization with two operational clefts linking through rotatable spacers in a single molecule could provide perfect sites for hosting two anions, each within one cleft. Because of the enhanced acidity of the thiourea groups, the receptor would possibly show preference to bind the first anion at the outer cleft (instead of inner cleft). This assumption was further supported by the electrostatic potential surfaces of L calculated at the M06-2X/6-31G(d,p) level of theory, showing more positive potential on the outer cleft than the inner cleft (Fig. 1). Such an assembled, exceptional anion receptor with an elite blend of urea-based cleft and thiourea-based cleft in a single tripodal receptor has not been reported previously.
Results and Discussion
As demonstrated by the electrostatic potential map as well as by the optimized structures (discussed later), the receptor adopts into a C 3 symmetric cone shape, owing to the presence of three identical (para-phenylene-bridged) arms linked to the tertiary amine (Fig. 1). A strong electrostatic positive potential is created within both the inner and outer clefts due to the urea and thiourea moieties, potentially making the molecule a ditopic receptor for anions. Through the analysis of 1H NMR binding isotherms and NMR NOESY experiments, we have shown that the receptor binds sulfate selectively over other oxoanions, forming a 1:2 stoichiometric complex. High level DFT calculations further support that the receptor efficiently encapsulates two sulfate ions in its inner cleft and outer cleft.
Synthesis
The new hexafunctional mixed urea/thiourea receptor L was prepared by three-step synthetic strategy (Fig. 1a), with about 40% overall yield. Tris(2-aminoethyl) amine (tren) (1) and p-nitrophenyl isocyanate were reacted to give the nitro-functionalized tris-urea 2 which was reduced with hydrazine hydrate and Pd/C (10%) to produce the amino-functionalized tris-urea 3. The final coupling was achieved by reacting 3 with p-cyanophenyl isothiocyanate to form the p-phenylene bridged hexafunctional mixed urea/thiourea L. The receptor is stable under normal conditions and soluble in DMSO, but hardly soluble in water and other common organic solvents. Attempts to isolate X-ray quality crystals of L with anions were unsuccessful.
NMR studies
The binding properties of L towards various oxoanions (SO4 2−, HSO4 −, H2PO4 −, ClO4 − and NO3 −), which were added as their tetrabutylammonium (TBA) salts, were investigated in DMSO-d 6 by using proton NMR titration techniques at room temperature. The free receptor shows four NH resonances at 6.16 (NH1), 8.58 (NH2), 9.99 (NH3) and 10.02 (NH4) ppm in its NMR spectrum: two for ureas (NH1 and NH2), and the other two for thioureas (NH3 and NH4), indicating the C 3 conformation of L (The NH signals were assigned by NOESY NMR spectroscopy, see below. See Fig. 2c for the numbering of the NH protons). Figure 2a shows the 1H NMR spectra of the free L and its mixture with 5 equivalents of different anions. After the addition of SO4 2− to L, the NH resonances significantly shifted downfield showing ΔNH2 = 0.89, ΔNH3 = 0.27 and ΔNH4 = 0.35, while H1 signal overlapped with one aromatic proton at 7.13 ppm (ΔNH1 = 0.97 ppm), suggesting the interactions of L with sulfate anions. In addition to the shifting of NH signals, the aromatic signals also shifted. In particular the upfield shift of peripheral signals (Hd and Hb) on p-cyanophenyl and p-phenylene groups were observed, which may be due to a shielding effect induced by the encapsulated sulfate inside the outer cleft 33, 43. Notably, the shift difference of NH resonances of urea groups is much larger than that of thiourea groups, suggesting a possible cavity strain due to the encapsulation of sulfate anion into the inner cavity. The addition of HSO4 − to L induced small but considerable changes in the chemical shifts of both urea and thiourea groups, showing ΔNH1 = 0.37, ΔNH2 = 0.28, ΔNH3 = 0.06 and ΔNH4 = 0.09 ppm. The larger shift change (ΔNH) in the respective NH signal due to the addition of SO4 2− than that of HSO4 − indicates stronger interactions of SO4 2− as it contains two charges. The addition of H2PO4 − to L resulted in downfield shifts of NH1 and NH2 signals, while both NH3 and NH4 signals were broadened as observed previously for related ligands43, 44. In contrast, the addition of ClO4 − or NO3 − to L did not show any noticeable change in the shifts of NH or aromatic protons (see the Supporting Information, Figs S12 and S13), thus indicating weaker interactions between the perchlorate or nitrate anions and the receptor.
(a) Partial 1H NMR spectra of L (2 mM) in the presence of 5 equivalents of different anions in DMSO-d 6 ; (b) partial 1HNMR titration of L showing changes in the NH chemical shifts of L (2 mM) with an increasing amount of SO4 2− (20 mM) in DMSO-d 6 . (H1 = CH2NHCO, H2 = CONHAr, H3 = ArNHCS, H4 = CSNHAr); and (c) proposed binding mechanism of L with SO4 2−.
Figure 2b shows the stacking of 1H NMR spectra of L with varying amount of SO4 2− (0 to 10 eq.) in DMSO-d 6 , exhibiting gradual downfield shifts of NH signals. The shift changes for NH resonances (Fig. 3), however, were not consistent with a purely 1:1 binding model as commonly observed for related molecules33. Therefore, they were analyzed with a 1:2 (L:sulfate) binding model using the EQNMR program46, displaying the binding constants (in log K) of 3.06(2) and 2.56(4) for L + SO4 2− = [L(SO4)]2− and [L(SO4)]2− + SO4 2− = [L(SO4)2]4−, respectively. These results clearly indicate the stepwise binding of two sulfates, one with the inner cleft and other with the outer cleft. Gunnlaugsson et al. reported ortho-, meta-, and para-phenylene bridged acyclic urea-amide based receptors, demonstrating that an anion recognition at the first binding moiety may lead to a “positive allosteric effect” for the second functionality toward anions, thus promoting the formation of a 1:2 complex47. A similar effect was recently described by Wu et al. for a ferrocenyl-functionalized hexaurea receptor that contains two urea groups separated by a meta-phenylene group, showing both 1:1 and 1:2 complexes with sulfate anions, as supported by 1H NMR and theoretical calculations44. In an earlier report, we also observed that a para-xylene bridged hexaprotonated azamacrocycle was capable of hosting two chlorides at its two binding moieties via trigonal recognition of two clefts48. In the present work, the receptor L featuring two clefts with different functionalities (urea and thiourea) can readily host two tetrahedral sulfate anions in its two clefts. Owing to an enhanced binding ability as well as the structural complementarity of thiourea functionalities, it is suggested that the first binding occurs at the outer cleft followed by the second binding at the inner cleft. This is further supported by optimizing the geometries and calculating the respective binding energies using high-level density functional theory (discussed later). As shown in Fig. 2b, the larger change in chemical shifts are observed within 0 to 1 equivalents of SO4 2−, implying a 1:1 complex, while the formation of the 1:2 species is dominant after one equivalent of the added anion. The stepwise binding constants of L for SO4 2− have been shown as log K 1 = 3.07(3) and log K 2 = 2.56(4) for the first and second sulfate, respectively. Since the first binding constant is higher than the second binding constant, this binding process can be considered as “non-cooperative”49. The titrations of L with HSO4 − or H2PO4 − also suggest the stepwise formation of both 1:1 and 1:2 complexes, and the calculated binding constants are provided in Table 1. The higher binding constant for the first step as compared to that for the second step for each complex implies that the outer cavity is the preferential binding site for sulfate, presumably the enhanced acidity of thioureas. Further, the overall binding trend in the order of SO4 2− > HSO4 − > H2PO4 − > ClO4 − or NO3 −, suggests that the receptor can selectively bind sulfate over other anions studied.
The solution binding mode of L for sulfate anion was further evaluated by 2D NOESY NMR experiments (Fig. 4), as reported before by us16 and others17, 50. As shown in Fig. 4a, the free receptor of L shows two strong cross peaks for NH1\(\cdots \)NH2 and NH3\(\cdots \)NH4 of urea and thiourea moieties, respectively; indicating that these protons are close in space16. In addition, two strong couplings for NH2\(\cdots \)CHa and NH3\(\cdots \)CHb with aromatic protons were observed. However, the addition of two equivalents of sulfate anions to L resulted in the complete loss of NH1\(\cdots \)NH2 contacts, implying a possible rotation of the two sites of a thiourea unit in order to bind a sulfate anion. On the other hand, the NH3\(\cdots \)NH4 contacts from urea groups were retained (Fig. 4b), suggesting that these protons remain in a close distance after the encapsulation of sulfate. Indeed, as shown in the optimized structure of the sulfate complexes (Fig. 5b,c, discussed later), the NH sites of a single urea are twisted to bind two oxygen atoms of sulfate inside the inner cleft, while this is not the case for thiourea groups, showing the respective sites bonded to a single oxygen atom.
Computational studies
In an effort to understand the interactions and structural aspects of the new receptor with sulfate, theoretical calculations based on density functional theory (DFT) were performed with hybrid meta-exchange correlation functional M06-2X51, using the Gaussian 09 package of programs52. Our previous work has shown that the M06-2X functional accurately predicts the binding energy trends for non-covalent interactions between anions and organic receptors33. To this end, the initial equilibrium geometry for the free receptor L was first optimized at the M06-2X/6-31G(d,p) level of theory53. From this equilibrium geometry, the sulfate anion with different orientations was placed in a single (inner or outer) cleft or both clefts, and molecular geometries of the various sulfate-bound complexes were fully optimized at the M06-2X/6-31G(d,p) level of theory and corrected for zero-point energies (ZPE) in gas phase as well as in a solvent phase to approximate a DMSO environment (dielectric constant = 46.8) using a polarizable continuum model (PCM). With the optimized geometry, the binding energies of L for SO4 2− were calculated using the equation: ΔE = E(complex) − [E(receptor) + E(anion)].
As shown in Fig. 5a, the optimized structure of the receptor adopts a perfect C 3 symmetric cone shape, due to the presence of three identical arms linked to the tertiary amine. The inner cleft of the receptor is decorated with six intra-molecular H-bonds, where each oxygen atom of one urea group is H-bonded with both NH of the adjacent urea unit. Further, all three NH groups of the outer cleft are pointed inside the cavity, making it a preferred binding site for a C 3 symmetric sulfate anion. With this optimized geometry, we first attempted to organize all NH groups of L around a tetrahedral sulfate; however, due to the lack of complementarity, the receptor could not be optimized with a single anion bonded to both clefts simultaneously. Therefore, we proceeded to optimize with one sulfate added separately at each cleft or two sulfates at both clefts of L. The optimized structure of the thiourea-bound sulfate complex as shown in Fig. 5b, reveals that the sulfate binds to the outer cleft through a total of six NH···O bonds (NH···O = 2.78–2.93 Å). The calculated binding energy of this complex was found to be −203 kcal/mol in gas phase, while it was much lower (−96 kcal/mol) in solvent phase, due to the polarity effect of DMSO solvent included in the calculations33.
In contrast to the thiourea-bound sulfate complex, the receptor significantly deformed in the urea-bound sulfate complex (Fig. 5c) to encapsulate a sulfate anion within its inner cleft, yielding the binding energies as −151 and −77 kcal/mol in gas and solvent phase, respectively. The calculated binding energies for thiourea-bound complex (ΔE = −203 kcal/mol) and for urea-bound complex (ΔE = −151 kcal/mol) are comparable to our previous report on sulfate binding with a tris-thiourea (ΔE = −200 kcal/mol) and a tris-urea (ΔE = −173 kcal/mol) in gas phase33. The higher binding energy for the thiourea-bound complex (Fig. 5b) than that for the urea-bound complex (Fig. 5c) demonstrates that the outer cleft is the preferential binding site for the first sulfate, which is in agreement with the experimental results. As mentioned previously, these results further support our assumption that the binding of the first sulfate at the outer cleft (Fig. 5b) may allow the second sulfate to bind at the inner cleft.
Considering that the first binding occurs at the outer cleft (thiourea groups), followed by the second binding at the inner cleft (urea groups), as proposed by NMR titration studies, we proceeded to re-optimize the receptor with two sulfate ions by incorporating both clefts, each with a single sulfate. The calculated binding energies were found to be −161 and −87 kcal/mol in gas and solvent phase, respectively. The optimized structure, as displayed in Fig. 6, reveals that both the inner cleft and the outer cleft are occupied by sulfate anions that are bound through strong H-bonding interactions (NH···O < 2.94 Å), thereby overcoming the expected electrostatic repulsion due to the encapsulation of two anions in a single molecule. It is noteworthy that the receptor, in a 1:1 complex (urea- or thiourea-bound sulfate), readjusted its geometry to implement maximum interactions for sulfate that is bonded through six NH···O bonds (see bond distances in Table 2). While the thiourea-bound complex adopted a perfect C 3 symmetry, leaving the urea-cleft open for a second sulfate (see Fig. 5b); the urea-bound complex deviated from its C 3 conformation, adopting a folded umbrella that could not allow to bind another sulfate due to the nonexistence of the outer cavity (Fig. 5c). On the other hand, the receptor is stabilized with two sulfates, each with six NH···O bonds from six NH binding sites from a single cleft (inner or outer), creating a perfect C 3 symmetric 1:2 complex.
Conclusion
We have designed and synthesized a novel para-phenylene-bridged hexafunctional tripodal receptor consisting of two different functionalized clefts (urea-based inner cleft and thiourea-based outer cleft). As demonstrated by experimental studies and theoretical calculations, the receptor can effectively bind sulfate anions in a two-step binding process, leading to a well-defined 1:2 stoichiometric complex that is stabilized through complementary H-bonding interactions. Our results suggest that the unique combination of two different functionalities makes the receptor ideal to bind the first sulfate at the thiourea-based outer cleft and the second sulfate at the urea-based inner cleft. The preferred binding at the outer cleft is due to the enhanced H-bonding ability as well as of the structural complementarity of thiourea functionalities, leading to stronger interactions with the anion than those with its urea analogue. This binding propagation was further supported by DFT calculations, illustrating that the thiourea-bound complex is energetically more favorable than the urea-bound complex. Therefore, we conclude that the binding of one sulfate at the outer cleft assists the receptor to bind the second sulfate at the inner cleft. To the best of our knowledge, such an assembled multifunctional anion receptor with the unique combination of a urea-based cleft and a thiourea-based cleft has not been reported previously. Understanding and being able to accurately predict the interactions between synthetic receptors and guests is a key step towards elucidating the complex mechanisms in living systems. Taken together, the results from our study may be useful in developing highly organized molecular receptors for extraction, catalysis and drug design for environmental and biomedical applications.
Methods
General
All reagents and solvents were purchased as reagent grade and were used without further purification. Nuclear magnetic resonance (NMR) spectra were recorded on a Varian Unity INOVA 500 FT-NMR. Chemical shifts for samples were measured in DMSO-d 6 and calibrated against sodium salt of 3-(trimethylsilyl) propionic-2,2,3,3-d 4 acid (TSP) as an external reference in a sealed capillary tube. NMR data were processed and analyzed with MestReNova Version 6.1.1-6384. The melting point was determined on a Mel-Temp (Electrothermal 120 VAC 50/60 Hz) melting point apparatus and was uncorrected. Elemental analysis was carried out by ECS 4010 Analytical Platform (Costech Instrument) elemental analyzer at Jackson State University.
Tris-(4-nitrophenyl)-urea (2)
Tris(2-aminoethyl)amine 1 (1.04 mL, 6.95 mmol) was mixed with three equivalents of p-nitrophenyl isocyanate (3.47 g, 21.14 mmol) in CH2Cl2 under reflux for 24 hours. A yellow precipitate was formed when the reaction mixture was cooled down. The precipitate was collected by filtration and washed with dichloromethane and diethyl ether solvent. The compound was dried over vacuum to give the analytically pure 2 as a yellow solid. Yield: 3.8 g (86%); 1H NMR (500 MHz, DMSO-d 6 ): δ 9.35 (s, 3H, Ar-NH), 8.07 (d, J = 8.6 Hz, 6H, ArH), 7.57 (d, J = 8.5 Hz, 6H, ArH), 6.43 (s, 3H, CH2NH), 3.22 (br, 6H, NHCH 2), 2.62 (br, 6H, NCH 2); 13C NMR (125 MHz, DMSO-d 6 ): δ 179.7, 145.9, 141.5, 124.35, 120.2, 51.2, 41.47.
Tris-(4-aminophenyl)-urea (3)
To a suspension of 2 in ethanol (1.0 L) containing 10% Pd/C (1.0 g) as a catalyst, hydrazine monohydrate (12.0 mL) was added drop-wise at room temperature. After refluxing for 4 hrs, the reaction mixture was filtrated through celite to remove Pd/C. The filtrate thus collected was evaporated to dryness. The white solid was washed with diethyl ether several times and dried over vacuum to 3. Yield: 2.9 g (89%); 1H NMR (500 MHz, DMSO-d 6 ): δ 8.03 (s, 3H, Ar-NH), 6.97 (d, J = 7.9 Hz, 6H, ArH), 6.44 (d, J = 8.0 Hz, 6H, ArH), 6.03 (s, 3H, CH2NH), 4.66 (s, 6H, Ar-NH), 3.11 (br, 6H, NHCH 2), 2.50 (br, 6H, NCH 2); 13C NMR (125 MHz, DMSO-d 6 ): δ 155.9, 143.4, 129.5, 120.4, 114.1, 54.1, 37.5.
Tripodal 4-amino phenyl tris-(4-cyanophenyl)-hexafunctional urea/thiourea ligand (L)
The compound 3 (2.5 g, 4.55 mmol) was added to three equivalent p-cyanophenyl isothiocyanate (2.2 g, 13.73 mmol) in methanol and the mixture was refluxed for overnight at 100–130 °C. A white precipitate was formed when the reaction mixture was cooled down. The precipitate was collected by filtration and washed with methanol and diethyl ether. The compound was dried over vacuum to give the hexafunctional receptor L as a white solid. Yield: 2.5 g (53%); mp: 245 °C; 1H NMR (500 MHz, DMSO-d 6 ): δ 10.02 (s, 3H, ArNHCS), 9.99 (s, 3H, ArNHCS), 8.58 (s, 3H, CONHAr), 7.75 (s, 12H, ArH), 7.36 (d, J = 8.7 Hz, 6H, ArH), 7.26 (d, 8.6 Hz, 6H, ArH), 6.16 (s, 3H, CH2NHCO), 3.19 (d, J = 4.8 Hz, 6H, NHCH 2), 2.60 (s, 6H, NCH 2); 13C NMR (125 MHz, DMSO-d 6 ): δ 179.4 (C=S), 155.3 (C=O), 144.3 (ArC), 137.9 (ArC), 132.7 (ArC), 132.1 (ArC), 124.9 (ArC), 122.4 (ArC), 119.2 (ArC), 117.9 (ArCN), 105.2 (ArC), 54.0 (NHCH2); ESI-MS (ESI+, CH3OH), m/z calcd for [M + H]+ 1029.32, found 1029.14; analysis (calcd., found for C51H48N16O3S3): C, 59.52, 59.29), H (4.70, H, 4.64), N (21.77, 21.69).
1H NMR Binding Studies
Binding constants were obtained by 1H NMR (Varian Unity INOVA 500 FT-NMR) titrations of L with the oxoanions (NO3 —, ClO4 —, H2PO4 —, HSO4 —, SO4 2—) at neutral pH. Initial concentrations were [ligand]0 = 2 mM, and [anion]0 = 20 mM. Each titration was performed by 13 measurements at room temperature. The association constant K was calculated by fitting of several independent NMR signals using a 1:2 (L:anion) binding model using the EQNMR program. Error limit in K was less than 15%.
Computational studies
Interaction energies and geometry optimization of sulfate complexes were performed with density functional theory (DFT) calculations51. All calculations were carried out using Gaussian 09 package of programs52.
Data Availability
All data generated or analysed during this study are included in this published article and its Supplementary Information files.
References
Wenzel, M., Hiscock, J. R. & Gale, P. A. Anion receptor chemistry: highlights from 2010. Chem. Soc. Rev. 41, 480–520 (2012).
Amendola, V., Fabbrizzi, L. & Mosca, L. Anion recognition by hydrogen bonding: urea-based receptors. Chem. Soc. Rev. 39, 3889–3915 (2010).
Carroll, C. N., Naleway, J. J., Haley, M. M. & Johnson, D. W. Arylethynyl receptors for neutral molecules and anions: emerging applications in cellular imaging. Chem. Soc. Rev. 39, 3875–3888 (2010).
Hargrove, A. E., Nieto, S., Zhang, T. Z., Sessler, J. L. & Anslyn, E. V. Artificial receptors for the recognition of phosphorylated molecules. Chem. Rev. 111, 6603–6782 (2011).
Hossain, M. A. Inclusion complexes of halide anions with macrocyclic receptors. Curr. Org. Chem. 12, 1231–1256 (2008).
Haque, S. A. et al. Experimental and Theoretical Aspects of Anion Complexes with a Thiophene-Based Cryptand. Comm. Inorg. Chem. 36, 305–326 (2016).
Ghosh, K., Masanta, G. & Chattopadhyay, A. P. Triphenylamine-Based Pyridine N-Oxide and Pyridinium Salts for Size-Selective Recognition of Dicarboxylates. Eur. J. Org. Chem. 26, 4515–4524 (2009).
Liu, W. X. & Jiang, Y. B. Intramolecular Hydrogen Bonding and Anion Binding of N-Benzamido-N’-benzoylthioureas. J. Org. Chem. 73, 1124–1127 (2008).
Duke, R. M., O’Brien, J. E., McCabe, T. & Gunnlaugsson, T. Colorimetric sensing of anions in aqueous solution using a charge neutral, cleft-like, amidothiourea receptor: tilting the balance between hydrogen bonding and deprotonation in anion recognition. Org. Biomol. Chem. 6, 4089–4092 (2008).
Basaran, I. et al. Synthesis and anion binding properties of a urea-based molecular cleft. Tetrahedron Lett. 56, 657–661 (2015).
Fowler, C. J. et al. Enhanced anion exchange for selective sulfate extraction: overcoming the Hofmeister bias. J. Am. Chem. Soc. 130, 14386–14387 (2008).
Young, R. W. The role of the Golgi complex in sulfate metabolism. J. Cell Biol. 57, 175–189 (1973).
Raboudi, N., Julian, J., Rohde, L. H. & Carson, D. D. Identification of Cell-surface Heparinmeparan Sulfate-binding Proteins of a Human Uterine Epithelial Cell Line (RL95). J. Biol. Chem. 267, 11930–11939 (1992).
Moyer, B. A. et al. A case for molecular recognition in nuclear separations: sulfate separation from nuclear wastes. Inorg Chem. 52, 3473–90 (2013).
Custelcean, R., Moyer, B. A. & Hay, B. P. A coordinatively saturated sulfate encapsulated in a metal-organic framework functionalized with urea hydrogen-bonding groups. Chem. Commun. 48, 5971–5973 (2005).
Pramanik, A. et al. Seven-coordinate anion complex with a tren-based urea: Binding discrepancy of hydrogen sulfate in solid and solution states. Org. Biomol. Chem. 9, 4444–4447 (2011).
Dey, S. K., Chutia, V. & Das, G. Oxyanion-encapsulated caged supramolecular frameworks of a tris (urea) receptor: evidence of hydroxide-and fluoride-ion-induced fixation of atmospheric CO2 as a trapped CO3 2– anion. Inorg. Chem. 51, 1727–1738 (2012).
Wu, B. et al. Sulfate ion encapsulation in caged supramolecular structures assembled by second-sphere coordination. Chem. Commun. 1762–1764 (2008).
Ravikumar, I., Lakshminarayanan, P. S., Arunachalam, M., Suresh, E. & Ghosh, P. Anion complexation of a pentafluorophenyl-substituted tripodal urea receptor in solution and the solid state: selectivity toward phosphate. Dalton Trans. 4160 –4168 (2009).
Custelcean, R., Remy, P., Bonnesen, P. V., Jiang, D. & Moyer, B. A. Sulfate Recognition by Persistent Crystalline Capsules With Rigidified Hydrogen-Bonding Cavities. Angew. Chem. Int. Ed. 47, 1866–1870 (2008).
Li, Y. et al. Sulfate anion templated synthesis of a triply interlocked capsule. Chem. Commun. 7134–7136 (2009).
dos Santos, C. M. G., Boyle, E. M., De Solis, S., Kruger, P. E. & Gunnlaugsson, T. Selective and tuneable recognition of anions using C 3v-symmetrical tripodal urea-amidereceptor platforms. Chem. Commun. 47, 12176–12178 (2011).
Jose, D. A., Kumar, D. K., Ganguly, B. & Das, A. Rugby-ball-shaped sulfate–water–sulfate adduct encapsulated in a neutral molecular receptor capsule. Inorg. Chem. 46, 5817–5819 (2007).
Akhuli, B., Ravikumar, I. & Ghosh, P. Acid/base controlled size modulation of capsular phosphates,hydroxide encapsulation, quantitative and clean extraction of sulfate with carbonate capsules of a tripodal urea receptor. Chem. Sci. 3, 1522–1530 (2012).
Bordwell, F. G. Equilibrium acidities in dimethyl sulfoxide solution. Acc. Chem. Res. 21, 456–463 (1988).
Moore, S. J. et al. Chloride, carboxylate and carbonate transport by ortho-phenylenediamine-based bisureas. Chem. Sci. 4, 103–117 (2013).
Haynes, C. J. E. et al. Tunable transmembrane chloride transport by bis-indolylureas. Chem. Sci. 3, 1436–1444 (2012).
Edwards, P. R., Hiscock, J. R., Gale, P. A. & Light, M. E. Carbamate complexation by urea-based receptors: studies in solution and the solid state. Org. Biomol. Chem. 8, 100–106 (2010).
Caltagirone, C., Bates, G. W., Gale, P. A. & Light, M. E. Anion binding vs. sulfonamide deprotonation in functionalised ureas. Chem. Commun. 61–63 (2008).
Busschaert, N. et al. Tripodal transmembrane transporters for bicarbonate. Chem. Commun. 46, 6252–6254 (2010).
Busschaert, N. et al. Structure–Activity Relationships in Tripodal Transmembrane Anion Transporters: The Effect of Fluorination. J. Am. Chem. Soc. 133, 14136–14148 (2011).
Basaran, I., Emami Khansari, M., Pramanik, A., Wong, B. M. & Hossain, M. A. An exclusive fluoride receptor: fluoride-induced proton transfer to a quinoline-based, thiourea. Tetrahedron Lett. 55, 1467–1470 (2014).
Khansari, M. E. et al. Synthesis and anion binding studies of tris(3-aminopropyl)amine-based tripodal urea and thiourea receptors: proton transfer-induced selectivity for hydrogen sulfate over sulfate. RSC Adv 5, 17606–17614 (2015).
Young, P. G. & Jolliffe, K. A. Selective recognition of sulfate ions by tripodal cyclic peptides functionalised with (thio)ureabinding sites. Org. Biomol. Chem. 10, 2664–2672 (2012).
Dey, S. K. & Das, G. Selective inclusion of PO4 3− within persistent dimeric capsules of a tris(thiourea) receptor and evidence of cation/solvent sealed unimolecular capsules. Dalton Trans. 41, 8960–8972 (2012).
Bose, P., Dutta, R., Santra, S., Chowdhury, B. & Ghosh, P. Combined Solution-Phase, Solid-Phase and Phase-Interface Anion Binding and Extraction Studies by a Simple Tripodal Thiourea Receptor. Eur. J. Inorg. Chem. 2012, 5791–5801 (2012).
Marques, I. et al. Tris–thiourea tripodal-based molecules as chloride trans membrane transporters: insights from molecular dynamics simulations. Soft Matter. 10, 3608–3621 (2014).
Horvat, D. et al. A synthetic thiourea-based tripodal receptor that impairs the function of human first trimester cytotrophoblast cells. Int. J. Environ. Res. Public Health 11, 7456–7469 (2014).
Birrell, J. A., Desrosiers, J.-N. & Jacobsen, E. N. Enantioselective acylation of silyl ketene acetals through fluoride anion-binding catalysis. J. Am. Chem. Soc. 133, 13872–13872 (2011).
Lin, S. & Jacobsen, E. N. Thiourea-catalysed ring opening of episulfonium ions with indole derivatives by means of stabilising, non-covalent interactions. Nat. Chem. 4, 817–824 (2012).
Burns, N. Z., Witten, M. R. & Jacobsen, E. N. Dual catalysis in enantioselective oxidopyrylium-based [5 + 2] cycloadditions. J. Am. Chem. Soc. 133, 14578–14581 (2011).
Hossain, M. A. & Schneider, H.-J. Flexibilty, association constants and salt effect in organic ion pairs: how single bonds affect molecular recognition. Chem. Eur. J. 5, 1284–1290 (1999).
Jia, C. et al. Highly efficient extraction of sulfate ions with a tripodal hexaurea receptor. Angew. Chem. Int. Ed. 50, 486–490 (2011).
Huang, X. et al. Stepwise Encapsulation of Sulfate Ions by Ferrocenyl-Functionalized Tripodal Hexaurea Receptors. Chem. Eur. J. 19, 9034–9041 (2013).
Pramanik, A., Emami Khansari, M., Powell, D. R., Fronczek, F. R. & Hossain, M. A. Absorption of atmospheric CO2 as carbonate inside the molecular cavity of a new tripodal hexaurea receptor. Org. Lett. 16, 366–369 (2014).
Hynes, M. J. EQNMR: a computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. Dalton Trans. 311–312 (1993).
dos Santos, C. M. G., McCabe, T., Watson, G. W., Kruger, P. E. & Gunnlaugsson, T. The Recognition and Sensing of Anions through “Positive Allosteric Effects” Using Simple Urea–Amide Receptors. J. Org. Chem. 73, 9235–9244 (2008).
Hossain, M. A. et al. Charge-assisted encapsulation of two chlorides by a hexaprotonated azamacrocycle. Crystal Growth & Design 10, 1478–1481 (2010).
Hunter, C. A. & Anderson, H. L. What is cooperativity? Angew Chem Int Ed Engl. 48, 7488–7499 (2009).
Werner, F. & Schneider, H. J. Complexation of anions including nucleotide anions by open-chain host compounds with amide, urea, and aryl functions. Helv. Chim. Acta 83, 465–478 (2000).
Zhao, Y. & Truhlar, D. G. Applications and validations of the Minnesota density functionals. Chem. Phys. Lett. 502, 1–13 (2011).
Frisch, M. J. et al. Gaussian 09, version D.01, Gaussian, Inc., Wallingford, CT (2009).
Cossi, M., Barone, V., Cammi, V. & Tomasi, J. Ab initio study of solvated molecules: a new implementation of the polarizable continuum model. Chem. Phys. Lett. 255, 327–335 (1996).
Acknowledgements
The National Science Foundation is acknowledged for a CAREER award (CHE-1056927) to M.A.H. Authors are thankful to the National Science Foundation (NSF/CREST HRD-1547754, for financial support and want to acknowledge the Extreme Science and Engineering Discovery Environment (XSEDE) by National Science Foundation grant number OCI-1053575 and XSEDE award allocation number DMR110088. Authors are also thankful to the Mississippi Center for Supercomputing Research (MCSR) for providing state-of-the-art high performance computing facilities for supporting this research. NMR core facility at Jackson State University was supported by the National Institutes of Health (G12MD007581).
Author information
Authors and Affiliations
Contributions
M.K. performed the synthesis and NMR studies. A.P. and C.R.J. undertook the fitting of the binding constants. A.M. and J.L. performed the computational studies. M.A.H. designed the experiments and wrote the manuscript. All authors revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Emami Khansari, M., Mirchi, A., Pramanik, A. et al. Remarkable hexafunctional anion receptor with operational urea-based inner cleft and thiourea-based outer cleft: Novel design with high-efficiency for sulfate binding. Sci Rep 7, 6032 (2017). https://doi.org/10.1038/s41598-017-05831-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05831-x
This article is cited by
-
Development of fluorescence sensors for quantifying anions based on polyhedral oligomeric silsesquioxane that contains flexible side chains with urea structures
Polymer Journal (2024)
-
Highly selective and sensitive macrocycle-based dinuclear foldamer for fluorometric and colorimetric sensing of citrate in water
Scientific Reports (2018)
-
Structural factors affecting 13C NMR chemical shifts of cellulose: a computational study
Cellulose (2018)
-
Recent advances in anion recognition
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.