Abstract
(E)-Methyl-N′-nitrobenzylidene-benzenesulfonohydrazide (MNBBSH) compounds were synthesized using a condensation procedure from the derivatives of nitrobenzaldehyde and 4-Methyl-benzenesulfonylhydrazine, which crystallized in ethanol and methanol as well as characterized by FTIR, UV-Vis, 1H-NMR, and 13C-NMR. MNBBSH structure was confirmed using a single crystal X-ray diffraction technique and used for the detection of selective yttrium ion (Y3+) by I-V system. A thin layer of MNBBSH was deposited onto a glassy carbon electrode (GCE) with 5% nafion for the sensitive and selective Y3+ sensor. The modified MNBBSH/GCE sensor is exhibited the better electrochemical performances such as sensitivity, limit of detection (LOD), linear dynamic range (LDR), limit of quantification (LOQ), short response time, and long term storage ability towards the selective metal ion (Y3+). The calibration curve of 2-MNBBSH/GCE sensor was plotted at +1.1 V over a broad range of Y3+ concentration. Sensitivity, LOD, LDR and LOQ of the fabricated sensor towards Y3+ were calculated from the calibration curve and found as 1.90 pAμM−1 cm−2, 10.0 pM, 1.0 nM~1.0 mM and 338.33 mM respectively. The 2-MNBBSH/Nafion/GCE sensor was applied to the selective determination of Y3+ in spiked samples such as industrial effluent and real water samples from different sources, and found acceptable and reasonable results.
Similar content being viewed by others
Introduction
Organic molecules having electron donor functional group is an immense attention in chemistry due to enormous number of applications in biological, catalysis, environmental, medicinal, and organometallic area by binding of diverse metal constituents1,2,3,4. Sulfonamides are acknowledged as sulfa drugs, and used as antibiotics in bacterial category infections in animals, and human beings. Sulfa drugs are also applied in the treatment of bacillary dysentery, conjunctivitis, eye & gut infections, malaria, meningitis, and urinary tract infections5, 6. Yttrium tungstate [Y2(WO4)3, orthorhombic structure] shows large negative thermal expansion beside all the three crystallographic axes7. Y-90 is a chaste β particle emitting radionuclide which is appropriate for the application in nuclear medicine such as radiolabeling of pharmaceuticals for bone palliation, cancer treatment, radiation synovectomy, rheumatoid arthritis, and tumor therapy. Several techniques for example extraction chromatography, ion exchange, precipitation, and solvent extraction used to purify and separate of Y-908, 9.
Sensors based on alkyl oxymercuration, DNA, oligonucleotide, protein, and little organic compounds have been developed and experienced for heavy metal ions (HMI). However, these techniques are inconvenience for Y3+ exposure in biological and environmental samples due to numerous limitations such as high functioning temperatures, lower-water solubility, nitrogen-purge buffer, time consuming, and too expensive. Hence it is necessary to build up an easy, sensitive, and dependable method for the detection of toxic metallic ions for environmental security, food quality control, protection, and human being health arena10. Electrochemical sensing of poisonous molecules represents a proficient advancement that can be used to harmonize earlier available techniques owing to combine features such as simple instrumentation, little cost, extremely selective and sensitive, and considerable for effectiveness in order to detect HMI11, 12. In this research work, an efficient sensor has been developed in order to detect the trivalent metal ion (Y3+) using (E)-Methyl-N′-nitrobenzylidene-benzenesulfonohydrazide derivatives. To best of our knowledge, it is the first report for selective and sensitive identification and determination of Y3+ using 2-MNBBSH by a dependable I-V performance with short response time.
Experimental
Material and methods
The chemicals of analytical ranking for example 2-Nitrobenzaldehyde, 3-Nitrobenzaldehyde, 4-Nitrobenzaldehyde, 4-Methyl-benzenesulfonylhydrazine, AgNO3, AuCl3, CdSO4, Co(NO3)2, Cr(NO3)3, HgCl2, NiCl2, Y(NO3)3, ZnSO4, EtOH, MeOH, NaH2PO4, Na2HPO4, and nafion (5% ethanolic solution) were purchased from the Sigma Aldrich company, and used as received. A mother solution of Y3+ (100.0 mM) was prepared from the purchased chemical, Y(NO3)3. A Stuart scientific SMP3 (version 5.0) melting point apparatus (Bibby Scientific Limited, Staffordshire, UK) was used to record the melting point (m.p.), and the reported m.p. was not corrected. 1H-NMR, and 13C-NMR spectra were recorded on an AVANCE-III instrument (400 MHz, Bruker, Fallanden, Switzerland) at 300 k, and chemical shifts were reported in ppm with reference with residual solvent signal. FTIR spectra were recorded as neat on a Thermo scientific NICOLET iS50 FTIR spectrometer (Madison, WI, USA). UV-V is study was conducted using Evolution 300 UV-Vis spectrophotometer (Thermo scientific). I-V method was carried out in order to detect Y3+ at a selective point using the modified 2-MNBBSH/GCE by Keithley electrometer (6517A, USA). Warning! Yttrium is poisonous. So, a minute amount of this chemical can be used for the preparation of the essential solution with care.
Synthesis of MNBBSH derivatives
(E)-4-Methyl-N′-(2-nitrobenzylidene)-benzenesulfonohydrazide (2-MNBBSH, 3)
A mixture of 2-nitrobenzaldehyde (514.3 mg, 3.38 mmol, 1.22 equiv), and 4-methyl-benzenesulfonylhydrazine (513.6 mg, 2.76 mmol, 1 equiv) in EtOH (30.0 mL) was stirred at room temperature (R.T.) for 3.30 h. Filtered, and the solution was then kept at open air to evaporate the solvent. The obtained product was crystallized from EtOH to give the title compound 3 as a yellow crystal (757.0 mg, 86%). EF = C14H13N3O4S, MW = 319.04, EA = C: 52.66, H: 4.10, N: 13.16, O: 20.04, S: 10.04. m. p. = 160.5–165.2 °C. 1 H-NMR (400 MHz, DMSO-d 6), δ: 11.91 (s, 1H), 8.31 (s, 1H), 8.03 (dd, J = 8.2, 1.2 Hz, 1 H), 7.84 (dd, J = 7.9, 1.5 Hz, 1H), 7.81–7.73 (m, 3H), 7.64 (ddd, J = 8.7, 7.4, 1.5 Hz, 1H), 7.44 (d, J = 8.0 Hz, 2H), 2.38 (s, 3H). 13 C-NMR (101 MHz, DMSO-d 6), δ: 189.82, 147.82, 143.64, 142.27, 136.06, 133.69, 130.66, 129.81, 129.75, 127.95, 127.81, 127.14, 124.59, 20.98. FTIR (neat) v max: 3200, 1900, 1805, 1710, 1605, 1470, 1335, 1195, 1085, 915, 845, 800, 725, 685, 535. UV-Vis (λ max): 287.5 nm.
(E)-4-Methyl-N′-(3-nitrobenzylidene)-benzenesulfonohydrazide (3-MNBBSH, 4)
A mixture of 3-nitrobenzaldehyde (513.3 mg, 3.37 mmol, 1.21 equiv), and 4-methyl-benzenesulfonylhydrazine (517.6 mg, 2.78 mmol, 1 equiv) in EtOH (30.0 mL) was stirred at R.T. for 2.30 h. Filtered, and subsequently the solution was kept at open air to evaporate the solvent. The found product was crystallized from EtOH to give the title compound 4 as a yellowish crystal (1.05 g, 97%). EF = C14H13N3O4S, MW = 319.34, EA = C: 52.66, H: 4.10, N: 13.16, O: 20.04, S: 10.04. m. p.0 = 166.2–179.8 °C. 1 H-NMR (400 MHz, DMSO-d 6), δ: 11.80 (s, 1H), 8.37 (t, J = 2.0 Hz, 1H), 8.23 (ddt, J = 8.2, 2.2, 1.0 Hz, 1H), 8.09–7.98 (m, 2H), 7.83–7.76 (m, 2H), 7.70 (t, J = 7.9 Hz, 1H), 7.43 (d, J = 8.0 Hz, 2H), 2.38 (s, 3H). 13 C-NMR (101 MHz, DMSO-d 6), δ: 148.14, 144.55, 143.63, 136.02, 135.44, 132.63, 130.39, 129.73, 127.15, 124.27, 121.00, 20.97. FTIR (neat) v max: 3200, 1905, 1807, 1705, 1610, 1485, 1330, 1265, 1193, 1090, 910, 855, 805, 720, 695, 540. UV-Vis. (λ max): 294.0 nm.
(E)-4-Methyl-N′-(4-nitrobenzylidene)-benzenesulfonohydrazide (4-MNBBSH, 5)
A combination of 4-nitrobenzaldehyde (512.8 mg, 3.37 mmol, 1.23 equiv), and 4-methyl-benzenesulfonylhydrazine (512.0 mg, 2.75 mmol, 1 equiv) in EtOH (25.0 mL) was stirred at R.T. for 3.0 h. Filtered, and MeOH was added with the precipitates. The obtained products were crystallized from MeOH to give the title molecule 5 as a white crystal (752.4 mg, 86%). EF = C14H13N3O4S, MW = 319.06 g/mol, EA = C: 52.66, H: 4.10, N: 13.16, O: 20.04, S: 10.04. m. p.0 = 166.3–169.2 °C. 1 H-NMR (400 MHz, DMSO-d 6), δ: 11.89 (s, 1H), 8.28–8.21 (m, 2H), 8.03 (s, 1H), 7.88–7.76 (m, 4H), 7.43 (d, J = 8.1 Hz, 2H), 2.38 (s, 3H). 13 C-NMR (101 MHz, DMSO-d 6), δ: 147.84, 144.32, 143.69, 139.80, 135.97, 129.75, 127.66, 127.15, 124.24, 124.00, 20.98. FTIR (neat) v max: 3200, 1910, 1803, 1700, 1615, 1490, 1325, 1255, 1197, 1075, 905, 820, 802, 715, 665, 545. UV-Vis. (λ max): 326.0 nm.
Crystallography investigation of MNBBSH molecules
Three novel benzenesulfonylhydrazides (3–5) were synthesized and crystallized from EtOH, EtOH, and MeOH respectively at R.T. under slow evaporation method. Extremely good-looking, grains like crystals were found in vials. Samples were screened out under microscope for good crystals to mount on instrument for data collection. The assembly used to mount samples consists of a glass fiber inserted in the wax fixed onto the hollow copper tube supported by magnetic base. The specific samples were glued over glass needle and mounted on agilent super nova (Dual source) diffractometer, equipped with microfocus Cu-Mo Kα radiation. The data collection was accomplished using CrysAlisPro software at 296 K under the Cu Kα radiation13. The structures were performed and refined by full–matrix least–squares methods on F 2 by means of SHELXL–97 in-built with WinGX14. The non-hydrogen atoms were also refined anisotropically using the same procedure. The crystal structure and hydrogen bonding of single crystals were generated through PLATON and ORTEP in built with WinGX15,16,17. All the C aromatic -H hydrogen atoms were positioned geometrically and treated as riding atoms with C–H = 0.93 Å and Uiso (H) = 1.2 Ueq (C) carbon atoms. The N-H hydrogen atoms were located through Fourier map and refined with N-H = 0.82 (3) Å with Uiso (H) was set to 1.2 Ueq for N atom. The Crystal data were deposited at the Cambridge crystallographic data centre and following deposition numbers have been assigned 1523454, 1523455 and 1523456 which are known as CCDC number for molecules 3–5 respectively. Crystal data can be received free of charge on application to CCDC 12 Union Road, Cambridge CB21 EZ, UK. (Fax: (+44) 1223 336–033; e-mail: data_request@ccdc.cam. ac.uk).
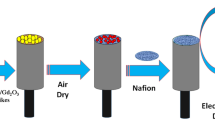
Modification of GCE with MNBBSH molecules
NaH2PO4 (93.5, 68.5, 39.0, 16.0, and 5.3 mL), Na2HPO4 (6.5, 31.5, 61.0, 84.0, and 94.7 mL), and distilled water (500.0 mL) were used for the preparation of a set of phosphate buffer, PB (pH = 5.7, 6.5, 7.0, 7.5, and 8.0). EtOH and nafion (coating binder) was used to modify GCE with MNBBSH compounds. The fabricated electrodes were then kept at R.T. for 3 h in order to development of uniform film with complete drying. The modified GCE and platinum (Pt) wire were used as a working, and counter electrode successively to examine the I-V responses (Fig. 1).
Results and Discussion
Spectroscopic studies of MNBBSH molecules
The title compounds (3–5) were prepared via a simple condensation method of 2-nitrobenzaldehyde, 3-nitrobenzaldehyde, and 4-nitrobenzaldehyde (1), and 4-methyl-benzenesulfonylhydrazine (2) with good yield (Fig. 2)18. Similar molecules already been reported in the earlier studies19,20,21,22,23,24,25,26.
The synthesized molecules 3–5 were characterized using diverse spectroscopic methods, and as a final point the structures were established by means of single crystal X-ray diffraction examination. The purity of compounds gave good spectra which helped us to identify the available proton in the molecules using chemical shift (δ), and coupling constant (J) values. One NH proton of the target molecules 3–5 shows singlet at δ 11.91, 11.80, and 11.89 respectively. Both of phenyl groups of the synthesized molecules (3–5) have eight protons that appeared in different signals. Aromatic protons found magnificently at their low field region at δ 7.44–8.31, 7.43–8.37, and 7.43–8.28 correspondingly for 3–5. One singlet observed at δ 2.38 shows three proton and this may be due to CH3. 13C-NMR spectra were also recorded and most of the carbon atoms was found in the aromatic region (Figs S1–6). FTIR spectroscopy has been examined at 4000–400 cm−1 for the structure by defining functional groups via different bending, and stretching peaks observed at a particular region in the spectra. UV-Vis spectra was recorded in DMSO quantitatively (200–800 nm), and the λmax were found at 287.5, 294.0, and 326.0 nm which is due to the π-π* transition of the imine functional group (C=NH) in the title molecules 3–5 (Fig. S7–9).
Crystallography studies of MNBBSH molecules
As we know the structural arrangements of molecules in crystal structure predicts their various properties so we crystallized the synthesized compounds and diffracted them on a single crystal diffractometer. Three (3–5) molecules were crystallized in different crystal system. The molecule 3 was crystallized in triclinic crystal system with P-1 space group, 4 adopted the orthorhombic crystal system with Pna2 space group and 5 accepted the monoclinic system with P21/a space group (Table 1). The compound 5 have two independent molecules in a single asymmetric unit cell. The molecules adopted the V shape having lower corner at S atom in each set. The S atom in the typical sulfonamide functional group adopted distorted tetrahedral geometry with <O1-S1-O2 = 120.43 (1)°, <O1-S1-O2 = 120.14 (2)°, <O1-S1-O2 = 120.08 (1)° and <O1-S1-O2 = 120.13 (1)° for molecules 3–5 respectively27,28,29,30. The dihedral angles between the two aromatic rings (C1-C6) and (C8-C13) are 86.41 (1)°, 73.12 (2)° in molecule 3, and 4. As compound 5 have two independent molecules so the dihedral angles between two aromatic rings are 72.85(2)° and 74.26(1)° in molecules 5a and 5b respectively. The nitro groups (NO2) are not planar with the aromatic rings to which these are attached and make dihedral angles of 28.86 (2)°, 16.32 (8)°, 6.01 (2)° and 1.93 (1)° for 3, 4, 5a, and 5b correspondingly (Fig. 3, and Table S1). The molecules also undergo the inter-molecular hydrogen bonding interactions to get them stabilize in their crystal structures. The molecules in crystal structure of 3 followed the classical and non-classical N-H…O and C-H…O interactions which connects them to build a two dimensional network along the plane bc. N-H…O interaction from the hydrazide and nitro group connect the molecules to form a sixteen member ring motif R 2 2(16) [31], while C-H…O interaction generated another eighteen member ring motif. Both of these rings connect to each other to form a polymeric chain along b axes. One hydrogen atom from methyl group connects these chains along c axes via C-H…O interaction. In compound 4, nitro group involved in non-classical hydrogen bonding interactions where O3 atoms from two different molecules connects the two different hydrogen atoms of same molecule and form long chain which runs across the a axes. There is another classical interaction through the hydrazide and SO2 group which connects the molecules along c axes, and we observed chains of two dimensional networks in molecule 5 which produced through the N-H…O and C-H…O interactions (Fig. 4, Table 2, and Table S2).
Application
Detection of trivalent Y3+ by 2-MNBBSH using I-V technique
Enhancement of the modified electrode with organic molecule is the beginning stage of application as a metal ion (MI) sensor. The significant use of MNBBSH fabricated onto the GCE was examined as a MI sensor for the detection and quantity of the desired cation, Y3+ in PB. The MNBBSH/GCE sensor comprised diverse compensation for example chemically inert, easy to modify, non-toxic, simple to assemble, steady in air and secure. Depending on the I-V technique theory, the current responses of 2-MNBBSH/GCE considerably changed during the adsorption of Y3+ by electrochemical approach. The probable mechanism towards 2-MNBBSH-Y complex formation is presented in Fig. 5. A similar interaction in perspective of yttrium complex formation has been reported in the previous studies32.
The significant application of MNBBSH assembled onto an electrode as a MI sensor used for the detection of target cation that are less useful in biological and environmental arena. At first pH of the different PB was optimized in perspective of 2-MNBBSH to find out which PB system was more suitable to detect the desired MI, and pH = 6.5 showed more response compared with others towards the modified electrode (Fig. 6a). After that, derivatives of MNBBSH were optimized in PB (pH = 6.5) and 2-MNBBSH appeared additional responses (Fig. 6b). The current signals for the bare GCE, GCE with nafion and coated with 2-MNBBSH on the working electrode were presented in Fig. 6c. The differences of the current responses among bare, with nafion and coated GCE occurred due to current signals had been increased by coated in compared with bare and GCE with nafion.
The metal ions such as Ag2+, Au3+, Cd2+, Co2+, Cr3+, Hg2+, Ni2+, Y3+, and Zn2+ were examined in order to find out the maximum current responses towards MNBBSH modified electrode, and consequently it was clearly observed that the sensor was more selective to Y3+ compared with other ions (Fig. 7a). The selectivity was optimized in perspective of MNBBSH derivatives and 2-MNBBSH appeared foremost response towards Y3+ (Fig. 7b). Figure 7c is the bar diagram presentation of selectivity optimization at +0.9 V. The current signal without metal ion (black-dotted) and with cation (green, brown, and blue dotted) were also performed (Fig. 7d). An increase of current responses observed regarding the modified 2-MNBBSH electrode with Y3+ which has given a large surface area with improved exposure in absorption, and adsorption potentiality onto the porous 2-MNBBSH surfaces of the target ion.
The I-V responses of the Y3+ with diverse concentration (100.0 pM~100.0 mM) towards 2-MNBBSH modified electrode were investigated with indication of the changes of current of the fabricated electrode was a function of Y3+ concentration under normal condition, and it was reported that the current responses increased gradually from lower to higher concentration of the marked MI [SD = 3.49, RSD = 25.91% at +1.5 V, and n = 10] (Fig. 8a). A good range of the Y3+ concentrations were conducted from the lower to higher potential (0.0 ~ +1.5 V) in order to find out the feasible analytical limit. The linear calibration curve was plotted at +1.1 V from a range of Y3+ concentration (CR = 100.0 pM~100.0 mM, SD = 2.03, and n = 10), and found linear (R 2 = 0.8480) (Fig. 8b). Sensitivity (1.90 pAμM−1 cm−2), LOD (10.0 pM), and LOQ (33.33.mM) of the proposed modified electrode, 2-MNBBSH/GCE towards Y3+ were calculated from the calibration curve using the equation (i–iii), where m = slope of the calibration line, A = Active surface area of GCE, and SD = standard deviation of the calibration point. LDR (1.0 nM ~ 1.0 mM) was also calculated from the calibration graph and found linear, R 2 = 0.8832 (Fig. 8c). Response time (r. t.) of Y3+ towards 2-MNBBSH/GCE was measured at 1.0 μM and found 10.0 s (Fig. 8d).
Sensor stability examination
The sensing ability of the 2-MNBBSH coated electrode was examined up to few weeks in order to the study of the reproducible (RP), and storage capabilities. Hence, a series of six successive magnitude of Y3+ solution (1.0 μM) was examined, and yielded outstanding reproducible responses with the 2-MNBBSH electrode at different environment (RP = 85%, SD = 0.90, and n = 6). It was recognized that the I-V responses were not comprehensively changes after washing of each experiment of the modified 2-MNBBSH electrode (Fig. 9a). The sensitivity remained similar around the original response up to two weeks, and after that the responses of the fabricated electrode become declined gradually. The responses of 2-MNBBSH sensor were considered with respect to storage time for the purpose of long term storage aptitude. The gratitude of storage ability of the 2-MNBBSH sensor was evaluated under regular array and the repeatability (RA) at calibrated potential (+1.1 V) was found 78% towards Y3+ for numerous days, SD = 0.84, and n = 6 (Fig. 9b, and Table S3). It was clearly reported that the modified sensor can be used without any considerable failure of sensitivity up to few weeks. A comparison of different MI detection using I-V method is presented in Table 3.
Interference effect examination
Examination of interference effect is one of the considerable practices in analytical science having the capability in order to make different the interfering agents from the MI bearing similar cationic nature. As3+, Au3+, Cr3+, and Sb3+ were usually used as interfering MI in the electrochemical Y3+ detection approach. I-V responses at 2-MNBBSH/GCE sensor toward the addition of Y3+ (1.0 μM), and interfering MI such as As3+, Au3+, Cr3+, and Sb3+ (1.0 and 10.0 μM, ~25.0 μL) in PB (pH = 6.5, and 10.0 mL) were investigated. The effects of interfering cations towards Y3+ were calculated from the experiential current (μA) at calibrated potential (+1.1 V), where the interfering effect of Y3+ was considered to be 100% (Fig. 10a–d, and Table S4). It was noticeable that 2-MNBBSH/GCE did not show any major current responses towards interfering MI. As a result, the expected sensor is proper for the finding of Y3+ with admirable sensitivity.
Prospective analysis of real samples
The real samples (RS) such as industrial effluent (IE), red sea water (RSW), surface water (SW), and tap water (TW) were examined in order to endorse the expected I-V scheme using 2-MNBBSH/GCE. A usual addition method was used to measure the concentration of Y3+ in true samples. A fixed amount (~25.0 µL) of each RS was analyzed in PB (10.0 mL, 100.0 mM) using the fabricated 2-MNBBSH/GCE. The results were included at calibrated potential (+1.1 V) regarding the determination of Y3+ in IE, RSW, SW, and TW which truly confirmed the projected I-V procedure is suitable, dependable, and appropriate for analyzing of RS (Fig. 11a–b, and Table 4).
Conclusion
Three novel MNBBSH compounds were synthesized, characterized, and potentially applied in order to detect the toxic trivalent metallic cation using I-V performance. Systematic performances of trivalent Y3+ sensor using 2-MNBBSH/GCE were examined by a reliable I-V technique in terms of sensitivity, LOD, LOQ, LDR, response time, and reproducibility. This sensor advancement was confirmed to the higher selectivity and fast detection of Y3+ using 2-MNBBSH modified GCE with nafion matrix. An innovative development regarding MI detection can be introduced from this novel approach for monitoring of toxic cations in biological and environmental field.
References
Mosa, A. I., Emara, A. A., Yousef, J. M. & Saddiq, A. A. Novel transition metal complexes of 4-hydroxy-coumarin-3-thiocarbohydrazone: Pharmacodynamic of Co(III) on rats and antimicrobial activity. Spectrochim Acat Part A: Molecul. Biomolecul. Spectroscopy 81, 35–43 (2011).
Fukuzumi, S. New development of photoinduced electron-transfer catalytic system. Pure Appl. Chem. 79, 981–991 (2007).
Zhang, X. B., Wang, Z., Xing, H., Xiang, Y. & Lu, Y. Catalytic and molecular beacons for amplified detection of metal ions and organic molecules with high sensitivity. Anal. Chem. 82, 5005–5011 (2010).
Roztocki, K., Matoga, D. & Szklarzewicz, J. Copper(II) complexes with acetone picolinoyl hydrazones: Crystallographic insight into metalloligand formation. Inorg. Chem. Commun. 57, 22–25 (2015).
Sartorelli, A. C. et al. Antineoplastic and biochemical properties of arylsulfonylhydrazones of 2-formylpyridine N-oxide. J. Med. Chem. 19, 830–833 (1976).
Ohtsuka, E. et al. Studies on tRNA and related compounds XXXVII.(1) Synthesis and physical properties of 2′-or 3′-O-(o-nitrobenzyl)nucleosides: the use of nitrophenyldiazomethane as a synthetic reagent. Chem. Pharm. Bull. 29, 318–324 (1981).
Karmakar, S., Deb, S. K., Tyagi, A. K. & Sharma, S. M. Pressure-induced amorphization in Y2(WO4)3: in situ X-ray diffraction and Raman studies. J. Solid State Chem. 177, 4087–4092 (2004).
Pichestapong, P., Sriwiang, W. & Injarean, U. Separation of Yttrium-90 from Strontium-90 by extraction chromatography using combined Sr resin and RE resin. Energy Procedia 89, 366–372 (2016).
Vyas, C. K., Joshirao, P. M. & Manchanda, V. K. Perchloric acid: A promising medium for the chromatographic separation of 90Y from 90Sr. Separat. Purificat. Technol. 124, 179–185 (2014).
Oriero, D. A., Gyan, I. O., Bolshaw, B. W., Cheng, I. F. & Aston, D. E. Electrospun biocatalutic hybrid silica-PVA-tyrosinase fiber mats for electrochemical detection of phenols. Microchem. J. 118, 166–175 (2015).
Kimmel, D. W., LeBlanc, G., Meschievitz, M. E. & Cliffel, D. E. Electrochemical Sensors and Biosensors. Anal. Chem. 84, 685–707 (2012).
Ronkainen, N. J., Halsall, H. B. & Heineman, W. R. Electrochemical biosensors. Chem. Soc. Rev. 39, 1747–1763 (2010).
A Crys. Alis. PRO Agilent Technologies. Yarnton, England (2012).
Sheldrick, G. M. A short history of SHELX. Acta Cryst. A64, 112–122 (2008).
Spek, A. L. PLATON-a Multipurpose Crystallographic Tool Utrecht University, Utrecht (2005).
Farrugia, L. J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Cryst. 32, 837–838 (1999).
Farrugia, L. J. WinGX and ORTEP for windows: an update. J. Appl. Cryst. 45, 849–854 (2012).
Hussain, M. M., Rahman, M. M., Arshad, M. N. & Asiri, A. M. Hg2+ sensor development based on (E)-N′-Nitrobenzylidine benzene sulfonohydrazide (NBBSH) derivatives fabricated on a glassy carbon electrode with a nafion matrix. ACS Omega 2, 420–431 (2017).
Zhao, J.-L. et al. Iron(III) phthalocyanine-chloride-catalyzed synthesis of sulfones from sulfonylhydrazones. Tetrahedron Lett. 57, 2375–2378 (2016).
Wang., T. et al. An efficient [3+2] cycloaddition for the synthesis of substituted pyrazolo[1,5-C]quinazolines. Tetrahedron 71, 4473–4477 (2015).
Xhang, X. et al. cycloaddition of N-iminoquiazoline ylides with allenoates for synthesis of tetrahydropyrazoloquinazoline derivatives. Tetrahedron 72, 8274–8281 (2016).
Xia, A.-J. et al. Metal free ring-expansion reaction of six-membered sulfonylimines with diazomethanes: An approach toward seven-membered enesulfonamides. Angew. Chem. Int. Ed. 55, 1441–1444 (2015).
Yadav, A. K., Srivastava, V. P. & Yadav, L. D. S. An easy access to fluoroalkanes by deoxygenative hydrofluorination of carbonyl compounds via their tosylhydraznes. Chem. Commun. 49, 2154–2156 (2013).
Inturi, S. B., Kalita, B. & Ahamed, A. J. I2-TBHP-catalyzed one-pot highly efficient synthesis of 4,3-fused 1,2,4-triazoles from N-tosylhydrazones and aromatic N-heterocycles via intermolecular formal 1,3-dipolar cycloaddition. Org. Biomol. Chem. 14, 11061–11064 (2016).
Ye, F. et al. C(sp)–C(sp3) Bond Formation through Cu-Catalyzed Cross-Coupling of N-Tosylhydrazones and Trialkylsilylethynes. J. Am. Chem. Soc. 134, 5742–5745 (2012).
Li, P., Wu, C., Zhao, J., Rogness, D. C. & Shi, F. Synthesis of Substituted 1H-Indazoles from Arynes and Hydrazones. J. Org. Chem. 77, 3149–3158 (2012).
Arshad, M. N. et al. 4-Hydroxy-2h-1,2-Benzothiazine-3-Carbohydrazide 1,1-Dioxide-Oxalohydrazide (1:1): X-Ray Structure and DFT Calculations. J. Struct. Chem. 54, 437–442 (2013).
Arshad, M. N. et al. Crystallographic Studies of Dehydration Phenomenon In Methyl 3-Hydroxy-2-Methyl-1,1,4-Trioxo-1,2,3,4-Tetrahydro-1λ6-Benzo[E][1,2]Thiazine-3-carboxylate. J. Chem. Cryst. 43, 671–676 (2013).
Arshad, M. N. et al. Synthesis, crystal structure and spectroscopic properties of 1, 2-Benzothiazine derivatives: An experimental and DFT study. Chinese J. Struct. Chem. 34, 15–25 (2015).
Shafiq, M. et al. Synthesis and Antioxidant Activity of a New Series of 2,1-Benzothiazine 2,2-Dioxide Hydrazine Derivatives. Asian J. Chem. 24, 4799–4803 (2012).
Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem. Int. Ed. Engl. 34, 1555–1573 (1995).
Liu, W., Wang, H., Zhao, H., Li, B. & Chen, S. Y(OTf)3-Catalyzed Cascade Propargylic Substitution/Aza-Meyer–Schuster Rearrangement: Stereoselective Synthesis of α,β-Unsaturated Hydrazones and Their Conversion into Pyrazoles. Synlett 26, 2170–2174 (2015).
Arshad, M. N. et al. Fabrication of cadmium ionic sensor based on (E)-4-Methyl-N′-(1-pyridin-2-yl)ethylene)benzenesulfunohydrazide (MPEBSH) by electrochemical approach. J. Organometal. Chem 827, 49–55 (2017).
Sheikh, T. A., Arshad, M. N., Rahman, M. M., Asiri, A. M. & Alamry, K. A. Development of highly efficient Co2+ ions sensor based on N,N′-(ethane-1,2-diyl)bis(2,5-dimethoxybenzenesulfonamide) (EMDMBS) fabricated glassy carbon electrode. J. Organometal. Chem 822, 53–61 (2016).
Rahman, M. M., Alamry, K. A., Saleh, T. S. & Asiri, A. M. Sensitive and selective Cu2+ sensor based on 4-(3-(thiophen-2-yl)-9H-carbazol-9-yl)benzaldehyde (TPCBZ) conjugated copper-complex. J. Organometal. Chem 817, 43–49 (2016).
Arshad, M. N., Rahman, M. M., Asiri, A. M., Sobahi, T. R. & Yu, S.-H. Development of Hg2+ sensor based on N′-[1-(pyridine-2-yl)ethylidene]benzenesulfonohydrazide (PEBSH) fabricated silver electrode for environmental remediation. RSC Adv 5, 81275–81281 (2015).
Acknowledgements
Center of Excellence for Advanced Materials Research (CEAMR), Chemistry Department, King Abdulaziz University, Jeddah, Saudi Arabia is highly acknowledged for instrumental support.
Author information
Authors and Affiliations
Contributions
M.M.H. designed, performed the experiment and wrote the manuscript. M.M.R. and M.N.A. also designed the experiment and revised the manuscript. A.M.A. also revised the manuscript and provided the technical supports. Finally, all the authors revised and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussain, M.M., Rahman, M.M., Arshad, M.N. et al. Trivalent Y3+ ionic sensor development based on (E)-Methyl-N′-nitrobenzylidene-benzenesulfonohydrazide (MNBBSH) derivatives modified with nafion matrix. Sci Rep 7, 5832 (2017). https://doi.org/10.1038/s41598-017-05703-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05703-4
This article is cited by
-
The Novel Fluorescent Probe Toward Yttrium(III) and its Bioimaging
Journal of Fluorescence (2023)
-
Functionalized polyethersulfone as PES-NH2-metal oxide nanofilers for the detection of Y3+
Polymer Bulletin (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.