Abstract
Nanoporous anatase TiO2 films were fabricated by a screen-printing method, and CuxO quantum dots (QDs) were deposited on the TiO2 films through successive ionic layer adsorption and reaction (SILAR). The amount of CuxO QDs on the TiO2 films are controlled by changing the number of SILAR cycles. The morphology, microstructure, optical, and photoelectrochemical properties of different CuxO sensitized TiO2 films (CuxO/TiO2) were investigated in detail. The nanoporous TiO2 film offers a large surface area for anchoring QDs. QD deposited samples exhibited a significant improvement in photoelectrochemical performance than the bare of TiO2. CuxO/TiO2, prepared with 7 SILAR cycles, showed the best photoelectrochemical properties, where the photocurrent density was enhanced to 500.01 μA/cm2 compared with 168.88 μA/cm2 of bare TiO2 under visible light. These results indicate that the designed CuxO/TiO2 structure possesses superior charge separation efficiency and photoelectrochemical properties.
Similar content being viewed by others
Introduction
Titanium dioxide (TiO2) has attracted great interest for water splitting1, 2, quantum dot-sensitized solar cells3, 4, optical sensors5, 6, photocatalytic degradation7 and other applications8,9,10, due to its unique optical and photoelectric properties. Nevertheless, the intrinsic band gap of TiO2 (3.0 eV for rutile and 3.2 eV for anatase) limits its photoelectrochemical utility11. Specifically, the wide band gap restricts the photoresponse of TiO2 to only ultraviolet region, with the wavelengths below 380 nm, which constitutes less than 5% of the solar spectrum. Therefore, many studies have been conducted to extend the TiO2 optical absorption range to the visible light region, in order to expand the portion of the solar spectrum for which TiO2 can be utilized.
In recent years, quantum dots (QDs) have become increasingly attractive for similar applications as TiO2, because of their unique electronic and optical properties12, 13, such as their high extinction coefficients14, 15, tunable band gap16, 17, multiple exciton generation18, 19, and an expandable optical absorption range by controlling particle size20, 21. Various QDs, including CdS22, CdSe23, PbS24, PbSe25, and so on26, 27, have been thoroughly studied. Although these studies have demonstrated that QDs-sensitized TiO2 can efficiently absorb visible light, inherent disadvantages of these QDs, such as a fixed visible light absorption range, the toxicity of elements, and the use of rare elements, limit their applications in biology, environment, medicine, and solar cells28. As a result, it is important to discover environmentally friendly QD materials. Recently, the development of copper oxides as both the core photocatalytic material and the photoelectrochemical material has drawn increasing amounts of attention29,30,31. Copper materials, which are multifunctional p-type semiconductors, with a band gap ranging between 1.5 and 2.4 eV32, are environmentally friendly and can absorb in the visible light region33, 34. Until now, to the best of our knowledge, previous studies have not reported the surface sensitization of a nanocrystalline TiO2 electrode with CuxO QDs.
Unlike many syntheses of QDs, such as electrode deposition35, photocatalytic reduction36, and sol-gel method37, our study uses a novel technique to fabricate CuxO QDs via successive ionic layer adsorption and reaction (SILAR) method38. SILAR is a simple fabrication methodology that combines successive layer adsorption with chemical redox reaction. The typical process involves successively immersing the TiO2 materials in \({\text{Cu}(\text{NH}}_{3}{)}_{4}^{2+}\) and H2O2 aqueous solutions successively for as many cycles as desired to achieve a uniform deposition of CuxO QDs. Not only is the overall process environmentally friendly, cost-effective, and can be carried out in normal atmospheric pressure and room temperature, but the density of QDs can also be easily controlled by merely varying the number of deposition cycles.
In this study, we report the preparation of nanoporous anatase TiO2 films on transparent conductive fluorine-doped tin oxide (FTO) substrates by screen-printing and the subsequent deposition of CuxO QDs on the TiO2 films via SILAR. The number of deposition cycles, and thus QD loading density, was varied to investigate the effect on the photoelectrochemical properties of nanocrystalline TiO2 electrode and derive the optimal density of CuxO QDs that enhanced the photoelectrochemical activity. The sample was also recycled 7 times to demonstrate its improved absorbance in the visible range and enhanced photoelectrochemical properties.
Experimental
Preparation of TiO2 films
All the chemical reagents were used as received. The colloidal were prepared by hydrolysis of titanium tetraisopropoxide as described by elsewhere39. The TiO2 films were synthesized directly on transparent fluorine-doped tin oxide (FTO, TEC-8, LOF) conducting glass substrates by screen-printing, followed by calcining the samples at 500 °C for 30 min in air.
Immobilization of CuxO quantum dots onto TiO2 films
The CuxO QDs were deposited onto the TiO2 photoanodes in situ by successive SILAR cycles. For CuxO deposition, the TiO2 photoanodes were successively immersed in two different solutions for 5 min in each solution, first, in \({\text{Cu}(\text{NH}}_{3}{)}_{4}^{2+}\) and second in H2O2 aqueous solutions. Following each immersion, the TiO2 photoanodes were rinsed with deionized water. The \({\text{Cu}(\text{NH}}_{3}{)}_{4}^{2+}\) were resulting from Cu(AC)2 and NH3 \(\bullet \)H2O. These sequential steps are considered as one SILAR cycle. The SILAR cycle was repeated for 0, 3, 5, 7, and 9 times. Samples are denoted as S0, S3, S5, S7, and S9, respectively. The relevant reactions for preparing CuxO QDs can be written as follows40:
Characterization techniques
Microstructures and the crystallinity of CuxO sensitized TiO2 films were characterized by field-emission scanning electron microscopy (FE-SEM, Hitachi, S4800), transmission electron microscopy (TEM), high resolution transmission electron microscopy (HR-TEM) (TEM, JME-2100, Japan), and X-ray diffraction (XRD, MAC, M18XHF) using CuKα radiation (1.54 Å). Absorption spectra were obtained by a UV-Vis spectrophotometer (UV-2550, Shimadzu). Photoluminescence (PL) and Raman spectra were recorded by a micro-Raman spectroscopy system (Renishaw PLC in Via-Reflex). X-ray photoelectron spectroscopy (XPS, Thermo, ESCALAB 250) was employed to analyze the surface composition of the samples. The elemental distributions and concentrations were analyzed by Energynergy dispersive spectroscopy (EDS, Oxford Inca) accompanying the FE-SEM.
Photoelectrochemical measurements
Photoelectrochemical measurements were carried out in a three-electrode configuration with the as-prepared sample as the working electrode, Pt foil as the counter electrode, and saturated Ag/AgCl as the reference electrode41. A 0.1 M Na2SO3 aqueous solution was used as the electrolyte. Photocurrent measurements were taken as a function of voltage by an electrochemistry workstation (CHI 660D, Shanghai Chenhua instrument). The working electrode was illuminated by a 300 W Xe lamp. An ultraviolet cutoff filter was inserted in between the light source and the quartz cell to exclude UV light with wavelength below 420 nm. Photoresponses of the different samples were determined by using a light on-off cycle of 60 s at a bias of 0 V versus the Ag/AgCl electrode.
Results and Discussion
XRD patterns
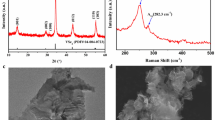
Figure 1 shows XRD patterns of S0 and S9. The XRD pattern of the substrate (FTO) was used as a reference. After the TiO2 products were formed on the FTO substrates, all FTO diffraction peaks became weaker. Both S0 and S9 samples have similar patterns, in that they display three peaks at 2θ = 36.02°, 62.56°, and 69.01°. These peaks can be attributed to the (101), (002), and (301) diffraction peaks of anatase TiO2, respectively, as they are in good agreement with the standard pattern of anatase TiO2 (PDF#65-1119). In addition, the diffraction peaks of CuxO QDs are not observed in S9, implying the low content and small size of QDs. Therefore, we can conclude that the formation of CuxO QDs does not influence the crystalline structure of the TiO2 electrodes.
Morphological analysis
Figure 2 presents SEM images of TiO2 films coated with CuxO QDs. The magnification and high magnification SEM images (Fig. 2a and b) show that the TiO2 film is porous and uniform. Figure 2c–f shows SEM images of the TiO2 after depositing 3, 5, 7, and 9 cycles of CuxO QDs. No morphological changes were observed among the samples deposited with CuxO QDs, due to the low content and small size of the CuxO QDs; however, EDS data of S7 (Fig. 2(g)) reveal that the sample consists of Ti, O, and Cu elements, confirming the presence of CuxO QDs.
TEM and SAED characterization was employed to examine the crystal structure and growth direction of TiO2 as well as the particle size of the CuxO QDs. Figure 3(a) shows that CuxO QDs were in contact with the TiO2 film. The size of TiO2 nanoparticles and CuxO QDs were approximately 34 nm and 10 nm, respectively. When the imaging was focused around the TiO2 and TiO2/CuxO interface, various crystalline facets were clearly observed, as shown in Fig. 3b and c. The larger crystalline region in Fig. 3b was confirmed to be TiO2. The observed lattice plane spacings are 0.17 nm and 0.34 nm corresponding to the (211) and (110) planes of anatase TiO2. Above the anatase TiO2 crystallites, we also observed the interplanar spacings of 0.21 and 0.23 nm (Fig. 3c), which could be indexed to elementary Cu (111) (d = 0.21 nm), CuO (111) (d = 0.23 nm), or Cu2O (002) (d = 0.21 nm). In addition, the SAED pattern in Fig. 3d demonstrates that the TiO2 electrodes exhibit a single crystal structure.
XPS analysis
In order to clarify the elemental composition and valence state of CuxO QDs, XPS characterizations was conducted. As shown in Fig. 4a, the general survey spectrum for CuxO QDs modified TiO2 electrodes contains Cu, O, Ti, and C elements. The small amount of carbon could have resulted from adventitious hydrocarbons from the XPS instrument itself and can be taken as the standard signal for the correction of other peaks42. From the Ti 2p spectrum (Fig. 4b), two main peaks at bonding energies of 458.6 and 461.4 eV were assigned to Ti 2p3/2 and Ti 2p1/2, respectively43, 44. Figure 4c shows a representative Cu 2p core level XPS spectrum with two peaks at 933.2 and 953.0 eV for atmospheric conditions at room temperature, and the oxidation products include Cu2O and CuO45, 46. Furthermore, two fitted peaks (Fig. 4d) from the O 1 s spectrum are observed around 529.7 and 531.9 eV, which can be assigned to the lattice oxygen and surface hydroxyl oxygen of TiO2 47, respectively.
UV-Visible absorption spectra
Figure 5 shows the absorption spectra of TiO2 electrodes sensitized with different SILAR cycles of CuxO QDs. The average absorbance can be calculated and the results are listed in Table 1. It can be seen that with an increase in the number of SILAR cycles, the absorbance increases at wavelengths 400 to 700 nm. This can be attributed to the SPR of Cu QDs and narrow band gap of CuO and Cu2O48. In addition, the red-shift of the absorption edge of CuxO/TiO2 is due to the broadening of size distribution of CuxO QDs49. Based on the UV-Visible absorption spectra, a plot of (αhv)2 versus energy (hv) is shown in Fig. 6, and the Eg values of different CuxO samples are shown in Table 1. It can be seen that the absorption bands of CuxO/TiO2 show large variation, which change from 2.90 to 2.50 eV. The band gap of CuxO/TiO2 (S9) was 2.50 eV, which was smaller than that of TiO2 (2.90 eV). These results suggest that the formation of the CuxO/TiO2 nanostructures decreased the recombination of photogenerated electrons and holes and improved the photoelectrochemical ability of the TiO2 electrodes.
PL spectra
To investigate charge transfer between photogenerated electctrons and hole pairs, photoluminescence (PL) emission spectroscopy was used to measure the recombination of free charge carriers. The emission peaks at 420 and 475 nm are assigned to exciton-casued PL resulting from band edge free excitons and defects of TiO2 50. PL peak intensity correlates directly with the defect densities in materials. The higher PL intensity typically indicates a higher recombination rate of the photo-generated electrons and holes51. As shown in Fig. 7, the PL intensity of CuxO/TiO2 reveals a significant decreases with increasing CuxO QDs. This is due to a decrease of radiative recombination processes52. When the CuxO QDs are deposited on TiO2 electrodes, TiO2 can easily bond with the CuxO QDs to form the CuxO/TiO2 composites. The photo-induced electrons can be trapped in the Cu 2p energy level below the conduction band in the CuxO/TiO2 composites, which inhibit the recombination of electron-hole pairs. The intensity of the peaks at 420 and 475 nm are the lowest for S7, which exhibit high quantum efficiency and higher photoelectrochemical properties.
Photoelectrochemical studies
Figure 8 shows the time-dependent photocurrent curves of CuxO/TiO2 under visible light illumination. It is generally known that transient photocurrent always reflect the transfer and separation of photoinduced charge carriers under intermittent illumination. As the light is turned on, the photocurrent values increase while the photocurrent values decrease rapidly as the light is turned off. This suggests that all samples have good reproducibility. In addition, photocurrents increase with more SILAR cycles, which indicate that the photocurrent of CuxO/TiO2 have a significant enhancement compared to bare TiO2. Moreover, after 7 SILAR cycles, the sample shows the highest photocurrent value of ca. 138 μA/cm2, which is about 13 times higher than that for bare TiO2. Nevertheless, when the SILAR cycles increase to 9, the photocurrent value decreases to ca. 120 μA/cm2. The increase in photocurrent may be attributed to the stronger SPR effect of CuxO QDs, which improves the light absorption of TiO2. With more than 9 SILAR cycles, the aggregation of CuxO QDs lead to a large particle size of CuxO QDs, which could block the surface active sites of TiO2 and act as potential barrier of charge transfer, resulting in a decrease of the photoelectrochemical properties53,54,55,56.
Figure 9 shows the LSV curves of the different samples in the dark and under light irradiation. Photocurrent values are shown in Table 1. The photocurrents of the CuxO/TiO2 samples improved compared with that of bare TiO2, suggesting that the CuxO/TiO2 exhibit a stronger ability to separate photo-generated electron-hole pairs. The CuxO/TiO2 prepared with a different number of SILAR cycles (0, 3, 5, 7 and 9 times) exhibited photocurrent values of 168.88, 248.95, 390.98, 500.01, and 424.15 μA/cm2 at 1.0 V (vs Ag/AgCl) under visible light irradiation, respectively. Clearly, photocurrent densities of the CuxO/TiO2 first increase then decrease with increasing the SILAR cycles. The S7 showed the strongest photocurrent value, and exhibits the best photoelectrochemical property, which is consistent with the PL results. When 9 SILAR cycles were used, the CuxO QDs aggregated to form a compact granular morphology, resulting in a lower surface area and reduced photocurrent57.
Conclusions
In summary, CuxO QDs are deposited on the nanoporous anatase TiO2 films by a screen-printing method, followed by successive ionic adsorption and reaction (SILAR). The microstructure, morphology, and loading amounts of the CuxO QDs on the TiO2 films are controlled by changing the number of SILAR cycles. The CuxO/TiO2 absorbs more light and exhibits enhanced photoelectrochemical properties compared to bare TiO2. Moreover, under visible light illumination, the TiO2 sensitized with 7 SILAR cycles of CuxO QDs shows the best photoelectrochemical properties, where the photocurrent density is increased to 500.01 μA/cm2, 2.96 times higher than the bare TiO2 electrode with 168.88 μA/cm2. The superior photoelectrochemical properties of the CuxO/TiO2 nanostructures could be ascribed to the large surface area of nanoporous TiO2 electrode and the SPR effect of CuxO QDs. The electrode design of CuxO/TiO2 will be beneficial for application of solar energy conversion and wastewater degradation.
References
Fujishima, A. & Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 238, 37–38 (1972).
Dinh, C. T., Nguyen, T. D., Kleitz, F. & Do, T. O. Shape-controlled synthesis of highly crystalline titania nanocrystals. ACS Nano 3, 3737–3743 (2009).
Wang, S. M. et al. CdS and CdSe quantum dot co-sensitized nanocrystalline TiO2 electrode: Quantum dot distribution, thickness optimization, and the enhanced photovoltaic performance. J. Power Sources 273, 645–653 (2015).
Hochbaum, A. I. & Yang, P. Semiconductor nanowires for energy conversion. Chem. Rev. 110, 527–546 (2010).
Zhang, Q., Yodyingyong, S., Xi, J., Myers, D. & Cao, G. Oxide nanowires for solar cell applications. Nanoscale 4, 1436–1445 (2012).
He, X. L., Cai, Y. Y., Zhang, H. M. & Liang, C. H. Photocatalytic degradation of organic pollutants with Ag decorated free-standing TiO2 nanotube arrays and interface electrochemical response. J. Mater. Chem. 21, 475–480 (2011).
Song, Y. Y., Stein, F. S., Berger, S. & Schmuki, P. TiO2 nano test tubes as a self-cleaning platform for high-sensitivity immunoassays. Small 6, 1180–1184 (2010).
Salari, M. et al. Enhancement of the electrochemical capacitance of TiO2 nanotube arrays through controlled phase transformation of anatase to rutile. Phys. Chem. Chem. Phys. 14, 4770–4779 (2012).
Agosta, R. et al. Electrochemical assessment of the band-edge positioning in shape-tailored TiO2-nanorod-based photoelectrodes for dye solar cells. J. Phys. Chem. C 117, 2574–2583 (2013).
Gao, Z. D. et al. Nickel hydroxide nanoparticle activated semi-metallic TiO2 nanotube arrays for nonenzymatic glucose sensing. Chem. A Eur. J 19, 15530–15534 (2013).
Labiadh, H. et al. Preparation of Cu-doped ZnS QDs/TiO2 nanocomposites with high photocatalytic activity. Appl. Catal. Environ. B 144, 29–35 (2014).
Sambur, J. B., Riha, S. C., Choi, D. & Parkinson, B. A. Influence of surface chemistry on the binding and electronic coupling of CdSe quantum dots to single crystal TiO2 surfaces. Langmuir 26, 4839–4347 (2010).
Jung, M. H. & Kang, M. G. Enhanced photo-conversion efficiency of CdSe-ZnS core-shell quantum dots with Au nanoparticles on TiO2 electrodes. J. Mater. Chem. 21, 2694–2700 (2011).
Semonin, O. E. et al. Peak external photocurrent quantum efficiency exceeding 100% via MEG in a quantum dot solar cell. Science 334, 1530–1533 (2011).
Gao, M. et al. Strongly photoluminescent CdTe nanocrystals by proper surface modification. J. Phys. Chem. B. 102, 8360–8363 (1998).
Yang, H., Holloway, P. H. & Ratna, B. B. Photoluminescent and electroluminescent properties of Mn-doped ZnS nanocrystals. J. Appl. Phys. 93, 586–592 (2003).
Zalfani, M., Mahdouani, M., Bourguiga, R. & Su, B. L. Experimental and theoretical study of optical properties and quantum size phenomena in the BiVO4/TiO2 nanostructures. Superlattices Microstruct. 83, 730–744 (2015).
Santra, P. K. & Kamat, P. V. Mn-doped quantum dot sensitized solar cells: a strategy to boost efficiency over 5%. J. Am. Chem. Soc. 134, 2508–2511 (2012).
Sharma, S. N., Kohil, S. & Rastogi, A. C. Quantum confinement effects of CdTe nanocrystals sequestered in TiO2 matrix: Effect of oxygen incorporation. Phys. E 25, 554–561 (2005).
Khan, S. U. M., Al-Shahry, M. & Ingler, W. B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 297, 2243–2245 (2002).
Asahi, R., Morikawa, T., Ohwaki, T., Aoki, K. & Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001).
Wang, H. et al. CdS quantum dots-sensitized TiO2 nanorod array on transparent conductive glass photoelectrodes. J. Phys. Chem. C 114, 16451–16455 (2010).
Luo, J. et al. Homogeneous photosensitization of complex TiO2 nanostructures for efficient solar energy conversion. Sci. Rep. 2, 451 (2012).
Etgar, L. et al. Light energy conversion by mesoscopic PbS quantum dots-TiO2 heterojunction solar cells. ACS Nano 6, 3092–3099 (2012).
Tisdale, W. A. et al. Hot-electron transfer from semiconductor nanocrystals. Science 328, 1543–1547 (2010).
Zaban, A., Mićić, Q. I., Gregg, B. A. & Nozik, A. J. Photosensitization of nanoporous TiO2 electrodes with InP quantum dots. Langmuir 14, 3153–3156, (1998).
Yu, P. et al. Nanocrystalline TiO2 solar cells sensitized with InAs quantum dots. J. Phys. Chem. B 110, 25451–25454 (2006).
Wang, Q. Y., Qiao, J. L., Zhou, J. & Gao, S. M. Fabrication of CuInSe2 quantum dotssensitized TiO2 nanotube arrays for enhancing visible light photoelectrochemical performance. Electrochim. Acta 167, 470–475 (2015).
Barreca, D. et al. Tailored vapor-phase growth of CuOx-TiO2 (x=2) nanomaterials decorated with Au particles. Langmuir 27, 6409–6417 (2011).
Barreca, D. et al. Novel synthesis and gas sensing performances of CuO-TiO2 functionalized with Au nanoparticles. J. Phys. Chem. C 115, 10510–10517 (2011).
Heciak, A., Morawski, A. W., Grzmil, B. & Mozia, S. Cu-modified TiO2 photocatalysts for decomposition of acetic acid with simultaneous formation of C1-C3 hydrocarbons and hydrogen. Appl. Catal. B Environ 140–141, 108–114 (2013).
Nagaoka, K., Ueyama, J. & Ogura, K. Photoelectrochemical behavior of electrodeposited CuO and Cu2O thin films on conducting substrates. J. Electrochem. Soc. 151, C661–C665 (2004).
Sun, Q., Li, Y., Sun, X. & Dong, L. An improved photoelectrical performance of single-crystal TiO2 nanorod arrays by surface sensitization with copper quantum dots. ACS Sustain. Chem. Eng. 1, 798–804 (2013).
Hua, Z. L. et al. Copper nanoparticles sensitized TiO2 nanotube arrays electrode with enhanced photoelectrocatalytic activity for diclofenac degradation. Chem. Eng. J. 283, 514–523 (2016).
Tsui, L. K. & Zangari, G. Modification of TiO2 nanotubes by Cu2O for photoelectrochemical, photocatalytic, and photovoltaic devices. Electrochim. Acta 128, 341–348 (2014).
Hou, Y., Zhao, Q., Quan, X., Li, X. & Chen, G. Fabrication of Cu2O/TiO2 nanotube heterojunction arrays and investigation of its photoelectrochemical behavior. Appl. Phys. Lett. 95, 093108 (2009).
Li, Y. S. et al. Enhanced photocatalytic activities of TiO2 nanocomposites doped with water-soluble mercapto-capped CdTe quantum dots. Appl. Catal. B: Environ 101, 118–129 (2010).
Wang, Q., Qiao, J., Xu, X. & Gao, S. Controlled synthesis of Cu nanoparticles on TiO2 nanotube array photoelectrodes and their photoelectrochemical properties. Mater. Lett. 131, 135–137 (2014).
Hu, L. H. et al. Microstructure design of nanoporous TiO2 photoelectrodes for dye sensitized solar cell modules. J. Phys. Chem. B 111, 358–362 (2007).
Li, G., Dimitrijevic, N. M., Chen, L., Rajh, T. & Gray, K. A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO-TiO2 nanocomposites. J. Phys. Chem. C 112, 19040–19044 (2008).
Luo, J. S. et al. TiO2/(CdS, CdSe, CdSeS) nanorod heterostructures and photoelectrochemical properties. J. Phys. Chem. C 116, 11956–11963 (2012).
Sun, Q., Li, Y., Sun, X. M. & Dong, L. F. Improved photoelectrical of single-crystal TiO2 nanorod arrays by surface sensitization with copper quantum dots. ACS Sustainable Chem. Eng. 1, 798–804 (2013).
Tian, H. et al. Retarded charge recombination in dye-sensitized nitrogen-doped TiO2 solar cells. J. Phys. Chem. C 114, 1627–1632 (2010).
Cao, J. L. et al. Hierarchical meso-macroporous titania-supported CuO nanocatalysts: preparation, characterization and catalytic CO oxidation. J. Mater. Sci. 44, 6717–6726 (2009).
Chan, G. H., Zhao, J., Hicks, E. M., Schatz, G. C. & Van Duyne, R. P. Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett. 7, 1947–1952 (2007).
Park, B. K. et al. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 311, 417–424 (2007).
Tian, B., Li, C. & Zhang, J. One-step preparation characterization and visible-light photocatalytic activity of Cr-doped TiO2 with anatase and rutile bicrystalline phases. Chem. Eng. J. 191, 402–409 (2012).
Sajjad, A. K. L., Shamaila, S., Tian, B., Chen, F. & Zhang, J. One step activation of WOx/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Catal. B: Environ 91, 397–405 (2009).
Hsu, C., Shen, Y., Wei, Z., Liu, D. & Liu, F. Anatase TiO2 nanobelts with plasmonic Au decoration exhibiting efficient charge separation and enhanced activity. J. Alloy. Compd. 613, 117–121 (2014).
Chen, Z., Berciaud, S., Nuckolls, C., Heinz, T. F. & Brus, L. E. Energy transfer from individual semiconductor nanocrystals to graphene. ACS Nano 4, 2964–2968 (2010).
Zhang, Y. H., Tang, Z. R., Fu, X. Z. & Xu, Y. J. TiO2-Graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 4, 7303–7314 (2010).
Toyoda, T., Hayakawa, T., Abe, K., Shigenari, T. & Shen, Q. Photoacoustic and photoluminescence characterization of highly porous, polycrystalline TiO2 electrodes made by chemical synthesis. J. Lumin. 87, 1237–1239 (2000).
Yu, J., Qi, L. & Jaroniec, M. Hydrogen production by photocatalytic water splitting over Pt/TiO2 nanosheets with exposed (001) facets. J. Phys. Chem. C 114, 13118–13125 (2010).
Yao, H. et al. Vertical growth of two-dimensional TiO2 nanosheets array films and enhanced photoelectrochemical properties sensitized by CdS quantum dots. Electrochim. Acta 125, 258–265 (2014).
Zhou, M., Zhou, X. W. D. & Xie, Y. Two-dimensional nanosheets for photoelectrochemical water splitting: Possibilities and opportunities. Nano Today 8, 598–618 (2013).
Hodes, G. Comparison of dye-and semiconductor-sensitized porous nanocrystalline liquid junction solar cells. J. Phys. Chem. C 112, 17778–17787 (2008).
Yang, L. et al. Photoelectrochemical properties of Ag/TiO2 electrodes constructed using vertically oriented two-dimensional TiO2 nanosheet array films. J. Electrochem. Soc. 163, H180–H185 (2016).
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 51472003), State Key Program for Basic Research of China (2013CB632705), the Anhui Provincial Natural Science Foundation (1608085ME95), the Anhui Provincial Natural Science Research Project (KJ2016A524) and the Higher Education Excellent Youth Talents Foundation of Anhui Province (gxyqZD2016328). The authors would like to thank Yonglong Zhuang, Zhongqing Lin, Yun Wu and Chao Cheng of the Experimental Technology Center of Anhui University, for electron microscope test and discussion.
Author information
Authors and Affiliations
Contributions
Jiajia Tao and Zhaoqi Sun wrote the main manuscript text. Miao Zhang prepared Figures 1 and 2. Jianguo Lv prepared Figures 3 and 4. Gang He and Xi Shunjiang prepared Figures 5 and 6. Xiaoshuang Chen prepared Figures 7–9. Yunlang Cheng, Shiwei Shi, Xingzhi Wang, Zhuang Wang, and Zezhou Gong reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tao, J., Sun, Z., Cheng, Y. et al. Enhanced photoelectrochemical properties of nanocrystalline TiO2 electrode by surface sensitization with CuxO quantum dots. Sci Rep 7, 5291 (2017). https://doi.org/10.1038/s41598-017-05645-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05645-x
This article is cited by
-
The effect of oxidative functionalization of carbon nanotubes on the morphological, optical, and photoelectrochemical properties of modified titanium dioxide photoanodes
Journal of Materials Science (2023)
-
Effect of vacuum annealing on solar light response and photocatalytic performance of Ag nanoparticle-modified ZnO thin films
Applied Physics A (2020)
-
Studies of the photocatalytic and electrochemical performance of the Fe2O3/TiO2 heteronanostructure
Journal of the Iranian Chemical Society (2020)
-
Surface morphology, electrochemical and electrical performances of ZnO thin films sensitized with Ag nanoparticles by UV irradiation
Journal of Materials Science: Materials in Electronics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.