Abstract
Renal biopsy has been widely recommended in clinic to determine the histological patterns of kidney disease. To prevent bleeding complications, patients should routinely stop anticoagulants prior to renal biopsy. However, patients with kidney disease are susceptible to thromboembolisms, particularly in those with severe hypoalbuminemia. This study was designed to investigate the application of serum D-dimer as a predictor for thrombotic events after renal biopsy. 400 consecutive native renal biopsies were prospectively included in this 2-month follow-up study. The overall incidence of bleeding and thrombotic complication is 4%, including hematuria or large perinephric hematoma (2.5%, n = 10) and thrombotic complication (1.5%, n = 6). Compared to low serum D-dimer (<2.00 μg/ml), subjects in the group of high serum D-dimer (≥2.00 μg/ml) were more incline to develop thrombotic complications (9.1% versus 0.3%; RR, 30.33; p < 0.001). D-dimer correlated positively with age (rs = 0.258, P < 0.001). Inverse correlations were found for albumin (rs = −0.339, P < 0.001). Taken together, patients with high serum D-dimer carry an increased risk of thrombotic complications after renal biopsy. Our findings suggest that serum D-dimer can serve as a potential predictor for thrombotic events in patients with kidney disease. Further cautions should be given to these subjects.
Similar content being viewed by others
Introduction
Percutaneous renal biopsy is an important diagnostic tool to obtain a histological diagnosis for specific patient treatment. Some studies have been done on renal biopsies to examine risk factors for biopsy complications1,2,3,4,5 Hemorrhage is the most common complication. Previous studies always took gross hematuria, perinephric hematoma as minor complications, bleeding requiring transfusion, a hemodynamically significant arteriovenous fistula, any radiological or surgical intervention and death as major complications. But rarely study reported thrombotic complications. Patients with kidney disease especially nephrotic syndrome can become hypercoagulable. In these patients, venous thromboembolism ranging from 2% in children6 to as high as 26.7% in adults7 and a relative risk of arterial thromboembolism ranging from 1 to 5.5 have been reported8, 9. The pathophysiological mechanisms of thromboembolism in patients with nephrotic syndrome have yet to be unraveled. Nevertheless, alterations in plasma levels of antithrombin III, protein C, and protein S, enhanced platelet aggregation, hyperviscosity, and hyperlipidemia, as well as treatment with corticosteroids and diuretics, are considered predisposing factors for the development of thrombotic events. Before and after renal biopsy should stop anticoagulants. The procedure also could activate coagulation system. At our institution, some patients admitted serious thrombotic complications after renal biopsy. It is important to select the patients who at high risk of thrombotic complication if taken renal biopsy.
D-dimer is a degradation product of cross-linked frbrin which exists in the blood after a thrombus is degraded by thrombin, coagulation factor XIIIa and fibrinolysin. D-dimer levels can reflect the activity of coagulation and the fibrinolytic system. Previous studies have demonstrated that expression levels of D-dimer in serum are significantly increased following thromblic diseases10,11,12,13,14,15,16. However, to our knowledge, no study has examined if D-dimer prior renal biopsy can also be used to predict thrombotic complications after renal biopsies.
Methods
In this study, 400 consecutive native renal biopsies (200 in men and 200 in women) were prospectively included (Table 1). The excluded criterions were as follow: patients with uncontrolled hypertension (systolic blood pressure ≥150 mmHg at the time before procedure); patients requiring ongoing anticoagulation; patients with significant liver disease (including known cirrhosis) or platelet counts of <100 × 10^9/L. Patients were told to stop any anticoagulants, nonsteroidal anti-inflammatory drugs and anti-platelet agents at least 3 days prior to the biopsy. Data were collected from our centre from May 1, 2016, until September 30, 2016. All of these patients were followed 2 months after renal biopsies.
The study was approved by the “Medical Ethics Review Committee of The Second Xiangya Hospital of Central South University”. Informed consent was obtained from all participants. All methods were performed in accordance with the guidelines and regulations of the National Natural Science Foundation of China and the Medical Ethics Review Committee of The Second Xiangya Hospital of Central South University.
All biopsies were performed with the use of real-time ultrasound guidance and an automated spring-loaded biopsy device. The size of the needles used were 16-gauge (Tochigi-Shi. Tochigi-Ken, Japan).
Clinical data including sex, age, systolic and diastolic blood pressure, weight, coagulation function, protein C, protein S and albumin were collected. D-dimer levels were measured before renal biopsy with ELISA method (Table 2). Kidney function was determined by means of estimated glomerular filtration rate (eGFR, Modification of Diet in Renal Disease [MDRD]).
After kidney biopsy, we followed these patients 2 months. Adverse effects after the biopsy procedure were divided into bleeding (bleeding requiring blood transfusion or invasive procedure, a hemodynamically significant arteriovenous fistula, any radiological or surgical internention and death) or large hematoma complications and thrombotic complications (e.g., deep venous thrombosis, pulmonary embolism, acute myocardial infarction) that diagnosed by clinical manifestations, imaging and laboratory test results.
For statistical analysis, the IBM SPSS Statistic 19 was used. Fisher’s exact test and χ2 analyses were used for the cross-tabulation of data. Correlation analyses were performed by use of Spearman’s test (rs). Multiple regression analyses were performed by use of Stepwise analysis. Binary logistic regression analysis was used. Data are presented as risk ratios (RR) and confidence intervals (CI). The Mann-Whitney U test was used. A 2-side P value of <0.05 was considered significant.
Data availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Results
The overall hematuria or large perinephric hematoma complication and thrombotic complication rate was 4% (n = 16), including bleeding or large hematoma (2.5%, n = 10) and thrombotic (1.5%, n = 6) complications. In the group of bleeding or large hematoma complications, nine patients had clinically significant hematoma (extended hospital care and treatment for pain) and one patients had extended hospital care for over half a month because of gross hematuria (prolonged observation, intravenous fluid replacement). In the group of thrombotic complication group, two patients had pulmonary embolism (PE) in the first week after renal biopsy, two patients had acute myocardial infarction in the first month after renal biopsy, one patient had deep venous thrombosis (DVT) of right lower limb, one had mesenteric thrombosis.
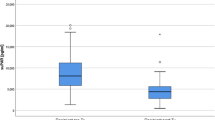
The median and mean of D-dimer level was 0.45 mg/l and 1.00 mg/l. There was no difference in D-dimer level between sexes. There was a cutoff-level for D-dimer as a predictor for thrombotic complications after renal biopsy 2 months, when D-dimer was ≥2.00 μg/ml versus <2.00 μg/ml. This resulted in different thrombotic complications (9.1% versus 0.3%; RR, 30.33; p < 0.001) (Fig. 1). Binary logistic regression analysis was performed, inclucing complications (bleeding or large hematoma complications and thrombotic complications) as dependent factor and as variables age, sex, systolic and diastolic blood pressure, weight, albumin, and D-dimer ≥2.00 μg/ml and <2.00 μg/ml. The significant variable in this model was D-dimer ≥2.00 μg/ml (P < 0.001) and systolic pressure <120 mmHg (P = 0.043) for thrombotic complications. It did not demonstrate a statistical difference for bleeding or large hematoma complications.
To assess what affected the D-dimer, we investigated for correlations to various variables. In the linear regression analysis, D-dimer correlated positively with age (rs = 0.258, P < 0.001). Inverse correlations were found for albumin (rs = −0.339, P < 0.001) (Fig. 2). No correlations were existed between D-dimer and sex, systolic and diastolic blood pressure, weight and eGFR.
A sub-analysis was performed for D-dimer levels of <2.00 μg/ml and ≥2.00 μg/ml. In the group D-dimer <2.00 μg/ml, D-dimer correlated negatively with eGFR (rs = −0.268, P < 0.001) and albumin (rs = −0.508, P < 0.001) (Fig. 3) but not with systolic and diastolic blood pressure, age, sex and weight. In the group ≥2.00 μg/ml, D-dimer had no correlation with systolic and diastolic blood pressure, age, sex, weight albumin, and eGFR.
Multiple regression stepwise analyses were performed with the D-dimer as the dependent factor and the independent variables ages, systolic and diastolic blood pressurem weight, albumin and eGFR. When including all D-dimer, the model was significant (r2 = 0.153, P < 0.001) with significant variables age (P < 0.001) and albumin (P < 0.001). When subgroup analyses were performed for D-dimer<2.00 μg/ml, the model’s r2 was 0.310 (P < 0.001), with eGFR (P < 0.001), sex (P = 0.037), albumin (P < 0.001) as the significant variables. For D-dimer ≥2.00 μg/ml, the model was not significant.
The coagulation function was analysed (Table 3). There were no differences in prothrombin time (PT), activated partial thromboplastin time (APTT), antithrombin III (AT-III) levels between D-dimer levels <2.00 μg/ml and ≥2.00 μg/ml groups. The protein C (P = 0.015) and fibrin degradation product (FDP) (P < 0.001) were higher in D-dimer levels ≥2.00 μg/ml group than D-dimer <2.00 μg/ml group (Table 4). The AT-III (P = 0.012) was higher in D-dimer <2.00 μg/ml group compared with D-dimer levels ≥2.00 μg/ml group.
One of the patient didn’t achieve sufficient tissue for diagnosis. The mean of D-dimer was more than 2.00 μg/ml in crescentic nephritis and lupus nephritis (II–V). The D-dimer was higher in crescentic nephritis (4.04 versus 0.96, P < 0.001) and lupus nephritis (II–V) (3.25 versus 0.95, P < 0.001) compared with other diagnoses (Table 5).
Discussion
As a degradation product of cross-linked fibrin, the presence of D-dimer indicates activation of the coagulation system. D-dimer has been become an important complementary tool for thrombotic disease diagnosis. The aim of our study was to investigate the association between the D-dimer level and the serious thrombotic complication after renal biopsy. To our knowledge, the present study is the first to examine whether correlations exist between D-dimer and thrombotic complications after renal biopsy. To get rid the effect of D-dimer level changes induced by surgery, we collected data before kidney biopsy, We indentified that elevated D-dimer, especially ≥2.00 μg/ml, was significantly associated with increased thrombotic complication after renal biopsy. Our finding motivates greater caution after native renal biopsies, especially in those with D-dimer ≥2.00 μg/ml measured before biopsy was performed. Perhaps these patients should be more closely observed after renal biopsies and may be use anticoagulants earlier.
Previous studies reported complications of renal biopies as gross hematuria, perinephric hematoma, a hemodynamically significant arteriovenous fistula, any radiological or surgical intervention3, 17, 18. They reported an incidence of major complications in the range of 6–8%1, 2, 19. Recent studies using realtime ultrasound and automated biopsy needles reported major complication rates of less than 5%5, 18. None of these studies connected thrombotic disease with renal biopies. The pathophysiology for the thrombophilic state in kidney disease is not fully understood. Regulatory coagulation proteins such as antithrombin III and protein S are oftern present in leakage that results from glomerular injury20. In response to protein loss into the urinary space, the liver compensates by increasing production of hemostatic proteins, especially factors I,VII,VIII,and X, shifting the hemostatic equilibrium in favor of pro-thrombosis21, 22. Elevation of platelet count has been regularly noted in patients with nephrotic syndrome, likely contributing to thrombotic risk. It has been shown that fibrin clot structure in nephrotic plasma is less porous than typical thrombi, possibly leading to increased resistance to fibrinolysis23. In our study, the higher FDP and the lower level AT-III in the group D-dimer levels ≥2.00 μg/ml which with higher rates of thrombotic complications also contribute to the hypercoagulable of patients with higher D-dimer level. The protein C pathway provides multiple important functions that regulate both haemostasis and host defence system in response to vascular injury. The anticoagulant protein C pathway is designed to regulate coagulation, maintain the fluidity of blood within the vasculature, and prevent thrombosis24, 25. The higher level of protein C in D-dimer ≥2.00 μg/ml group reflects the activated of anticoagulation system. Before and after the renal biopsy procedure the routine is stop any anti-platelet agents or anticoagulants and after the procedure the patient should lie flat on strict bed for 4 to 24 hours to reduce the bleeding complication, which could increase the risks of thrombotic complications in some patients. The renal biopsy procedure itself also stimulate the coagulation system.
D-dimer tests have been used in the evaluation of suspected thrombtic disorders for more than 20 years10, 13. D-dimer are typically elevated in patients with acute vein thromboembolism, myocardial infarction, stroke and other prothrombotic conditions16, 26,27,28. D-dimer is a sensitive but nonspecific marker for venous thrombosis. Recent guidelines recommend the use of initial D-dimer testing when evaluating patients with either a low or moderate pretest probability of DVT or PE29. If the D-dimer assay result is negative, DVT or PE is excluded and no further testing is necessary.
Levels of D-dimer are not only elevated with acute VTE, but also present in a wide variety of inflammatory conditions and connective tissue disorders, which contributes to the high level of D-dimer in lupus nephritis and crescentic nephritis. The main mechanisms of the coagulation derangement during systemic inflammatory activity are tissue factor-mediated thrombin generation and an imbalance of dysfunction of the normal physiologic anticoagulant mechanisms, such as the antithrombin system and the protein C system30. Inflammation and coagulation often interact. Inflammation activates coagulation, and vice versa, coagulation also modulates inflammatory activity. D-dimer as an end product of fibrinolysis, can promote the inflammatory cascade by activating neutrophils and monocytes, which induces inflammatory cytokines secretion and promotes hepatic synthesis of acute-phase proteins31. In addition to its diagnostic value, D-dimer levels are also of great prognostic significance and are associated with outcomes in many diseases. Serum D-dimer levels are associated with outcomes in patient with stroke, acute myocardial infarction, A acute aortic dissection, carcinoma, COPD32,33,34,35.
We do recognize that our study has several limitations. Firstly, this is a single-center study performed in a limited number of subjects of Chinese ethnicity. Only patients received renal biopsy between May 1, 2016 and September 30, 2016 were enrolled in this study. It might be insufficient to draw the same conclusion in other populations. Secondly, the different pathogenesis of kidney diseases as well as the therapeutics can affect the coagulation system. Thirdly, the incidence of thrombotic events in the whole patients after renal biopsy is relatively low. It could be attributed to the early anti-coagulant treatment on severe hypoalbuminemia. In light of its apparent clinical benefits in kidney disease, however, it will be viewed as unethical to conduct control trials in patients with uncontrolled hypercoagulation. Lastly, in an open-label study, our data may be subject to observer and selection bias as well. We acknowledge that a well-designed randomized, controlled trial could be more convincing to determine the diagnosis value of serum D-dimer in thrombotic complications.
In this study, we demonstrate that high serum D-dimer is associated with increased risk of thrombotic events in patients with renal biopsy. Our findings also indicate that serum D-dimer is a potential predictor for the incidence of venous thrombosis in kidney disease. Further cautions should be given to these subjects including consideration of early anticoagulant therapy after renal biopsy. It is of clinical significance to further determine if the pattern of serum D-dimer can link to the thrombotic complications in acute and chronic kidney diseases.
References
Whittier, W. L. & Korbet, S. M. Timing of complications in percutaneous renal biopsy. J Am Soc Nephrol 15, 142–7 (2004).
Liu, H. et al. Renal biopsy findings of patients presenting with isolated hematuria: disease associations. Am J Nephrol 36, 377–85 (2012).
McMahon, G. M. et al. Development of an outpatient native kidney biopsy service in low-risk patients: a multidisciplinary approach. Am J Nephrol 35, 321–6 (2012).
Waldo, B., Korbet, S. M., Freimanis, M. G. & Lewis, E. J. The value of post-biopsy ultrasound in predicting complications after percutaneous renal biopsy of native kidneys. Nephrol Dial Transplant 24, 2433–9 (2009).
Stratta, P. et al. Risk management of renal biopsy: 1387 cases over 30 years in a single centre. Eur J Clin Invest 37, 954–63 (2007).
Lilova, M. I., Velkovski, I. G. & Topalov, I. B. Thromboembolic complications in children with nephrotic syndrome in Bulgaria (1974–1996). Pediatric nephrology 15, 74–78 (2000).
Kerlin, B. A., Ayoob, R. & Smoyer, W. E. Epidemiology and pathophysiology of nephrotic syndromeassociated thromboembolic disease. Clin J Am Soc Nephrol 7, 513–20 (2012).
Ordonez, J. D., Hiatt, R. A., Killebrew, E. J. & Fireman, B. H. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney Int 44, 638–42 (1993).
Chen, G., Liu, H. & Liu, F. A glimpse of the glomerular milieu: from endothelial cell to thrombotic disease in nephrotic syndrome. Microvasc Res 89, 1–6 (2013).
Bates, S. M. D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost 38, 673–82 (2012).
Adam, S. S., Key, N. S. & Greenberg, C. S. D-dimer antigen: current concepts and future prospects. Blood 113, 2878–87 (2009).
Adams, D., Welch, J. L. & Kline, J. A. Clinical utility of an age-adjusted D-dimer in the diagnosis of venous thromboembolism. Ann Emerg Med 64, 232–4 (2014).
Righini, M., Perrier, A., De Moerloose, P. & Bounameaux, H. D-Dimer for venous thromboembolism diagnosis: 20 years later. J Thromb Haemost 6, 1059–71 (2008).
Bounameaux, H. et al. Measurement of D-dimer in plasma as diagnostic aid in suspected pulmonary embolism. Lancet 337, 196–200 (1991).
Bounameaux, H., Schneider, P. A., Reber, G., de Moerloose, P. & Krahenbuhl, B. Measurement of plasma D-dimer for diagnosis of deep venous thrombosis. Am J Clin Pathol 91, 82–5 (1989).
Di Nisio, M. et al. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost 5, 296–304 (2007).
Tang, L., Fang, Z. F. & Zhou, S. H. Paradoxical embolism causing acute embolic events in a patient with hereditary thrombophilia. Herz 40, 314–7 (2015).
Manno, C. et al. Predictors of bleeding complications in percutaneous ultrasound-guided renal biopsy. Kidney Int 66, 1570–7 (2004).
Marwah, D. S. & Korbet, S. M. Timing of complications in percutaneous renal biopsy: what is the optimal period of observation? American journal of kidney diseases: the official journal of the National Kidney Foundation 28, 47–52 (1996).
Hanevold, C. D., Lazarchick, J., Constantin, M. A., Hiott, K. L. & Orak, J. K. Acquired free protein S deficiency in children with steroid resistant nephrosis. Annals of clinical and laboratory science 26, 279–282 (1996).
al-Mugeiren, M. M. et al. Coagulopathy of childhood nephrotic syndrome–a reappraisal of the role of natural anticoagulants and fibrinolysis. Haemostasis 26, 304–310 (1996).
Schlegel, N. Thromboembolic risks and complications in nephrotic children. Semin Thromb Hemost 23, 271–80 (1997).
Colle, J. P. et al. Abnormal fibrin clot architecture in nephrotic patients is related to hypofibrinolysis: influence of plasma biochemical modifications: a possible mechanism for the high thrombotic tendency? Thrombosis and aemostasis 82, 1482–1489 (1999).
Bouwens, E. A., Stavenuiter, F. & Mosnier, L. O. Mechanisms of anticoagulant and cytoprotective actions of the protein C pathway. J Thromb Haemost 11(Suppl 1), 242–53 (2013).
Bruley, D. F. & Streiff, M. B. Nature’s “silver bullet” for anticoagulation: mechanism of Zymogen Protein C to Activated Protein C. Adv Exp Med Biol 765, 15–21 (2013).
Brotman, D. J., Segal, J. B., Jani, J. T., Petty, B. G. & Kickler, T. S. Limitations of D-dimer testing in unselected inpatients with suspected venous thromboembolism. The American journal of medicine 114, 276–282 (2003).
Siragusa, S. D-dimer testing: advantages and limitations in emergency medicine for managing acute venous thromboembolism. Internal and emergency medicine 1, 59–66 (2006).
Chen, G. et al. High dose urokinase against massive pulmonary embolism in nephrotic syndrome. Blood Coagul Fibrinolysis 24, 439–43 (2013).
Bates, S. M. et al. Diagnosis of DVT: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141, e351S–e418S (2012).
Levi, M. & van der Poll, T. Inflammation and coagulation. Crit Care Med 38, S26–34 (2010).
Shorr, A. F., Thomas, S. J., Alkins, S. A., Fitzpatrick, T. M. & Ling, G. S. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest 121, 1262–1268 (2002).
Huang, Y. et al. The prognostic value of D-dimer levels in metastatic osteosarcoma patients treated with second-line chemotherapy. Oncotarget 7, 65568–65576 (2016).
Huang, B. et al. Impact of d-Dimer Levels on Admission on Inhospital and Long-Term Outcome in Patients With Type A Acute Aortic Dissection. Am J Cardiol 115, 1595–600 (2015).
Shen, K., Tang, H., Jing, R., Liu, F. & Zhou, X. Application of triple-branched stent graft for Stanford type A aortic dissection: potential risks. Eur J Cardiothorac Surg 41, e12–7 (2012).
Hu, G. et al. Prognostic role of D-dimer for in-hospital and 1-year mortality in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis 11, 2729–2736 (2016).
Acknowledgements
The authors thank the patients who participated in this study and their relatives. This work was supported by a research grant (81470947 and 81400721) from the National Natural Science Foundation of China.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Hong Liu and Xia Tan. Performed the experiments: Xia Tan, Yu Liu, Letian Zhou, Liyu He, Di Liu, Yexin Liu, Fan Zhang, Huiqiong Li. Analysed the data: Xia Tan and Guochun Chen. Wrote the paper: Xia Tan and Guochun Chen.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tan, X., Chen, G., Liu, Y. et al. Serum D-dimer is a potential predictor for thromboembolism complications in patients with renal biopsy. Sci Rep 7, 4836 (2017). https://doi.org/10.1038/s41598-017-05210-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05210-6
This article is cited by
-
Coagulation parameters are associated with the prognosis of immunoglobulin a nephropathy: a retrospective study
BMC Nephrology (2020)
-
D-dimer Levels in Chronic Kidney Illness: A Comprehensive and Systematic Literature Review
Proceedings of the National Academy of Sciences, India Section B: Biological Sciences (2020)
-
Manual compression and reflex syncope in native renal biopsy
Clinical and Experimental Nephrology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.