Abstract
A major challenge for agriculture is to provide sufficient plant nutrients such as phosphorus (P) to meet the global food demand. The sufficiency of P is a concern because of it’s essential role in plant growth, the finite availability of P-rock for fertilizer production and the poor plant availability of soil P. This study investigated whether biofertilizers and bioenhancers, such as arbuscular mycorrhizal fungi (AMF) and their associated bacteria could enhance growth and P uptake in maize. Plants were grown with or without mycorrhizas in compartmented pots with radioactive P tracers and were inoculated with each of 10 selected bacteria isolated from AMF spores. Root colonization by AMF produced large plant growth responses, while seven bacterial strains further facilitated root growth and P uptake by promoting the development of AMF extraradical mycelium. Among the tested strains, Streptomyces sp. W94 produced the largest increases in uptake and translocation of 33P, while Streptomyces sp. W77 highly enhanced hyphal length specific uptake of 33P. The positive relationship between AMF-mediated P absorption and shoot P content was significantly influenced by the bacteria inoculants and such results emphasize the potential importance of managing both AMF and their microbiota for improving P acquisition by crops.
Similar content being viewed by others
Introduction
In the next decades, a major challenge for agriculture will be the sustainable production of enough food crops to meet the growing global demand. Current agricultural systems strongly depend on continuous applications of chemical fertilizers, mainly nitrogen (N), phosphorus (P) and potassium (K), which contribute to the decline of biological soil fertility1,2,3. In particular, the use of phosphate-based fertilizers has increased from approximately 5 million tons P per year in 1961 to approximately 20 million tons in 20134. Phosphorus is required in relatively large amounts by plants for optimal growth, as it is a structural constituent of biomolecules involved in several key plant processes, including photosynthesis, nucleic acid and phospholipid biosynthesis, respiration and energy transfer5, 6. Most agricultural soils contain high concentrations of P, in both inorganic and organic forms, that is poorly available for plant uptake due to its immobilization and precipitation with other soil minerals, such as iron (Fe) and aluminium (Al) in acid soils and calcium (Ca) in alkaline soils. Thus, only a small percentage of P (<1%) is directly available for plant uptake7, 8. The abundant plant root-associated microbiota represents one sustainable strategy for the exploitation and mobilization of the soil P pool9.
Arbuscular mycorrhizal (AM) fungi (AMF, Glomeromycota) are important beneficial soil microorganisms establishing mutualistic associations with most food crops. These associations increase plant nutrient uptake and tolerance to root pathogens and drought. AMF are obligate biotrophs and colonize host roots to obtain sugars in exchange of mineral nutrients, absorbed and translocated through a fine network of extraradical mycelium (ERM) spreading from colonized roots into the soil10. Such belowground networks, which show length densities, up to about 25 m g−1 soil11, 12, function as auxiliary absorbing systems that transfer mineral nutrients, such as phosphorus (P), nitrogen (N), sulfur (S), potassium (K), calcium (Ca), iron (Fe), copper (Cu), and zinc (Zn), from the soil outside the roots to the host plants13,14,15,16. AMF mycorrhizal networks may differentially affect P and N supply to host plants depending on their structural and functional properties17,18,19,20, including the occurrence and differential expression of P transporter and N assimilation fungal genes21,22,23. The improved P nutrition of mycorrhizal plants is also the result of hyphal P uptake beyond the P depletion zone caused by the fast root absorption of P from the soil solution, which cannot be rapidly replenished, given the poor mobility of P in the soil8, 10. The P taken up by ERM is translocated to intraradical hyphae in the form of polyphosphates24,25,26. High P fluxes in AMF hyphae have been detected, ranging from 2 to 20 × 10−6 mol m−2 s−1 27,28,29, with bidirectional protoplasmic flows, measured on the basis of cellular particles movement (vacuoles, nuclei, fat droplets, organelles, granules), ranging from 3.0 to 4.3 μm s−1 30, 31.
The development and performance of AMF may be mediated by a third component of the symbiosis, represented by highly diverse bacterial communities living associated with AMF spores and mycelium (mycorrhizospheric microbiota)32,33,34,35,36. Such beneficial bacteria showed key plant growth promoting (PGP) functions, encompassing nitrogen fixation, P solubilization, the production of indole acetic acid (IAA), siderophores and antibiotics, supplying fundamental nutrients and growth factors37,38,39,40,41,42. Recently, a culture-dependent approach allowed the isolation in pure culture of 374 bacterial strains strictly associated with Rhizophagus irregularis (formerly Glomus intraradices) spores, and the selection of Actinobacteria, Bacillaceae and Sinorhizobium meliloti strains showing several PGP activities, including P mineralization from phytate and mineral P solubilization43.
This work, for the first time, investigated to which degree growth and P uptake of mycorrhizal and non-mycorrhizal maize plants would benefit from inoculation with a selection of bacterial strains isolated from AMF spores, showing multiple PGP activities in vitro. Bacillus spp., Sinorhizobium meliloti and Streptomyces spp. were inoculated alone or in combination with R. irregularis in two experiments using compartmented pots with a root hyphal compartment (RHC) and a hyphal compartment (HC). Labelling of HC soil with radioactive P (32P or 33P) made it possible to distinguish between P uptake by AM hyphae and P uptake by roots and hyphae in combination. While AM colonization resulted in large plant growth responses, the bacteria inoculants increased the abundance of AMF hyphae in soil and four of them also root P content and dry weight.
Results
Root growth and AMF abundance in roots and hyphal compartment soil
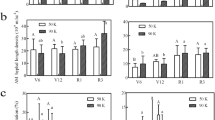
Here we report data on total and colonized root length of maize plants grown in the RHC and on hyphal length occurring in the HC, obtained from two successive experiments where the PGP bacterial strains (3 strains in Exp. 1, 8 strains in Exp. 2) were inoculated alone and in combination with the AM fungus R. irregularis. Roots of AMF inoculated plants were well colonized in both experiments and had a distinct yellow-orange root colour due to apocarotenoid accumulation44, while roots of non-inoculated plants remained uncolonized and pale. Bacterial inoculation had no effects on mycorrhizal colonization, which ranged from 78% to 87% in Exp. 1, and from 58 to 67% in Exp. 2 (Table 1). The root length was strongly and consistently increased by AM colonization, in particular in Exp. 2. Maize plants inoculated with R. irregularis alone or in combination with bacteria, showed root length increases of 62 and 211% in Exp. 1 and in Exp. 2, respectively, compared with non-mycorrhizal (NM) controls (Table 1).
The extraradical mycelium, but not roots, proliferated into all HCs of AMF-inoculated pots and the measured hyphal length densities (HLD) were corrected by subtraction of the average background values measured in HCs of NM treatments (0.15 ± 0.04–0.90 ± 0.10 m g−1 soil). Inoculation with bacteria increased HLD significantly in both experiments (Exp. 1, P = 0.020; Exp. 2, P < 0.001; Table 1). This stimulation of HLD was caused by S. meliloti TSA41 in Exp. 1 and by all bacteria except Streptomyces sp. W77 in Exp. 2. Interestingly, S. meliloti TSA41 was the most stimulatory bacterium in both Exp. 1 and 2, where HLD increased by 19 and 25% over the levels measured in mycorrhizal plants without bacterial inoculation.
P content and P uptake
Here, data are reported on P uptake by AM hyphae and by roots and hyphae in combination, as revealed by the use of radioactive P (32P or 33P) added to HC soil. In Exp. 1, radioactivity (32P) was consistently detected in shoots already 14 days after planting (Fig. 1). Even though the monitoring allowed for only semi-quantitative measurement of radioactivity, the 32P content in shoots differed clearly between plants inoculated with R. irregularis and uninoculated controls. Moreover, mycorrhizal plants showed higher counts when co-inoculated with bacteria than when non-co-inoculated, at four early time points (16, 18, 21 and 23d).
Time course of radioactivity (32P, counts per second) monitored in the shoots of Zea mays L. genotype Oh43 inoculated (red squares) or not (blue circles) with Rhizophagus irregularis alone or in combination with different bacterial strains, in Exp. 1. TSA3: Sinorhizobium meliloti TSA3; TSA41: Sinorhizobium meliloti TSA41; CH19: Lysinibacillus fusiformis CH19. Bars represent standard errors.
In Exp. 1, shoot and root P content, and root length specific P uptake were 177%, 196% and 80% greater in mycorrhizal than in NM treatments (P < 0.001) (Table 2). In Exp. 2, as AMF inoculation strongly increased plant variables, not allowing the satisfaction of data normality and homogeneity of variances, values of mycorrhizal and NM plants were analysed separately by one-way ANOVA, followed by Student’s t test to compare the pooled means (Table 2). As expected, shoot and root P content, as well as root specific P uptake of maize plants responded strongly to mycorrhizal colonization (P < 0.001). Bacterial inoculation of NM plants significantly increased root P content (P = 0.036), with increases higher than 80% in plants inoculated with Streptomyces sp. W43N and W94, Bacillus sp. CH10 and S. meliloti TSA26. In contrast, root length specific P uptake was not influenced by bacterial inoculation (P = 0.087). In mycorrhizal plants, inoculation with Streptomyces sp. W94 significantly increased 33P content in shoots (P < 0.001), compared with the corresponding control. Bacterial inoculation significantly increased hyphal length specific 33P uptake (P < 0.001) by 37 and 39% in plants inoculated with Streptomyces sp. W94 and W77, respectively, while it decreased by 28% in plants inoculated with S. meliloti N29.
Growth responses
Here we report data on plant height, stem diameter, shoot dry weight (DW) and root DW of maize plants grown for 30 days in the experimental trials. In Exp. 1, stem diameter and shoot DW were consistently higher in mycorrhizal plants than in non-mycorrhizal controls, either inoculated or not-inoculated with bacteria. Variable responses were obtained for height and root DW (Table 3). In Exp. 2, as AMF inoculation strongly increased plant variables, not allowing the satisfaction of data normality and homogeneity of variances, values of mycorrhizal and control plants were analysed separately by one-way ANOVA, followed by Student’s t test to compare the pooled means (Table 3). Shoot and root DW, as well as maize plants height, responded strongly to AMF colonization (P < 0.001) (Table 3). In NM plants, neither shoot DW (P = 0.055) nor root DW (P = 0.069) were significantly influenced by bacterial inoculation (Table 3). In AM-colonized plants, shoot DW (P = 0.016) and root DW (P = 0.012) were significantly affected by bacterial inoculation, depending on the strains. The highest shoot DW was found in plants inoculated with Streptomyces sp. W77, which significantly differed from shoot DW of S. meliloti TSA41 and S. meliloti N29 inoculated plants. Inoculation with Streptomyces sp. W43N was the only treatment producing a significantly higher root DW, compared with AMF control plants (Table 3).
Relationships between plant growth and P uptake as influenced by microbial inoculants
In accordance with the P-limiting growth conditions, plant growth responses to mycorrhizal colonization overall mirrored the increases in shoot P contents (Fig. 2). Similar correlations between DW and P content in shoots were observed within each individual group of NM or AM-colonized plants in both experiments (Fig. 2). These correlations between shoot DW and shoot P content were significant in both NM (P < 0.001) and AMF treatments (P < 0.001) in the two experiments, suggesting that the consistent and strong effects of bacteria on maize shoot growth depend on their ability to improve plant P uptake. Shoot P content was positively correlated with 32P shoot content in Exp. 1 (P = 0.001) (Fig. 3), showing that the effect of bacterial inoculation on plant P uptake was actually related to its effect on the contribution of AMF hyphae to P uptake. A similar, but not significant (P = 0.126) correlation was observed for total P and 33P in shoots of Exp. 2.
Linear relationship between shoot dry weight and shoot P content of mycorrhizal (AM) and non mycorrhizal (NM) Z. mays L. genotype Oh43 inoculated with different bacterial strains. Results of the regression analysis are as follows: Exp. 1, NM y = 0.542x + 0.796, R2 = 0.631; AMF y = 0.768x − 2.106, R² = 0.609; Exp. 2, NM, y = 0.678x + 0.111, R² = 0.835; AMF y = 0.619x + 0.851, R² = 0.483. For abbreviations see Table 1.
Linear relationship between total 32P/33P and total P content of mycorrhizal Z. mays L. genotype Oh43 inoculated with R. irregularis in combination with different bacterial strains. Result of the regression analysis is as follows: Exp. 1, y = 0.077x + 5.748, R² = 0.535; Exp. 2, y = 0.085x + 6.831, R² = 0.303. For abbreviations see Table 1.
Discussion
This work provides the first evidence of the positive interaction among AMF and bacteria isolated from their spores, leading to increased abundance of root-external mycelium. In several cases, this resulted in increased growth and P uptake via mycorrhizal pathway in maize plants while a particularly efficient strain, Streptomyces sp. W94, facilitated P uptake by AMF hyphae from a root free soil compartment.
The marked AMF-related enhancement in P uptake and growth of the inbred maize line Oh43 is typical for plants grown under P-limiting conditions10 and the magnitude of the responses is in good agreement with previous results obtained with the same maize genotype45, 46. The key role of the root-external mycelium in AMF effects on plant P uptake was confirmed by the large uptake of 32P or 33P from the root free HC into shoots; this uptake corresponded to 12–15% and 4–9% of the radioactivity that had been added to and equilibrated with the native P pools in the HC soil in Exp. 1 and 2, respectively.
The differential effects of inoculated bacterial strains on plant performance were consistent with their previous functional characterization43. Hence, our findings that Streptomyces sp. W43N showed the higher values of root DW in AM plants and that S. meliloti TSA26, Bacillus sp. CH10, Streptomyces sp. W43N and Streptomyces sp. W94 increased root P content of NM plants are in agreement with their functional traits, as they showed the largest phosphate solubilisation and a high phytate mineralization43. Indeed, a growing number of PGPB, including Bacillus and rhizobia, have been reported to mobilize soil P from insoluble to soluble forms through two main processes: the secretion of organic anions (such as gluconic acid) chelating cations bound to phosphate and the production of phytase/phospathase enzymes able to hydrolyse organic forms of phosphate compounds47,48,49,50. Since P was the main growth limiting factor in our work, as evidenced by the low P concentration found in NM plants, approximately 1.3 mg g−1 and 1.4 mg g−1 of P in Exp. 1 and Exp. 2 respectively, the bacterial strains utilized here can be considered promising inoculants, providing a possible strategy to enhance P availability and root uptake.
In addition to their possible direct effects on P mobilization, the bacteria also influenced the AMF symbiosis. Hence, seven out of 10 bacterial strains significantly improved hyphal length growth and two of the strains, S. meliloti TSA41 and Streptomyces sp. W43N, increased hyphal length as much as 24% over the levels measured in AMF plants without bacterial inoculation. Such strains, belonging to the gram-negative Proteobacteria and gram-positive Firmicutes and Actinomycetes, could thus be classified as mycorrhiza helper bacteria (MHB) for their promotion of mycorrhizal functioning51. Previous studies showed that different species of Streptomyces stimulated spore germination of G. margarita through the production of volatile compounds52, that Brevibacillus sp. promoted mycorrhizal colonization by F. mosseae in Trifolium pratense 53 and that Paenibacillus rhizosphaerae, Azospirillum sp., and Rhizobium etli significantly increased the growth of extraradical hyphae and the number of newly formed spores of R. irregularis cultivated in vitro 54. Another recent study reported that several Pseudomonas strains could stimulate the in vitro growth of R. irregularis ERM55. Our data are particularly interesting, as they were obtained in vivo, by a method which, though labour intensive13, allows the quantification of the actual abundance of AMF hyphae in the soil. Bacterial enhancement of their growth is consistent with a previous study where inoculation of P. putida stimulated the growth of Glomus fistulosum extraradical hyphae, although AM colonization was not improved56. Similarly, P. fluorescens DF57 and Burkolderia cepacia Bc2 enhanced mycelial development of G. caledonium and R. irregularis, respectively57, 58. So far, the mechanisms underlying such outcome remain unknown. However, there is evidence to support the hypothesis that bacterial production of IAA and indole butyric acid (IBA) play roles in AMF hyphal elongation. For example, IAA-producing Paenibacillus strains increased Glomus intraradices development59 while the exogenous application of IBA promoted the ERM of Diversispora versiformis in association with trifoliate orange60.
In this work, plants inoculated with AMF, alone or in combination with bacteria, significantly enhanced root development. Specifically, in Exp. 2, root length of mycorrhizal maize plants was three fold the values for non-mycorrhizal plants. Interestingly, the root length in NM plants was increased up to 53% by inoculation with Bacillus sp. CH10. Such results are consistent with recent reports on the in vitro production of IAA by Bacillus sp. CH1043. Indole acetic acid is an important phytohormone, playing a key role in plant growth by affecting many functional activities, such as cell division, elongation, root initiation and fruit development61. So far, a number of strains belonging to different species, including several Bacillus species, have been reported to synthesize IAA. For instance, IAA producing B. flexus P4 and Bacillus sp. S6 increased Solanum tuberosum root length by 40 and 50%, respectively, compared with non-inoculated control62, while S. meliloti TSA41 and Streptomyces sp. W43N highly improved the levels of IAA in Dark Opal basil plants, in comparison with controls63.
Radioactive P tracers (32P and 33P) are widely applied to study soil P availability as well as P uptake from different soil and fertilizer P sources64. However, no studies have utilized isotopic techniques to assess the interactions between AMF and their strictly associated bacteria and to determine their contribution to plant P uptake, while only a few works investigated the effects of rhizobacteria on P nutrition65,66,67. Here, the labelling of HC soil with radioactive P (32P or 33P) made it possible to distinguish between P uptake by AM hyphae and P uptake by roots and hyphae in combination and to detect a wide variation among the bacterial strains in their ability to improve P uptake via mycorrhizal pathway. These findings are consistent with the monitoring data for shoot radioactivity, showing that AM plants had higher counts in the presence than in the absence of inoculated bacteria at 16, 18, 21 and 23 d after sowing. Interestingly, both shoot 33P and hyphal length specific 33P uptake was greater in AM plants inoculated with Streptomyces sp. W94, than in the corresponding no bacteria control, while Streptomyces sp. W77 enhanced hyphal length specific 33P uptake. In our work, the bacterial strains were applied directly to the seed and after 15 days to the seedlings instead to the hyphal compartment. As such bacteria may be associated not only to AMF spores, but also to plant roots, further studies could show whether their effects on plant growth and P uptake are maintained when inoculated far from the roots, for example in the root-free compartment.
The influence of bacterial inoculation on the AMF-mediated P uptake from the HC was further supported by the significant positive correlation between shoot P content and shoot 32P. These data suggest that the mechanism underlining the higher shoot 33P content of bacteria inoculated plants was less related to phosphate solubilization than to the bacterial induction of greater root length and/or hyphal abundance. Our results are consistent with previous findings obtained using isotopic-labeling technique, where P. protegens CHA0 did not enhance P release from soil, although it was able to solubilize P in vitro 67. A number of studies reported that in vitro P solubilization by microorganisms does not necessarily imply that inoculated plants will grow better. For instance, Taurian et al.68 screened 110 potential P solubilizing bacteria in vitro but only one of them promoted peanut growth. Thus, P mobilization by PGP bacteria is a complex phenomenon that can be ascribed to multiple functional traits differentially affecting plant growth. Indeed, a large array of bacteria belonging to different genera was reported to indirectly improve P uptake by altering root architecture through IAA production69.
In conclusion, root colonization by AMF produced large plant growth responses, while seven bacterial strains further facilitated root growth and P uptake by promoting the development of AMF extraradical mycelium. Among the tested strains, Streptomyces sp. W94 produced the largest increases in uptake and translocation of 33P, while Streptomyces sp. W77 highly enhanced hyphal length specific uptake of 33P. The positive relationship between AMF-mediated P absorption and shoot P content was significantly influenced by the bacteria inoculants and such results emphasize the potential importance of managing both AMF and their microbiota for improving P acquisition by crops. Our findings show that interactions among plants, AMF and mycorrhizospheric bacteria are complex and may produce different outcomes, depending on the composition of the microbial inoculum. A future focus on the interactions between different plant, AMF and bacteria genotypes, including effects of growth conditions will deepen our knowledge of the functioning of such multipartite interactions and help us to select the most efficient consortia to develop biofertilizers and bioenhancers for sustainable agriculture systems.
Methods
Experiment 1
The Exp. 1 was set up using compartmented pots with a root hyphal compartment (RHC) and a hyphal compartment (HC)17, 70. RHC was a 1.5 kg plastic pot lined with a plastic bag, while HC was a small plastic cylinder capped at both ends with a 25 µm nylon mesh. This mesh allowed fungal hyphae to pass through and absorb nutrients but prevented the ingrowth of roots from RHC. The growth medium used was 1:1 mixture of γ-irradiated soil (15 kGy) and autoclaved quartz sand. The soil was a local low P sandy loam and had the following characteristics: 10% clay, 12% silt, 46% fine sand, 30% coarse sand, 1.2% total Organic Carbon, 10 mg P kg−1 0.5 M NaHCO3 extractable P71. In order to ensure an adequacy of all nutrients but P, the growth medium, hereafter called soil, was supplemented with basal nutrients minus P. Soil for the HC, was uniformly labelled with 5 kBq g−1 soil of carrier-free H3 32PO4. The RHC was filled with 1445 g of unlabelled soil while the HC was filled with 55 g of 32P-labelled soil and placed horizontally at the same depth in all pots.
Uniformly sized seeds of Zea mays L., inbred line Oh43, were surface sterilized twice in 1.5% (v/v) sodium hypochlorite solution for 10 min and rinsed thoroughly with sterile MilliQ water. The seeds were imbibed 2 h in sterile water and pre-germinated on moist paper in Petri dishes kept in darkness for 2 days at 27 °C until radicles emerged. The germinated seeds were then sown, one per 1.5 kg pot, at approximately 2 cm depth.
Half of the pots were inoculated with the AM fungus R. irregularis Schenck and Smith BEG 87 that had been propagated in pot cultures with Trifolium subterraneum L. for four months in the same soil as described above. The trap plants were well colonized and had produced high quantities of spores and extraradical mycelium. Pot cultures were air dried, shoots were removed and a crude inoculum was prepared by mixing the soil with chopped mycorrhizal roots, extraradical mycelium, and spores and thereafter stored at 4 °C. Ten per cent (w/w) of this crude inoculum was mixed into the RHC soil. RHCs of non-mycorrhizal plants received a mock inoculum produced by sterilizing the appropriate amount of mycorrhizal inoculum. All pots, non-mycorrhizal and mycorrhizal, received 20 mL of soil filtrate (through Whatman paper), made by dissolving 200 g of BEG 87 inoculum in 1500 mL water, to ensure a common microbiota to all treatments.
Three bacterial strains, S. meliloti TSA3, S. meliloti TSA41 and Lysinibacillus fusiformis CH19 were used to inoculate the plants, alone or in combination with the mycorrhizal inoculum. The bacteria were previously isolated from R. irregularis IMA6 (formerly G. intraradices)43 spores and were selected for their plant growth promoting (PGP) properties, such as the production of indole acetic acid (IAA) and siderophores, phosphatase-solubilizing and phytase-mineralizing activities. Each bacterial strain was cultured and inoculum prepared as previously reported72. For each strain, bacterial density of the suspension was assessed using a Thoma cell chamber to obtain a final cell concentration of 109 cells mL−1. At sowing, inoculation was performed by adding on the surface of sterilized seeds 1.5 mL of 109 cells mL−1. Ten days after sowing, seedlings were inoculated with the same amount of bacterial suspension. The same amount of physiological saline solution was provided to uninoculated plants.
The experiment combined mycorrhizal and bacterial inoculation. The four AMF treatments were: inoculation with R. irregularis and inoculation with the bacterial strains S. meliloti TSA3, S. meliloti TSA41, L. fusiformis CH19. The corresponding non-AMF treatments were set up. Each of the resulting eight treatments had five biological replicates.
Plants were grown in a controlled environment chamber at the Technical University of Denmark, Risø Campus, under a day/night cycle of 14/10 h, 25/20 °C, 60% relative humidity and an average light intensity of 500 μmol m−2 sec−1. Plants were watered by weight to 60% of water-holding capacity over the course of the experiment and nitrogen (as NH4NO3) was added to the pots 16, 19, and 22 days after sowing, providing a total of 195 mg N per pot. Uptake of 32P from HC compartments was monitored by a G-M tube (Mini 900 Meter, Thermo Fisher Scientific, Waltham, Ma, USA) on the middle part of the youngest fully developed leaf at 1–3 days intervals between 13 and 28 days after planting.
Experiment 2
The set up and growth conditions of Exp. 2 were similar to those of Exp. 1, except that nine bacterial treatments were included: inoculation with S. meliloti TSA41, S. meliloti TSA26, Streptomyces sp. W43N, Streptomyces sp. W64, Streptomyces sp. W77, Streptomyces sp. W94, S. meliloti N29, Bacillus sp. pumilus group CH10 or no inoculation. As for Exp. 1, each treatment had five biological replicates. In Exp. 2, the HC soil was labelled with 5 kBq g−1 soil of carrier-free H3 33PO4 and plants were maintained in a growth chamber at University of Copenhagen.
Harvest and samples analysis
Harvest procedures and measurements were similar in the two experiments. Plant height and stem diameter (only in Exp. 1) were measured just before harvest, 30 days after sowing. Shoots were cut at soil surface level and weighed and HCs were removed from the pots and stored frozen. Soil from the HCs was dried at 50 °C for 24 h and used for the determination of hyphal length density (HLD). HLD was measured following the membrane filter procedure described in Jakobsen et al.13.
Roots were carefully washed free of soil, blotted and total fresh weight was determined. A weighed representative subsample for assessment of AM colonization was stored in 50% ethanol. Dry weights of shoots and roots were recorded after 48 h drying at 70 °C in a ventilated oven. Shoot and root tissues were ground to powder and wet oxidised in a solution of nitric and perchloric acids (4:1, v-v). Digests were analysed for P concentration by the molybdenum blue method (Murphy and Riley, 1962) using AutoAnalyzer 3 (Bran + Luebbe, Norderstedt, Germany) for Exp. 1 and FiaStar 5000 Analyzer (Foss Analytical AB, Höganäs, Sweden) for Exp. 2. The radioactivity (32P or 33P) in the digests was determined by liquid scintillation counting using the scintillation mix Ultima Gold ™ with TriCarb 1900 TR for Exp. 1 and TriCarb 2910 TR for Exp. 2 (Perkin Elmer, Waltham, MA, USA); counts were corrected for decay and background. Radioactivity and P concentration was used to calculate the specific activity (SA) in the shoots (32P/31P or 33P/31P).
Percentages of AMF colonization and total root length were assessed under a dissecting microscope using the gridline intersect method73 after clearing of roots in 10% KOH and staining with Trypan blue in lactic acid (0.05%).
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM Corp., Armon, NY Inc, USA). Data were analysed by one-way ANOVA, using treatment as factor, followed by Fisher’s least significant difference (LSD) with cut-off significance at P < 0.05. Data were transformed as needed to obtain normality and homogeneity of variances. If a normal distribution and/or homogeneity of data could not be guaranteed, we performed a Student’s t test to compare means between NM and AMF plants. The means within each mycorrhizal treatment were then analysed by one-way ANOVA followed by LSD or Dunnett’s T3 post-hoc test (where equal variances were not assumed). Correlations among shoot biomass, P content, P uptake and HDL were examined by linear regression analysis (P < 0.05). In Exp. 1 four seedlings that died were treated as missing values whilst in Exp. 2 two extreme outliers and two plants with abnormal growth were excluded from all analyses. This resulted in 36 and 86 pots in Exp. 1 and Exp. 2, respectively.
References
Gruhn, P., Goletti, F. & Yudelman, M. Integrated nutrient management, soil fertility, and sustainable agriculture: current issues and future challenges. International Food Policy Research Institute (2000).
Lal, R. Climate strategic soil management. Challenges 5, 43–74 (2014).
Plenchette, C., Clermont-Dauphin, C., Meynard, J. M. & Fortin, J. A. Managing arbuscular mycorrhizal fungi in cropping systems. Can. J. Plant Sci. 85, 31–40 (2005).
Chen, M. & Graedel, T. E. A half-century of global phosphorus flows, stocks, production, consumption, recycling, and environmental impacts. Glob. Environ. Change 36, 139–152 (2016).
Raghothama, K. G. Phosphate acquisition. Annu. Rev. Plant Biol. 50, 665–693 (1999).
Schachtman, D. P., Reid, R. J. & Ayling, S. M. Phosphorus uptake by plants: from soil to cell. Plant Physiol. 116, 447–453 (1998).
Richardson, A. E. et al. Utilization of soil organic phosphorus by higher plants. Organic phosphorus in the environment, 165–184 (CABI, Wallingford, 2005).
Karandashov, V. & Bucher, M. Symbiotic phosphate transport in arbuscular mycorrhizas. Trends Plant Sci. 10, 22–29 (2005).
Philippot, L., Raaijmakers, J. M., Lemanceau, P. & van der Putten, W. H. Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 11, 789–799 (2013).
Smith, S. E. & Read, D. J. Mycorrhizal symbiosis (Academic Press, San Diego, 2008).
Giovannetti, M. & Avio, L. Biotechnology of arbuscular mycorrhizas. Applied mycology and biotechnology: agriculture and food production. (eds) Khachatourians, G. G. & Arora, D. K. 275–310 (Elsevier, Amsterdam, 2002).
Mikkelsen, B. L., Rosendahl, S. & Jakobsen, I. Underground resource allocation between individual networks of mycorrhizal fungi. New Phytol. 180, 890–898 (2008).
Jakobsen, I. & Rosendahl, L. Carbon flow into soil and external hyphae from roots of mycorrhizal cucumber plants. New Phytol. 115, 77–83 (1990).
Giovannetti, M. et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 107, 242–251 (2012).
Garcia, K. & Zimmermann, S. D. The role of mycorrhizal associations in plant potassium nutrition. Front. Plant Sci. 5, 337 (2014).
Lehmann, A. & Rillig, M. C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops–A meta-analysis. Soil Biol. Biochem. 81, 147–158 (2015).
Smith, S. E., Smith, F. A. & Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. New Phytol. 162, 511–524 (2004).
Avio, L., Pellegrino, E., Bonari, E. & Giovannetti, M. Functional diversity of arbuscular mycorrhizal fungal isolates in relation to extraradical mycelial networks. New Phytol. 172, 347–357 (2006).
Schnepf, A., Roose, T. & Schweiger, P. Impact of growth and uptake patterns of arbuscular mycorrhizal fungi on plant phosphorus uptake—a modelling study. Plant Soil 312, 85–99 (2008).
Pepe, A., Giovannetti, M. & Sbrana, C. Different levels of hyphal self-incompatibility modulate interconnectedness of mycorrhizal networks in three arbuscular mycorrhizal fungi within the Glomeraceae. Mycorrhiza 26, 325–332 (2016).
Harrison, M. J. & van Buuren, M. L. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 378, 626–629 (1995).
Maldonado-Mendoza, I. E., Dewbre, G. R. & Harrison, M. J. A phosphate transporter gene from the extraradical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol. Plant Microbe In. 14, 1140–1148 (2001).
Govindarajulu, M. et al. Nitrogen transfer in the arbuscular mycorrhizal symbiosis. Nature 435, 819–823 (2005).
Viereck, N., Hansen, P. E. & Jakobsen, I. Phosphate pool dynamics in the arbuscular mycorrhizal fungus Glomus intraradices studied by in vivo 31P NMR spectroscopy. New Phytol. 162, 783–794 (2004).
Hijikata, N. et al. Polyphosphate has a central role in the rapid and massive accumulation of phosphorus in extraradical mycelium of an arbuscular mycorrhizal fungus. New Phytol. 186, 285–289 (2010).
Kikuchi, Y. et al. Aquaporin‐mediated long‐distance polyphosphate translocation directed towards the host in arbuscular mycorrhizal symbiosis: application of virus‐induced gene silencing. New Phytol. 211, 1202–1208 (2016).
Pearson, V. & Tinker, P. B. Measurements of phosphorus fluxes in the external hyphae of endomycorrhizas [Sanders, F. E., Mosse, B., Tinker, P. B. (eds)] (Endomycorrhizas Academic, London, 1975).
Cooper, K. M. & Tinker, P. B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas II. Uptake and translocation of phosphorus, zinc and sulphur. New Phytol. 81, 43–52 (1978).
Cooper, K. M. & Tinker, P. B. Translocation and transfer of nutrients in vesicular-arbuscular mycorrhizas IV. Effect of environmental variables on movement of phosphorous. New Phytol. 88, 327–339 (1981).
Logi, C., Sbrana, C. & Giovannetti, M. Cellular events involved in survival of individual arbuscular mycorrhizal symbionts growing in the absence of the host. Appl. Environ. Microb. 64, 3473–3479 (1998).
Giovannetti, M., Sbrana, C. & Logi, C. Microchambers and video-enhanced light microscopy for monitoring cellular events in living hyphae of arbuscular mycorrhizal fungi. Plant Soil 226, 153–159 (2000).
Roesti, D. et al. Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl. Environ. Microb. 71, 6673–6679 (2005).
Long, L., Zhu, H., Yao, Q. & Ai, Y. Analysis of bacterial communities associated with spores of Gigaspora margarita and Gigaspora rosea. Plant Soil 310, 1–9 (2008).
Naumann, M., Schüssler, A. & Bonfante, P. The obligate endobacteria of arbuscular mycorrhizal fungi are ancient heritable components related to the Mollicutes. ISME J. 4, 862–871 (2010).
Desirò, A. et al. Detection of a novel intracellular microbiome hosted in arbuscular mycorrhizal fungi. ISME J. 8, 257–270 (2014).
Agnolucci, M., Battini, F., Cristani, C. & Giovannetti, M. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol. Fert. Soils 51, 379–389 (2015).
Rouphael, Y. et al. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 196, 91–108 (2015).
Barea, J. M., Azcon, R. & Azcon-Aguilar, C. Mycorrhizosphere interactions to improve plant fitness and soil quality. Anton. Van Leeuw. 81, 343–351 (2002).
Xavier, L. J. C. & Germida, J. J. Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol. Biochem. 35, 471–478 (2003).
Bharadwaj, D. P., Lundquist, P. O., Persson, P. & Alström, S. Evidence for specificity of cultivable bacteria associated with arbuscular mycorrhizal fungal spores. FEMS Microbiol. Ecol. 65, 310–322 (2008).
Bharadwaj, D. P., Lundquist, P. O. & Alström, S. Arbuscular mycorrhizal fungal spore-associated bacteria affect mycorrhizal colonization, plant growth and potato pathogens. Soil Biol. Biochem. 40, 2494–2501 (2008).
Cruz, A. F. & Ishii, T. Arbuscular mycorrhizal fungal spores host bacteria that affect nutrient biodynamics and biocontrol of soil-borne plant pathogens. Biol. Open 1, 52–57 (2011).
Battini, F., Cristani, C., Giovannetti, M. & Agnolucci, M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol. Res. 183, 68–79 (2016).
Klingner, A., Bothe, H., Wray, V. & Marner, F. J. Identification of a yellow pigment formed in maize roots upon mycorrhizal colonization. Phytochemistry 38, 53–55 (1995).
Sawer, R. J. H. et al. Phosphorus acquisition efficiency in arbuscular mycorrhizal maize is correlated with the abundance of root-external hyphae and the accumulation of transcripts encoding PHT1 phosphate transporters. New Phytol. doi:10.1111/nph.14403 (2017).
Kaeppler, S. M. et al. Variation among maize inbred lines and detection of quantitative trait loci for growth at low phosphorus and responsiveness to arbuscular mycorrhizal fungi. Crop Sci. 40, 358–364 (2000).
Jones, D. L., Oburger, E. Solubilization of phosphorus by soil microorganism: Phosphorus in Action [Buenemann, E. K., Oberson, A., Frossard, E. (eds)] [169–198] (Springer, New York, 2011).
Jorquera, M. A. et al. Identification of β-propeller phytase-encoding genes in culturable Paenibacillus and Bacillus spp. from the rhizosphere of pasture plants on volcanic soils. FEMS Microbiol. Ecol. 75, 163–172 (2011).
Oberson, A., Pypers, P., Bünemann, E. K., Frossard, E. Management impacts on biological phosphorus cycling in cropped soils. Phosphorus in Action. [Buenemann, E.K., Oberson, A., Frossard, E. (eds)] [169–198] (Springer, New York, 2011).
Owen, D., Williams, A. P., Griffith, G. W. & Withers, P. J. A. Use of commercial bio-inoculants to increase agricultural production through improved phosphorous acquisition. Appl. Soil Ecol. 86, 41–54 (2015).
Frey‐Klett, P., Garbaye, J. A. & Tarkka, M. The mycorrhiza helper bacteria revisited. New Phytol. 176, 22–36 (2007).
Tylka, G. L., Hussey, R. S. & Roncadori, R. W. Axenic germination of vesicular–arbuscular mycorrhizal fungi: effects of selected Streptomyces species. Phytopathology 81, 754–759 (1991).
Vivas, A., Azcón, R., Biró, B., Barea, J. M. & Ruiz-Lozano, J. M. Influence of bacterial strains isolated from lead-polluted soil and their interactions with arbuscular mycorrhizae on the growth of Trifolium pratense L. under lead toxicity. Can. J. Microbiol. 49, 577–588 (2003).
Bidondo, L. F. et al. Cultivable bacteria associated with infective propagules of arbuscular mycorrhizal fungi. Implications for mycorrhizal activity. Appl. Soil Ecol. 105, 86–90 (2016).
Ordoñez, Y. M. et al. Bacteria with phosphate solubilizing capacity alter mycorrhizal fungal growth both inside and outside the root and in the presence of native microbial communities. PloS one 11, e0154438 (2016).
Gryndler, M. & Vosátka, M. The response of Glomus fistulosum–maize mycorrhiza to treatments with culture fractions from Pseudomonas putida. Mycorrhiza 6, 207–211 (1996).
Ravnskov, S. & Jakobsen, I. Effects of Pseudomonas fluorescens DF57 on growth and P uptake of two arbuscular mycorrhizal fungi in symbiosis with cucumber. Mycorrhiza 8, 329–334 (1999).
Ravnskov, S., Larsen, J. & Jakobsen, I. Phosphorus uptake of an arbuscular mycorrhizal fungus is not effected by the biocontrol bacterium Burkholderia cepacia. Soil Biol. Biochem 34, 1875–1881 (2002).
Bidondo, L. F. et al. Pre-symbiotic and symbiotic interactions between Glomus intraradices and two Paenibacillus species isolated from AM propagules. In vitro and in vivo assays with soybean (AG043RG) as plant host. Soil Biol. Biochem. 43, 1866–1872 (2011).
Liu, C.-Y., Srivastava, A. K., Zhang, D.-J., Zou, Y.-N. & Wu, Q.-S. Exogenous phytohormones modulate mycorrhiza-induced changes in root hair configuration of trifoliate orange. Not. Bot Horti Agrobo 44, 548–556.
Duca, D., Lorv, J., Patten, C. L., Rose, D. & Glick, B. R. Indole-3-acetic acid in plant–microbe interactions. Anton. Van Leeuw. 106, 85–125 (2014).
Ahmed, A. & Hasnain, S. Auxin-producing Bacillus sp.: auxin quantification and effect on the growth of Solanum tuberosum. Pure Appl. Chem. 82, 313–319 (2010).
Battini, F. et al. Dual inoculation with AMF and associated bacteria improves nutraceutical value of sweet basil grown under commercial conditions. Agrochimica 60, 81–99 (2016).
Frossard, E. et al. The use of tracers to investigate phosphate cycling in soil plant systems. Phosphorus in Action (eds.) Bünemann, E., Oberson, A. & Frossard, E. 59–91 (Springer, New York, 2011).
Toro, M., Azcon, R. & Barea, J. Improvement of arbuscular mycorrhiza development by inoculation of soil with phosphate-solubilizing rhizobacteria to improve rock phosphate bioavailability 32P and nutrient cycling. Appl. Environ. Microbiol. 63, 4408–4412 (1997).
Barea, J. M., Toro, M., Orozco, M. O., Campos, E. & Azcón, R. The application of isotopic (32P and 15N) dilution techniques to evaluate the interactive effect of phosphate-solubilizing rhizobacteria, mycorrhizal fungi and Rhizobium to improve the agronomic efficiency of rock phosphate for legume crops. Nutr. Cycl. Agroecosys. 63, 35–42 (2002).
Meyer, G. et al. Gross phosphorus fluxes in a calcareous soil inoculated with Pseudomonas protegens CHA0 revealed by 33P isotopic dilution. Soil Biol. Biochem. 104, 81–94 (2017).
Taurian, T. et al. Phosphate-solubilizing peanut associated bacteria: screening for plant growth-promoting activities. Plant Soil 329, 421–431 (2010).
Marschner, P., Crowley, D. & Rengel, Z. Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol. Biochem. 43, 883–894 (2011).
Smith, S. E., Smith, F. A. & Jakobsen, I. Mycorrhizal fungi can dominate phosphate supply to plants irrespective of growth responses. Plant Physiol. 133, 16–20 (2003).
Olsen, S. R., Cole, C.V., Watanabe, W. S. & Dean, L. A. Estimation of available phosphorus in soil by extraction with sodium bicarbonate. USDA Circular 939 (U.S. Government Printing Office Washington, DC., 1954).
Battini, F., Bernardi, R., Turrini, A., Agnolucci, M. & Giovannetti, M. Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza 26, 699–707 (2016).
Giovannetti, M. & Mosse, B. An evaluation of techniques for measuring vesicular–arbuscular mycorrhizal infection in roots. New Phytol. 84, 489–500 (1980).
Acknowledgements
The authors are thankful to Mette Flodgaard, Lena Asta Byrgesen and Ayse Gül Ata for their excellent technical assistance, to Ruairidh Sawers, LANGEBIO, CINVESTAV-IPN, Irapuato, Guanajuato, Mexico for kindly providing the Oh43 seeds, and to Luciano Avio for his assistance in statistical analyses. Funding was provided by a University of Pisa grant (Fondi di Ateneo) and by Innovation Fund Denmark, grant number 1308-00016B.
Author information
Authors and Affiliations
Contributions
Experiments were conceived by F.B., I.J. and Ma.Gio. and conducted by F.B. and Me.Grø. F.B. drafted the original manuscript and M.A., Ma.Gio. and I.J. reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Battini, F., Grønlund, M., Agnolucci, M. et al. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci Rep 7, 4686 (2017). https://doi.org/10.1038/s41598-017-04959-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04959-0
This article is cited by
-
Mass Production of Arbuscular Mycorrhizal Fungi on the Sorghum Plants Inoculated with Burkholderia arboris Using Soybean Mill Waste and Vermicompost-Amended Soil–Sand Substrate
Current Microbiology (2024)
-
Bacterial community changes in the presence of AMF in the context of maize with low phosphorus content
Journal of Soils and Sediments (2024)
-
Enhancing Phyllostachys edulis seedling growth in phosphorus-deficient soil: complementing the role of phosphate-solubilizing microorganisms with arbuscular mycorrhizal fungi
Plant and Soil (2024)
-
Short-Term Evaluation of the Spatial Distribution of Trophic Groups of Amoebae in the Rhizosphere of Zea mays Inoculated with Rhizophagus intraradices
Microbial Ecology (2023)
-
Lemon balm and kidney bean intercropping: the potential for incorporating AMF for sustainable agricultural production
International Journal of Environmental Science and Technology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.