Abstract
Previous studies have demonstrated that alcohol consumption impairs neuroplasticity in the motor cortex. However, it is unknown whether alcohol produces a similar impairment of neuroplasticity in the dorsolateral prefrontal cortex (DLPFC), a brain region that plays an important role in cognitive functioning. The aim of the current study was to evaluate the effect of alcohol intoxication on neuroplasticity in the DLPFC. Paired associative stimulation (PAS) combined with electroencephalography (EEG) was used for the induction and measurement of associative LTP-like neuroplasticity in the DLPFC. Fifteen healthy subjects were administered PAS to the DLPFC following consumption of an alcohol (1.5 g/l of body water) or placebo beverage in a within-subject cross-over design. PAS induced neuroplasticity was indexed up to 60 minutes following PAS. Additionally, the effect of alcohol on PAS-induced potentiation of theta-gamma coupling (an index associated with learning and memory) was examined prior to and following PAS. Alcohol consumption resulted in a significant impairment of mean (t = 2.456, df = 13, p = 0.029) and maximum potentiation (t = −2.945, df = 13, p = 0.011) compared to the placebo beverage in the DLPFC and globally. Alcohol also suppressed the potentiation of theta-gamma coupling by PAS. Findings from the present study provide a potential neurophysiological mechanism for impairment of cognitive functioning by alcohol.
Similar content being viewed by others
Introduction
Alcohol consumption produces a number of neurochemical and neurophysiological changes in the brain. Acute alcohol consumption is broadly defined as consumption of any given volume of alcohol over a short period of time. Binge drinking is defined more specifically by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) as “…a pattern of drinking that brings blood alcohol concentration (BAC) levels to 17.4 mM (0.08% BAC). This typically occurs after 4 drinks for women and 5 drinks for men—in about 2 hours”. (http://www.niaaa.nih.gov/)”. Multiple lines of evidence suggest that acute consumption of alcohol (including binge drinking) results in an increase in GABAA receptor mediated neurotransmission1,2,3,4 and a decrease in NMDA receptor mediated transmission5,6,7. These aberrancies in neurotransmission with alcohol use may adversely affect neuroplasticity, which is dependent on both GABAA and NMDA receptor activities8, 9.
Paired associative stimulation (PAS) is a transcranial magnetic stimulation (TMS) protocol that can be used to index long-term potentiation (LTP)-like neuroplasticity in vivo. Previous PAS studies have demonstrated that alcohol impairs neuroplasticity indexed from the motor cortex among healthy subjects10, 11. Our group examined the effect of alcohol intoxication (at doses of alcohol high enough to constitute heavy drinking) in 15 subjects up to 60 minutes and 24 hours following PAS in a within-subject crossover design study. Consumption of high doses of alcohol significantly suppressed LTP-like neuroplasticity at 30 minutes and 60 minutes following PAS administration compared to placebo11.
Findings from previous studies suggest that alcohol consumption affects neurophysiology in the dorsolateral prefrontal cortex (DLPFC) (for review, see ref. 12). The DLPFC is a region in the brain within the mesocortico-limbic pathway that is implicated in the pathophysiology of addiction13. The DLPFC plays an important role in reward processing to guide behaviour and mediates cognitive functioning including working memory (for review, see ref. 14). Given that neuroplasticity is the principle mechanism underlying learning and memory important in cognitive functioning15, 16, indexing the effect of consuming high doses of alcohol on DLPFC neuroplasticity may reveal a potential mechanism of cognitive dysfunction observed during alcohol intoxication.
Despite the important implications of neuroplasticity impairment by alcohol in the DLPFC, no study to date has examined the effect of alcohol intoxication on neuroplasticity in this region in humans in vivo. Our group has demonstrated that LTP-like neuroplasticity can be indexed from the DLPFC by combining PAS with electroencephalography (EEG)17. EEG allows for the measurement of cortical evoked activity (CEA), which is the net brain activity to a stimulus. Potentiation of CEA by PAS is therefore indicative of an increase in brain activity, reflective of LTP-like neuroplasticity. This potentiation of CEA was associated with an increase in coupling of cortical oscillations of theta amplitude and gamma phase (theta-gamma coupling)17, believed to be associated with the working memory function of the DLPFC18. This novel technique holds the promise of revealing the effect of alcohol on neuroplasticity in the DLPFC and allows for the indexing theta-gamma coupling.
The goal of our study was to examine the effect of alcohol intoxication on neuroplasticity in the DLPFC in fifteen healthy subjects using PAS with EEG. Additionally, the effect of alcohol intoxication on PAS potentiation of theta-gamma coupling was examined. By the definition provided by the NIAAA, the acute alcohol consumption in the present study would also meets criteria for a binge drinking episode.
Materials and Methods
Study Design
The current study was a within-subject, randomized cross-over study design consisting of a total of two study visits following enrollment into the study. These visits consisted of one study visit where subjects received the alcohol beverage and one study visit where subjects received the placebo beverage. During both study visits, PAS was administered to the DLPFC and EEG was collected. The order of the alcohol and placebo study visits was randomized with a minimum one-month washout period between beverage testing visits.
Study Visits
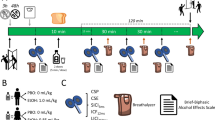
At the beginning of all study visits, breath measures of BAC and carbon monoxide were obtained and urine drug screens were administered. Females of child bearing age were administered urine pregnancy tests before study visits. The resting motor threshold (RMT) and the stimulus intensity required to produce an average motor evoked potential (MEP) amplitude of 1 mV (1mVT1) was obtained. The DLPFC was identified using the F5 electrode as the marker19. To assess baseline cortical evoked activity (CEA), a train of 100 pulses at 0.1 Hz were delivered at stimulus intensity 1mVT1 pre PAS to the DLPFC (PrePAS) and EEG was collected. Subjects were then given 15 min to consume the beverage to achieve a rapid increase in BAC. Their BAC was obtained via breath measures every 15 minutes after beverage consumption and when BAC was ≥ 17.4 mM (≥0.08%), the intensity required to produce an average motor evoked potential (MEP) amplitude of 1 mV (1mVT2) was reassessed. To assess CEA following the beverage, another 100 pulses were administered to the DLPFC at stimulus intensity 1mVT2. PAS was then administered to the DLPFC. Immediately after the termination of PAS, a train of 100 pulses of TMS at 0.1 Hz frequency were delivered to the DLPFC at (Post 0), 15 minutes later (Post 15), 30 minutes post PAS (Post 30) and 60 minutes post PAS (Post 60) while EEG was collected to assess potentiation of CEA following PAS (Fig. 1).
Study design. Study visits (alcohol/placebo) were randomized with a 1 month washout period between study visits. 100 TMS pulses were administered to the DLPFC at 1 mV stimulus intensity prior to beverage consumption (SI1mVT1). Subjects were given 15 minutes to consume the beverage. BAC was obtained every 15 minutes following beverage consumption (except during PAS). Another 100 TMS pulses were administered following beverage consumption and stimulus intensity was adjusted if necessary (SI1mVT2). PAS was then administered using SI1mVT2, followed by 100 TMS pulses also at SI1mVT2 at Post 0 (immediately following PAS), Post 15 (15 minutes following PAS), Post 30 (30 minutes following PAS) and Post 60 (60 minutes following PAS). EEG was collected at all time points to measure CEA.
Subjects
Subjects were recruited through ads on-line and ads posted at the University of Toronto. All subjects provided written informed consent prior for participation in the study. All experiments were conducted in accordance with the Declaration of Helsinki and were approved by the research ethics board at the Centre for Addiction and Mental Health. Fifteen healthy drinkers participated in the study (mean age 33.42, ± 7.52, 23–46 years of age, 10 Males). Subjects had endorsed at least one heavy drinking episode (defined at 5 standard drinks for men and 4 standard drinks for women) (“NIAAA Guidelines,” 2004), within the last month, as assessed using the Alcohol Timeline Follow-Back20. This criteria was used to ensure that subjects would be able to tolerate the high doses of alcohol administered in the study. Subjects were between the age of 19 and 60 years of age and were of legal drinking age in Ontario, Canada. Subjects were non-smokers (had not smoked any cigarettes in the last three months) and did not meet DSM-IV criteria for any current drug abuse or dependence or any psychiatric disorders. Subjects were excluded if they had a history of seizures, neurological disease or cognitive impairment21 and none reported regular use of any therapeutic or recreational psychoactive drugs during the last three months. Subjects reported having had on average 4.5 ± 4.9 heavy drinking episodes. Therefore while the subjects were otherwise healthy, they would be classified as binge drinkers with an increased risk for developing AUDs. Table 1 includes the demographic information of the subjects.
Beverages
Beverages were prepared as described in Loheswaran et al., 201522. The alcohol beverage was made using 95% United States Pharmacopeia (USP) alcohol at a dose of 1.5 g/l of body water and mixed in a 1:5 ratio with orange juice and tonic water. The placebo beverage was made from an equivalent volume of orange juice and tonic water. Absolut Vodka (0.2 mL of 40% alcohol) was added to both beverages immediately prior to administering the beverage to the subject to produce the odour of alcohol but is minimal enough to not produce any additional alcohol effects. Investigators were blinded to the beverage type until after the first BAC measure was obtained 15 minutes following beverage consumption. Subjects were also blinded to the beverage type. All subjects were asked to guess which beverage they had received 15 minutes after beverage consumption and all subjects correctly guessed which beverage they had received.
BAC and CO Measurements
BAC was measured with an Alco-Sensor FST (DAVTECH Analytic Services, Canada) at the beginning of all study visits and at 15 min intervals following beverage consumption (except during PAS administration). A Micro + ™ Smokerlyzer® CO monitor (Bedfont Scientific Ltd.) was used to obtain CO measures at the beginning of all study visits.
Neurophysiological Assessment
TMS Stimulation
TMS pulses were administered to the left motor cortex to obtain the resting motor threshold and 1 mV intensity and to the left DLPFC (for PrePAS, PAS and PostPAS) using a 7 cm figure-of-eight coil, and a Magstim 200 stimulator (Magstim Company Ltd., UK) connected via a Bistim module and electromyography (EMG) data was collected using dedicated software (Cambridge Electronics Design, UK). The RMT was determined according to the protocol outlined by Rossini et al., 199423. The RMT was defined as the minimum stimulus intensity that elicits a MEP of more than 50 µV in five of ten trials. The intensity of stimulation was estimated based on the RMT (120% RMT) from the left motor cortex. The intensity of stimulation was then adjusted as necessary to produce a mean peak-to-peak MEP amplitude of 1 mV24 in the left motor cortex. In stimulating the left motor cortex, the TMS coil was placed at the optimal position for eliciting MEPs from the right abductor pollicis brevis (APB) muscle. EMG was captured by placing two disposable disc electrodes over the right APB muscle. The signal was amplified using a Model 2024F amplifier (Intronix Technologies Corporation, Bolton, Ontario Canada). The signal was filtered at band pass of 2 Hz to 2.5 kHz and digitized using the Micro 1401 (Cambridge Electronics Design, Cambridge UK).
Cortical Evoked Activity
CEA was measured using EEG signals acquired through a 64-channel Synamps 2 EEG system. All electrodes were referenced to an electrode positioned posterior to the Cz electrode. EEG signals were recorded using DC and a lowpass filter of 100 Hz at 20 kHz sampling rate25, (see Supplementary Materials and Methods for description of EEG cleaning and analysis). To quantify the PAS-induced potentiation for each session, we calculated the ratio of TMS-evoked potential (TEP) power at each time post-PAS over pre- PAS responses. This ratio was used as an index of potentiation. As the post-PAS timing of maximum potentiation of CEA could vary among subjects, we selected the maximum CEA ratio for each subject after PAS. To assess PAS-induced potentiation in the DLPFC, the 4 left frontal electrodes encompassing the DLPFC (F1, F3, F5 and F7) were used. To assess global PAS-induced potentiation, all 62 electrodes were averaged.
Paired Associative Stimulation
PAS was administered to the DLPFC in accordance with the protocol first described by Rajji et al., 201317. Briefly, PAS administration consisted of 180 TMS stimuli delivered over the F5 electrode at a frequency of 0.1 Hz. The TMS stimuli over the DLPFC were preceded by peripheral nerve stimulation (PNS) delivered to the right median nerve by 25 ms. Electrical median nerve stimulation was delivered at 300% of the sensory threshold. The sensory threshold was identified as the minimum detectable PNS stimulus. As described by Rajji et al., 2013, given that the post timing for maximum potentiation varies among subjects, the maximum ratio for CEA for each subject following PAS was selected17. One outlier (with CEA values 3 standard deviations above the mean during the placebo visit) was removed from CEA calculations.
Attention
Attention was assessed by having subjects attend to their hand and count the total number of stimulations delivered. Subjects were asked to report the count randomly throughout the 30 minute session and subjects were asked for a final count at the end of the PAS session.
Theta-Gamma Coupling
The analysis of coupling of theta-phase and gamma amplitude was performed as described by Rajji et al. 201317. Briefly, an entropy based modulation index (MI) was used to quantify coupling: MI = [log(N) − H(P)]/log(N)] (see Supplementary Materials and Methods for description of theta-gamma coupling analysis). The choice of the post-PAS time-point for the analysis of theta-gamma coupling for each subject was based on the maximum time of potentiation.
Statistics
Statistical analyses were performed using IBM SPSS Statistics (Version 22). Beverage effects on potentiation and theta-gamma coupling were analyzed using a general linear model repeated measures (ANOVA) with beverage (alcohol versus placebo) as the within-subjects factor. For evaluation of mean potentiation, potentiation from all Post-PAS time-points were averaged (Post 0, Post 15, Post 30 and Post60). For evaluation of maximum potentiation, the time point with the maximal potentiation index was selected. Post-hoc analyses were conducted using paired t-tests. Mean 1 mV peak-to-peak intensities (TMS test stimulus intensity) before and after beverage were compared using paired t-tests.
Results
1 mV Intensity (% stimulator output)
A repeated measures ANOVA was used examine if the alcohol or placebo beverage had an effect on 1 mV peak-to-peak intensity between T1 and T2. There was no significant effect of time (F = 1.431; df = 1,14, p = 2.51) and no significant beverage by time interaction effect (F = 3.332; df = 1,14, p = 0.573) (Table 2), suggesting that neither alcohol or placebo beverage has an effect on corticospinal excitability at baseline.
Breath Alcohol Concentration
The mean peak blood alcohol concentration (BAC) was 23.6 mM ± 4.1 mM (range 18.5 mM–34.2 mM). BAC was always at 0 mM at SI1mVT1 and peaked during T2. BAC remained above 17.4 mM (the legal intoxication level), with the exception of two subjects who were just below this level during PAS administration.
Attention
Attention has been shown to affect neuroplasticity26. Paired t-tests revealed no significant difference in number of sensory stimuli detected during PAS in the alcohol and placebo conditions, suggesting that attention levels were not significantly different between the two conditions (t = −0.946;df = 14; p = 0.360) (Table 2).
Effect of Beverages on Cortical Evoked Activity
A repeated measures ANOVA revealed a significant beverage by time interaction effect on cortical evoked activity (F = 5.919; df = 1,13, p = 0.030). No significant effect of beverage (F = 3.451; df = 1,13, p = 0.086) or time (F = 0.11, df = 1,3, p = 0.920) was observed. Paired t-tests revealed that neither the placebo beverage (t = −1.641, df = 13, p = 0.125) or alcohol beverage (t = 2.054, df = 13, p = 0.061) produced a significant change in CEA in the DLPFC compared to before beverage. Paired t-tests also revealed that there was also no significant difference in cortical activity before beverage between the placebo and alcohol conditions (t = −1.263, df = 13, p = 0.229). Following beverage consumption, there was a significant difference between cortical evoked activity in the alcohol and placebo groups (t = −2.696, df = 13, p = 0.018). These findings suggest that acute alcohol consumption produces a decrease in cortical evoked activity (Fig. 2).
Cortical evoked activity (CEA) before (white bars) and after (black bars) beverage (μV) for the placebo and alcohol conditions (n = 14). Error bars represent the standard deviations. Neither placebo or alcohol beverage produced a significant change in CEA in the DLPFC compared to before beverage. Following beverage consumption, there was a significant difference between cortical evoked activity in the alcohol and placebo groups.
Effect of Alcohol on Neuroplasticity in the DLPFC
The repeated measures ANOVA revealed a main effect of beverage (F = 6.034; df = 1,13, p = 0.029), reflective of decreased PAS-induced neuroplasticity in the DLPFC, compared to placebo. Alcohol intoxication resulted in a significant impairment of the mean potentiation compared to placebo beverage (t = 2.456, df = 13, p = 0.029) in the DLPFC (Fig. 3). Alcohol intoxication also resulted in impaired peak PAS-induced neuroplasticity in the DLPFC compared to the placebo beverage, as indexed using the mean maximum CEA ratio in the DLPFC (t = −2.945, df = 13, p = 0.011; Fig. 3). Alcohol significantly impaired mean (t = −3.051, df = 13, p = 0.009) and maximum DLPFC PAS-induced neuroplasticity globally (t = −3.260, df = 13, p = 0.006). A one sample t-test confirmed that potentiation occurred under the placebo condition, as the mean maximum CEA ratio was significantly greater than 1 in the DLPFC (t = 2.432, df = 13, p = 0.030) and globally (t = 2.325, df = 13, p = 0.037).
(a) Mean ratio of 100 TMS pulses to the DLPFC across all post-PAS timepoints (Post 0 min, Post 15 min, Post 30 min, Post 60 min) to 100 TMS pulses to the DLPFC pre-PAS for the placebo (white bar) and alcohol(black bar) conditions (n = 14). Error bars represent the standard deviations. Alcohol significantly impaired mean PAS-induced neuroplasticity. (b) Mean ratio of 100 TMS pulses to the DLPFC at time point of maximum potentiation compared to 100 TMS pulses to the DLPFC pre-PAS for the alcohol(black bar) and placebo (white bar) conditions (n = 14). Error bars represent the standard deviations. Alcohol significantly impaired maximal PAS-induced neuroplasticity. The panels on the bottom represents average topoplots of alcoholcompared to placebo, with hotter colours representing greater CEA following PAS.
Alcohol’s Effects on Theta-Gamma Coupling
An exploratory analysis was conducted to explore whether PAS had an effect on theta-gamma coupling. The repeated measures ANOVA revealed a significant effect of time (PrePAS vs max potentiation time Post-PAS), reflective of increased theta- gamma coupling following PAS (F = 7.516, df = 1,14, p = 0.016). No significant effect of beverage (F = 0.111, df = 1,14, p = 0.744) or beverage by time interaction was (F = 2.720, df = 1,14, p = 0.121). Paired t-tests revealed that PAS to the DLPFC resulted in an increase in MI, indicating increased theta-gamma coupling during the placebo visit (t = 2.954, df = 14, p = 0.010). This significant increase following PAS was not observed during the alcohol visit (t = 1.486, df = 14, p = 0.159) (Fig. 4).
Theta-gamma coupling. Theta-gamma coupling is indexed through the modulation index (MI). There was a significant increase in MI following PAS with placebo beverage. This significant increase in MI was not observed following PAS with the alcohol beverage. Error bars represent the standard deviations.
Discussion
Impairment of mean and maximum potentiation from this study indicate that alcohol intoxication significantly impairs PAS-induced neuroplasticity in left DLPFC and globally. The impairment of neuroplasticity was accompanied by impairment of the PAS-induced increase in theta-gamma coupling in the left DLPFC. These findings provide a possible mechanism by which alcohol intoxication impairs cognitive function.
Alcohol’s Impairment of Neuroplasticity in the DLPFC
Intoxication by alcohol impaired PAS-induced neuroplasticity in the left DLPFC and globally. Previously, it has been demonstrated that a single heavy drinking episode11 and even low doses of alcohol10 impair PAS-induced neuroplasticity in the motor cortex. Using DLPFC PAS and EEG, current findings demonstrate that this impairment of neuroplasticity by alcohol intoxication occurs in DLPFC. Interestingly, we found that the impairment in neuroplasticity induced by alcohol was not just localized to the DLPFC. Rather, we also observed a global impairment of neuroplasticity. It is not well understood whether this global impairment of neuroplasticity is a result of neuroplasticity induction within regions outside the DLPFC or a phenomena secondary to modulation of neuroplasticity in the DLPFC. Given that the DLPFC is functionally connected to a number of cortical and subcortical regions, the global effect observed may be due to downstream neuroplasticity mechanisms. Global evoked response potential (ERP) deficits have been reported in binge drinkers compared to non-drinkers during basic and high level cognitive states27. Global aberrancies in neuronal responses may highlight neural inefficiencies in heavy drinkers (as subjects in the study reported at least one previous binge drinking episode in the last month) that contribute to a less localized induction of neuroplasticity in this population. Induction of less localized neuroplasticity in this population along with alcohol’s widespread action on the brain1 may explain the global impairment of neuroplasticity seen in the present study. Interestingly, we observed that alcohol produced a suppression of CEA following beverage consumption, suggesting that alcohol suppresses both CEA and PAS-induced neuroplasticity.
Mechanisms for Alcohol’s Impairment of Neuroplasticity
The mechanisms of alcohol’s impairment of neuroplasticity in the DLPFC is likely related to its antagonistic effect on NMDA receptor mediated neurotransmission and potentiating effect on GABAA receptor mediated neurotransmission28,29,30,31. Using paired pulse TMS paradigms, Ziemann et al., 1995 demonstrated that alcohol consumption results in an increase of TMS indices of GABAA and GABAB receptor mediated inhibition and a decrease in TMS indices of NMDA receptor mediated excitability in healthy subjects30. Studies using rat hippocampal slices have demonstrated that both GABAA and NMDA receptors are necessary for the complete reduction of neuroplasticity by alcohol. Pharmacological isolation of NMDA receptors by antagonism of GABAA receptors via picrotoxin did not produce the complete impairment of neuroplasticity otherwise produced by alcohol in hippocampal slices. Rather, alcohol produced greater impairments of LTP when GABAA receptors were functional (as seen in the absence of picrotoxin)29. Taken together, these studies reveal that both GABAA and NMDA receptor activity contribute to the neuroplasticity deficits produced by alcohol.
Alcohol’s Impairment of Theta-Gamma Coupling
An increase in theta-gamma coupling following PAS with placebo beverage was observed in the present study. These findings are consistent with Rajji et al., 2013 who demonstrated that PAS potentiated theta-gamma coupling in the DLPFC among healthy subjects. Interestingly, this increase in theta-gamma coupling following PAS was not observed with alcohol. Theta-gamma coupling is believed to be an index of DLPFC functioning, specifically working memory18, 32. Theta-gamma coupling has been shown to be increased during trials that required ordering information compared to trials that do not require ordering17. Previous studies have demonstrated that acute alcohol intoxication produces an impairment of working memory across several domains33,34,35. Increased theta-gamma coupling following PAS observed in the current study is speculated to be due to PAS activating the same neuronal networks in the DLPFC involved in working memory17. Therefore, alcohol’s impairment of PAS induced potentiation of theta-gamma coupling may be associated with alcohol’s effects on neuroplasticity in neuronal networks involved in learning and memory.
Implications for Cognitive and Executive Function
Current findings demonstrate that alcohol intoxication impairs PAS-induced neuroplasticity in the DLPFC, a brain region that plays an important role in cognitive and executive functioning. Finding of alcohol’s impairment of potentiation of theta-gamma coupling suggest that alcohol intoxication impairs neuroplasticity in neuronal networks involved in cognitive functioning. Impaired cognitive function is a commonly reported effect of alcohol intoxication36. The BAC level required to impair cognitive function varies with the type of task. Complex cognitive tasks (ie. tasks requiring divided attention) are impaired at BACs as low as 0.01%37. At higher BACs, as seen following a heavy drinking episode (≥0.08%BAC), a much wider range of cognitive impairments are seen, including a slowing of simple reaction time38, 39. The impairment of neuroplasticity in the DLPFC observed in the present study provides a potential mechanism by which alcohol intoxication impairs cognitive function.
Given that the DLPFC is a part of the brain reward circuitry as a part of the mesocortico-limbic pathway, impairment of neuroplasticity in the reward circuitry by alcohol may also play a role in the transition to alcohol use disorders (AUDs) following repetitive heavy drinking. The DLPFC regulates the integration of goal-motivated behavior by assimilating information regarding the potential negative and positive outcomes of selecting a behavior. Aberrant neuroplasticity in this regions result in the selection of inappropriate behaviors despite their negative consequences, such as compulsive drug taking40. Indirect evidence for impaired neuroplasticity in the DLPFC of alcohol dependent individuals comes from findings of neuromodulatory brain stimulation studies that have demonstrated that modulation of neuroplasticity in the DLPFC through repetitive transcranial magnetic stimulation shows promise as a treatment of AUDs41, 42. Identifying the existence, localization and direction of neuroplasticity impairment in the DLPFC of alcohol dependent individuals through PAS with EEG can help inform the future use of neuromodulatory brain stimulation for the treatment of individuals with AUDS.
The present study has a number of limitations. Firstly, it would have been informative to examine the effect of alcohol intoxication on working memory. This would have allowed us to determine if alcohol’s impairment of neuroplasticity and theta-gamma coupling is associated with working memory dysfunction. However, given that theta-gamma coupling has been demonstrated to be related to working memory load, we can infer that alcohol is acting on the same neuronal networks involved in working memory to impair potentiation of theta-gamma coupling. Another limitation to the current study is that we used breath samples to calculate BAC levels rather than measuring from blood samples. However, it would have been unnecessarily invasive and disruptive to take blood samples from patients throughout the beverage visits as breath measures correlate highly with BAC43. Another limitation of the study was the sample size of the present study is relatively small. However, the within-sample design of the study allowed for the use of the current sample size. Lastly, while we attempted to blind the subjects to their beverage type, all subjects correctly guessed which beverage they had received during both beverage visits. However, we have no reason to believe that being aware of which beverage they had received would affect their neurophysiological measurements and neuroplasticity.
Conclusions
Findings from the current study revealed that alcohol intoxication impaired neuroplasticity in the DLPFC and throughout the cortex. Additionally, the increase in coupling of the theta-gamma coupling following PAS was not observed following alcohol consumption. As theta-gamma coupling is believed to be related to working memory, these findings provide a potential mechanism for impairment of cognitive function by alcohol. Furthermore, as the DLPFC is a part of the brain reward circuitry, impairment of neuroplasticity in this region may play a role in the transition to AUDs following repetitive heavy drinking. Future research is needed to determine the role of DLPFC neuroplasticity in the pathophysiology of AUDs.
References
Nevo, I. & Hamon, M. Neurotransmitter and neuromodulatory mechanisms involved in alcohol abuse and alcoholism. Neurochemistry international 26, 305–336; discussion 337–342 (1995).
Koob, G. F. A role for GABA in alcohol dependence. Adv Pharmacol 54, 205–229 (2006).
Sundstrom-Poromaa, I. et al. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nature neuroscience 5, 721–722 (2002).
Liljequist, S. & Engel, J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology 78, 71–75 (1982).
Lovinger, D. M., White, G. & Weight, F. F. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. The Journal of neuroscience: the official journal of the Society for Neuroscience 10, 1372–1379 (1990).
Weight, F. F., Peoples, R. W., Wright, J. M., Lovinger, D. M. & White, G. Ethanol action on excitatory amino acid activated ion channels. Alcohol Alcohol Suppl 2, 353–358 (1993).
Ehlers, C. L., Kaneko, W. M., Wall, T. L. & Chaplin, R. I. Effects of dizocilpine (MK-801) and ethanol on the EEG and event-related potentials (ERPS) in rats. Neuropharmacology 31, 369–378 (1992).
Aroniadou, V. A. & Keller, A. Mechanisms of LTP induction in rat motor cortex in vitro. Cereb Cortex 5, 353–362 (1995).
Hess, G., Aizenman, C. D. & Donoghue, J. P. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol 75, 1765–1778 (1996).
Lucke, C. et al. Deleterious effects of a low amount of ethanol on LTP-like plasticity in human cortex. Neuropsychopharmacology 39, 1508–1518 (2014).
Loheswaran, G. et al. Alcohol Intoxication by Binge Drinking Impairs Neuroplasticity. Brain Stimul 9, 27–32 (2016).
Loheswaran, G. et al. Brain Stimulation in Alcohol Use Disorders: Investigational and Therapeutic Tools. Biological Psychiatry: Cognitive Neurosciene and Neuroimaging 1, 5–13 (2016).
Park et al. Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol 42, 417–422 (2007).
Owen, A. M., McMillan, K. M., Laird, A. R. & Bullmore, E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp 25, 46–59 (2005).
Rosenzweig, M. R., Breedlove, S. M. & Leiman, A. L. Biological Psychology. (Sinauer Associates, 2002).
Weinberger, N. M. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5, 279–290 (2004).
Rajji, T. K. et al. PAS-induced potentiation of cortical-evoked activity in the dorsolateral prefrontal cortex. Neuropsychopharmacology 38, 2545–2552 (2013).
Lisman & Idiart. Storage of 7 + /− 2 short-term memories in oscillatory subcycles. Science 267, 1512–1515 (1995).
Rusjan, P. M. et al. Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp (2010).
Sobell, L. C., Sobell, M. B., Leo, G. I. & Cancilla, A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict 83, 393–402 (1988).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12, 189–198 (1975).
Loheswaran, G. et al. Alcohol Intoxication by Binge Drinking Impairs Neuroplasticity. Brain Stimul In Press (2015).
Rossini, P. M. et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91, 79–92 (1994).
Valls-Sole, J., Pascual-Leone, A., Wassermann, E. M. & Hallett, M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalography and clinical neurophysiology 85, 355–364 (1992).
Daskalakis, Z. J. et al. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology 33, 2860–2869 (2008).
Stefan, K., Wycislo, M. & Classen, J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol 92, 66–72 (2004).
Maurage, P. et al. Cerebral effects of binge drinking: respective influences of global alcohol intake and consumption pattern. Clin Neurophysiol 123, 892–901 (2012).
Schummers, J., Bentz, S. & Browning, M. D. Ethanol’s inhibition of LTP may not be mediated solely via direct effects on the NMDA receptor. Alcoholism, clinical and experimental research 21, 404–408 (1997).
Schummers, J. & Browning, M. D. Evidence for a role for GABA(A) and NMDA receptors in ethanol inhibition of long-term potentiation. Brain research. Molecular brain research 94, 9–14 (2001).
Ziemann, U., Lonnecker, S. & Paulus, W. Inhibition of human motor cortex by ethanol. A transcranial magnetic stimulation study. Brain 118(Pt 6), 1437–1446 (1995).
Lucke, C. et al. Deleterious effects of a low amount of ethanol on LTP-like plasticity in human cortex. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 39, 1508–1518 (2014).
Canolty, R. T. & Knight, R. T. The functional role of cross-frequency coupling. Trends Cogn Sci 14, 506–515 (2010).
Grattan-Miscio, K. E. & Vogel-Sprott, M. Effects of alcohol and performance incentives on immediate working memory. Psychopharmacology (Berl) 181, 188–196 (2005).
Schweizer, T. A. & Vogel-Sprott, M. Alcohol-impaired speed and accuracy of cognitive functions: a review of acute tolerance and recovery of cognitive performance. Exp Clin Psychopharmacol 16, 240–250 (2008).
Saults, J. S., Cowan, N., Sher, K. J. & Moreno, M. V. Differential effects of alcohol on working memory: distinguishing multiple processes. Exp Clin Psychopharmacol 15, 576–587 (2007).
Koob, G. F. & Le Moal, M. Neurobiology of Addiction. (Elsevier, 2006).
Moscowitz, H. & Fiorentino, D. A Review of the Literature on the Effects of Low Doses of Alcohol on Driving-Related Skills. (U.S. DEPARTMENT OF TRANSPORTATION, 2000).
Fillmore. Acute alcohol-induced impairment of cognitive functions: past and present findings. International Journal on Disability and Human Development 6, 115–125 (2007).
Holloway, F. A. Low-dose alcohol effects on human behavior and performance. Alcohol, Drugs Driving 11, 39–56 (1995).
Feil, J. & Zangen, A. Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev 34, 559–574 (2010).
Mishra, B. R., Nizamie, S. H., Das, B. & Praharaj, S. K. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: a sham-controlled study. Addiction 105, 49–55 (2010).
De Ridder, D., Vanneste, S., Kovacs, S., Sunaert, S. & Dom, G. Transient alcohol craving suppression by rTMS of dorsal anterior cingulate: an fMRI and LORETA EEG study. Neurosci Lett 496, 5–10 (2011).
Peleg. Comparison of blood alcohol levels with breath alcohol levels measured using the Drager 7110 MKIII breathalyzer. Injury Prevention (2010).
Acknowledgements
This work was funded by the Centre for Addiction and Mental Health (CAMH) Foundation and the Campbell Institute.
Author information
Authors and Affiliations
Contributions
G.L. was involved in conception and designing of the study, recruiting and enrolling the subjects, running the study visits and acquiring the EEG data, cleaning the EEG data, the analysis and interpretation of the data and drafting and editing the manuscript. M.S.B. contributed to the conception and designing of the study, the analysis and interpretation of the data and the critical reviewing of the manuscript. R.Z. contributed to the analysis and interpretation of the data and critical reviewing of the manuscript. T.K.R. was involved with the conception and designing of the study, the analysis and interpretation of the data and the critical reviewing of the manuscript. D.M.B. contributed to the conception and designing of the study and the critical reviewing of the manuscript. B.L.F. was involved with the conception and designing of the study and the critical reviewing of the manuscript. Z.J.D. was involved with the conception and designing of the study, the analysis and interpretation of the data and the critical reviewing of the manuscript. All authors have given final approval of the version of the article to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loheswaran, G., Barr, M.S., Zomorrodi, R. et al. Impairment of Neuroplasticity in the Dorsolateral Prefrontal Cortex by Alcohol. Sci Rep 7, 5276 (2017). https://doi.org/10.1038/s41598-017-04764-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04764-9
This article is cited by
-
Neuroimaging in psychedelic drug development: past, present, and future
Molecular Psychiatry (2023)
-
High-wearable EEG-based distraction detection in motor rehabilitation
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.