Abstract
A zinc trisporphyrinate has been developed as a chirality sensor for chiral mono-alcohols. In its structure, there are two “spaces” surrounded by three porphyrin moieties, which allow guests to fill in. It has shown extremely high CD sensitivity for a chiral mono-alcohol with a naphthyl substituent, 1-(1-naphthyl)ethanol, at μM level, which is at least three orders of magnitude lower concentration than previous reports. A crystallographic study of the host-guest complex reveals the binding of 1-(1-naphthyl)ethanol to the zinc trisporphyrinate is greatly enhanced by multipoint interactions, such as coordination interactions, hydrogen bonding, π-π and CH···π interactions etc. Spectroscopic studies suggest the corresponding binding constant K1 is over 105 M−1, which is two or three orders of magnitude larger than other mono-alcohols. Among porphyrin systems, this trisporphyrin have the strongest binding affinity for 1-(1-naphthyl)ethanol, which leads to the highest CD sensitivity.
Similar content being viewed by others
Introduction
Chiral alcohols are commonly encountered structural elements in pharmaceutical drugs, biologically active natural products and synthetic intermediate1,2,3. Determination of their absolute configurations is crucial to understand their functions, and the rapid determination of absolute configuration and enantiomeric excess for chiral molecules are also important for high-throughput screening of chiral catalysts4. Among these alcohols, mono-alcohols are more difficult to investigate due to their limited binding site and the weak binding affinity to metal centres. In past years, many efforts have been devoted to developing highly sensitive systems for recognition of chiral mono-alcohols. Some of them require the mono-alcohols to be derivatized5,6,7. Obviously, without chemical derivation, the determination of absolute configurations will be more convenient and rapid, especially through CD measurements.
Because of the intensive absorption in the Soret band, metalloporphyrins have been widely studied as good candidates for chirality related research8,9,10,11. However, because of the weak binding affinity of alcohols to metal centres, very few studies were reported12,13,14. For example, Borovkov and co-workers reported the ethane-bridged bisporphyrin system. In that case, the short ethane-bridge causes the steric interactions between the 3,7-ethyl groups of the porphyrin and the substituents of the ligand, resulting in the induced supramolecular chirality12. Another more sensitive system is an ester-linked bisporphyrin developed by Takanami et al., which improves the detecting concentration of mono-alcohols to mM level through the simultaneous double coordination of the hydroxyl group to the two metals14.
Although considerable progress has been made with respect to the recognition of chiral mono-alcohols, the search for highly sensitive sensors still remains challenge. In order to improve the sensitivity, one important strategy is to increase the host-guest interactions. In view of molecular recognition, such a purpose can be achieved through multipoint host-guest interactions.
We have recently reported a trisporphyrin system as shown in Fig. 1 15, 16. In this trisporphyrin, the linking group is surrounded by three porphyrin moieties, which leads to the formation of two “spaces”, one above the linking phenyl and another below it. So this host has potential for chirality sensing of specific guests through multipoint interactions, such as coordination interactions, hydrogen bonding, π-π interactions and CH···π interactions etc., which will enhance host-guest interactions, and the disadvantage of mono-alcohols can be overcome.
In our previous study16, the crystal structure suggests the “space” formed by porphyrin moieties is a little big for 1-phenylethanol, and that “space” can accommodate one 1-phenylethanol and one H2O. A bulkier guest could match the “space” better. Herein, we examined several mono-alcohols. This Zn trisporphyrinate showed the highest sensitivity to 1-(1-naphthyl)ethanol (the guest 2), the one with a naphthyl substituent. To obtain good CD signals, the concentration of the guest 2 only need to be at μM level, which is at least three orders of magnitude smaller than previous reports. To our knowledge, this is the most CD sensitive porphyrin system for chiral mono-alcohols so far.
Results and Discussion
Circular dichroism spectra
The CD spectra for the mixture of [Zn3-BTATPP] and the guest 2 were shown in Fig. 2. When the concentration of guest 2 is 0.55 µM, there are remarkable bisignate CD signals in the Soret band region. As shown in Figure S5, when its concentration is 2.1 × 10−5 M, the A value reaches 1054 cm−1M−1, the strongest CD value in the reported porphyrin systems for mono-alcohols. For 2S, the longer-wavelength peak of the Soret band was negative and the shorter-wavelength peak was positive. For 2R, the CD spectra showed similar shapes and intensities but opposite signs in the Soret band region. So this host can be used to determine the absolute configuration of 1-(1-naphthyl)ethanol. The spectra for other mono-alcohols are shown in the supporting information. The corresponding guest concentrations at which the Aobs values are about 100 cm−1M−1 are also listed in Table 1. For the guest 2, the detecting concentration is three orders of magnitude lower than those reported by Takanami et al.14. We also noticed there were limitations for this trisporphyrin system. For example, the signs of these CD spectra for these mono-alcohols did not show clear patterns as literatures reported. And this system is only highly sensitive to the specific case of 1-(1-naphthyl)ethanol. Except the guest 2, the required concentrations for other guests are much higher, ranging from 10−3 to 10−4 M, which are similar to Takanami’s results.
Rationalization of the CD sensitivity for the guest 2
What causes that the CD sensitivity for the guest 2 is significantly higher than other mono-alcohols? The magnitude of CD signals relies on the following two factors: 1) The binding affinity of guests to hosts. It determines the amount of host-guest complexes formed at low concentrations. 2) The CD magnitude of the corresponding host-guest complex per unit. In the following studies, we have evaluated the contributions from both factors.
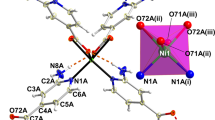
In order to evaluate the first factor, the binding affinity, we did studies on the crystal structure and the binding constants. When large excess of the guest 2 was mixed with the host, the suitable single crystals for the 1:2 host-guest complex were obtained. The structure was solved in P1, a chiral space group. In one asymmetric unit, there are two independent zinc trisporphyrinate molecules, mol A and mol B. The structure of mol A is displayed in Fig. 3, and the other is displayed in Figure S11. The main framework consists of three zinc porphyrinate subunits with a benzene tricarboxamide group as the linker. In each trisporphyrin, only two Zn centres are five-coordinate with 2S as an axial ligand, while the third Zn is four-coordinate without any axial ligand. The overall formula is [Zn3-BTATPP]·(2S)2. Each 2S adopts the “inside” binding mode. Besides the coordination interactions, hydrogen bonding and π-π interactions are also found between hosts and guests as shown in Fig. 3A. The carbonyl oxygens form hydrogen bonds with the coordinated OH, the corresponding O···O distances are 2.67 and 2.74 Å. The corresponding data are provided in Table S3. There are π-π interactions formed between the naphthyl rings of 2S and the linking phenyl ring. The centroid-centroid distances are 3.55 Å and 3.72 Å, the dihedral angles are 2.0° and 8.4°, respectively. The two guests fit the two “spaces” surrounded by three porphyrin moieties to form a “sandwich” like structure.
Crystal structure of [Zn3-BTATPP]·(2S)2. (mol A). (A) Showing hydrogen bonds (red dash line) and π-π interactions (blue dash line). Some phenyl groups at meso-positions and all hydrogen atoms except those for the guest are omitted for clarity. (B) Showing CH···π interactions (yellow dash line and brown dash line).
As expected, 1-(1-naphthyl)ethanol is bulkier than 1-phenylethanol, which makes it match the “space” better, and also causes other interactions between hosts and guests. Besides coordination interactions, π-π interactions and hydrogen bonding interactions, there are also C-H···π interactions as shown in Fig. 3B. Obviously, the C-H···π interactions cause the blocking of the “inside” coordination position of the third zinc (the four-coordinate zinc), which leads to the 1:2 complex when the guests are in large excess.
The chirality of the crystal is confirmed by the solid CD spectrum in Figure S12, which shows clear signals in the Soret band region. The solid CD spectrum has a similar shape to the solution CD spectrum, which indicates the host-guest complex in solution could have a similar structure to that in the solid state. But we can not rule out other possibilities since intermolecular exciton couplings in the solid state could occur due to crystal packing17, 18. The crystal structure reveals there are multipoint interactions among the host and guests, which could increase the overall interactions, and thus increase the binding affinity. This is further confirmed by binding constant determination.
The binding constants were determined from UV-vis titration spectra. As shown in Fig. 4, the UV-vis spectral change showed two steps: (1) When the concentrations of the guest 2 change from 0 to 3.1 × 10−6 M, the peak at 419 nm keep decreasing; (2) When the concentrations increase from 7.4 × 10−6 to 4.2 × 10−4 M, the peak at 419 nm starts to increase and a shoulder appears at 424 nm, then two isobestic points are observed at 415 and 431 nm. These spectral changes indicate there are the following two equilibriums in solution, where L present the guest. There could be three equilibriums since there are three Zn centres. But since the binding of the first and second guests blocks the “inside” coordination position of the third Zn, the third Zn has much weaker binding affinity than other two. Under our experimental conditions, the concentrations of the guest 2 is not high enough to cause the formation of the detectable amount of the 1:3 complex, and thus the third equilibrium can be ignored. Actually, the 1:2 complex in solution is confirmed by the Job’s plot based on the 1H NMR titrations at relative high concentrations (from 10−3 to 10−2 mol/L) (Figure S15). The peak at 0.33 mole fraction corresponds to a 1:2 host-guest complex.
The nonlinear least-squares program SQUAD19 was used to calculate the binding constants for the two equilibriums. More fitting details were provided in the supporting information (Figure S20). For 2S, the binding constants K1 and K2 are found to be 1.8 (±0.1) × 105 M−1 and 1.4 (±0.1) × 104 M−1. The fitting of the CD spectral changes (Figs S25–S29) also gave these binding constants, which provided similar values (Table S6). For the guest 2, K1 is the largest value in the reported porphyrin-monoalcohol complexes, which is 2 or 3 orders of magnitude larger than other mono-alcohols in Table 2, and two orders of magnitude larger than that in Takanami’s system14. The strong binding affinity to the guest 2 is consistent with the multipoint interactions in the structure.
The binding constant K1 values follow this order: 2 ≫ 5 ≈ 4 > 1 > 6 > 3. For mono-alcohols 5, 4, 1 and 6, this order is consistent with their bulkiness. For the case of 2, its size is comparable with 5 (its overall volume is smaller than 5, but its long axis (the longest distance between two atom) is actually longer than 5). More importantly, the π-π or C-H···π interactions are absent for the guest 5, which make its binding affinity much less than 2. For the case of 3, it is a tertiary alcohol, others are secondary alcohols, which probably causes its binding affinity much weaker than others.
The high binding constants for the guest 2 suggest that more host-guest complexes are formed than other mono-alcohols when their concentrations of guests (and hosts) are the same. To further evaluate the CD results, we have also to consider the second factor - the CD contribution of the corresponding host-guest complexes per unit.
We can actually obtain some direct information about CD contributions from experimental data. CD signals are contributed from both 1:1 and 1:2 host-guest complexes. For the CD sensitivity, we are concerned about the CD signals at low guest concentrations. Under such a condition, the amount of 1:1 complexes are much more than 1:2 complex as shown in Table 2. Thus the CD magnitude is mainly contributed from the 1:1 complexes, the CD magnitude per unit could be roughly treated as Aobs/P1. As shown in Table 2, the CD contributions range from 176 to 1254 cm−1M−1 for different mono-alcohols. Obviously, among them, the 1:1 complex for the guest 2 leads to the largest CD magnitude per unit. So for the same amount of 1:1 complex, the CD amplitude for 1-(1-naphthyl)ethanol is several times (below 10) as other alcohols.
The above studies suggest that this zinc trisporphyrinate has the strongest binding affinity to 1-(1-naphthyl)ethanol and the host-guest complex has the largest CD magnitude per unit, which leads to the highest CD sensitivity. If we consider the binding constants for 1-(1-naphthyl)ethanol is over 100 times larger than others and its CD contribution per unit is only a few times than others, the major factor causing the highest CD sensitivity for the guest 2 should be the strong bonding affinity, which is due to the strong host-guest interactions.
Conclusion
In conclusion, a zinc trisporphyrinate has shown high CD sensitivity at µM level for 1-(1-naphthyl)ethanol. The crystallographic study reveals that coordination interactions, hydrogen bonding, π-π and CH…π interactions in the host-guest complex cause strong host-guest interactions, which is confirmed by the large binding constant. Our studies suggest the “space” formed by porphyrin moieties is “recognized” best by the chiral mono-alcohol with the naphthyl substituent, the guest 2, which is crucial to chiral recognition of mono-alcohols. We could adjust the substituents in hosts to create the right “space” for the specific guest, which can improve the corresponding recognition ability and sensitivity. This type of hosts can be used as powerful tools for molecular recognition of some specific chiral molecules, and have also potential applications in catalytic synthesis. Further studies on other guests and derived hosts are under investigation.
Experimental section
Material and Physical Methods
Reactions involving moisture sensitive reagents were carried out under a nitrogen atmosphere using standard vacuum line techniques and glassware that was flame-dried and cooled under nitrogen before use. CH2Cl2 and n-hexane were distilled over CaH2. Triethylamine (Et3N) was distilled over KOH and then used immediately. Zinc 5-(2-aminophenyl)-10,15,20-triphenyl-porphyrinate was prepared as described in the literature20. The porphyrin host [Zn3-BTATPP] was prepared according to the reported method15. Elemental analyses were performed on an Elementar Vario EL III analytical instrument. A Shimadzu UV-3150 spectrometer was used to record UV−vis spectra. A MT Model J-815 spectrophotometer was used to record CD spectra at 25 °C in 10% CH2Cl2/hexane (scan speed: 50 nm/min, date pitch: 0.5 nm, bandwidth: 2 nm, response time: 1 seconds).
CD and UV-vis measurements
Solutions of various guests in 10% CH2Cl2/hexane were added to the [Zn3-BTATPP] solutions at 25 °C. Then CD and UV-vis spectra were recorded. The corresponding CD spectra were normalized based on the [Zn3-BTATPP] concentration.
According to the following method, the measurement of the solid CD spectrum was performed. A single crystal and some solid KBr were grinded under high pressure to get the KBr pellets (the thickness is 0.3 mm), and then CD spectrum was recorded.
Job’s continuous plot analysis to determine complex stoichiometry
The complex stoichiometry for the host-guest complexes was determined through 1H-NMR titration of [Zn3-BTATPP] and the chiral guest 2R. Upon complexation of 2R with [Zn3-BTATPP]1,HNMR spectra undergo changes in chemical shift (Δδ) due to the anisotropic effect of the porphyrin rings. These changes can be used to determine the composition of host-guest complexation in solution. The 1H-NMR titration [Zn3-BTATPP] with 2R (Figure S14) demonstrates changes in the chemical shifts of the protons upon binding of the guest to the host.
For each titration, 4.7 mg of [Zn3-BTATPP] (2.1 × 10−6 mol) was dissolved in CDCl3 (0.50 mL), and the NMR spectrum was recorded (TMS was used as internal standard). Following this, 0.3, 0.6, 1.0, 1.2, … up to 20 equivalents of 2R was added to the above solution; the NMR spectra was recorded after each addition.
Job’s plot was obtained with respect to changes in chemical shift (Δδ) of one of the [Zn3-BTATPP] protons (peak at 3.25 ppm, Figure S14). The molar fraction of [Zn3-BTATPP] (Xp) was multiplied by the changes in 1H-NMR chemical shift of [Zn3-BTATPP] (Δδp) for each titration data point. The resulting value was plotted against the molar fraction of [Zn3-BTATPP]. Peaking at 0.33 mol fraction corresponds to a 1:2 host-guest complex (Figure S15).
Preparation of crystals of [Zn3-BTATPP]·(2S)2
The solution of [Zn3-BTATPP] (10 mg, 0.004 mmol) in toluene (4 mL) was mixed with the solution of S-1-(1-naphthyl)ethanol (9.4 mg, 0.05 mmol) in CH2Cl2 (1 mL), and the mixture was stirred for 3 min, then transferred into glass tubes (8 mm × 250 mm). n-Heptane was added as the nonsolvent at room temperature. After one months, purple crystals were then isolated (5.8 mg, yield 51%). Anal. Calcd for C330H222N30O10Zn6: C, 76.92; H, 4.34; N, 8.10; Found: C, 74.89; H, 4.40; N, 8.83.
X-ray structure determination
A Bruker APEX-II CCD was used for data collection at 293(2) K, which is equipped with graphite monochromated Mo Kα (λ = 0.71073 nm). Direct methods were used to solve the structure, further refinements were done on F 2 by full-matrix least-squares technique using the SHELXL-2014 program package21. In the asymmetric unit, there are two molecules of [Zn3-BTATPP]·(2S)2. Non-hydrogen atoms were refined anisotropically and all hydrogen atoms were included in calculated positions. In the structure, several highly disordered solvent molecules were present, which could not be modeled successfully and, therefore, SQUEEZE22 was used. The electron count within the interporphyrin voids was 86 e, which corresponds to one molecule of dichloromethane per [Zn3-BTATPP]·(2S)2. Data collection parameters are listed in Table S1. The CIF file for the crystal structures were deposited at the Cambridge Crystallographic Data Centre (CCDC). The CCDC deposition number is 1504576.
References
Zhou, Y., Luan, P., Liu, L. A. & Sun, Z. P. Chiral derivatizing reagents for drug enantiomers bearing hydroxyl-groups. J. Chromatogr. B-Biomed. Appl. 659, 109–126 (1994).
Mozga, T., Prokop, Z., Chaloupkova, R. & Damborsky, J. Chiral aliphatic hydroxy compounds in nature: a review of biological functions and practical applications. Collect. Czech. Chem. Commun. 74, 1195–1278 (2009).
Collados, J. F., Sola, R., Harutyunyan, S. R. & Macia, B. Catalytic Synthesis of Enantiopure Chiral Alcohols via Addition of Grignard Reagents to Carbonyl Compounds. ACS Catal. 6, 1952–1970 (2016).
You, L., Zha, D. & Anslyn, E. V. Recent Advances in Supramolecular Analytical Chemistry Using Optical Sensing. Chem. Rev. 115, 7840–7892 (2015).
Kurtan, T. et al. Chiral Recognition by CD-Sensitive Dimeric Zinc Porphyrin Host. 1. Chiroptical Protocol for Absolute Configurational Assignments of Monoalcohols and Primary Monoamines. J. Am. Chem. Soc. 123, 5962–5973 (2001).
Kurtan, T. et al. Chiral Recognition by CD-Sensitive Dimeric Zinc Porphyrin Host. 2. Structural Studies of Host-Guest Complexes with Chiral Alcohol and Monoamine Conjugates. J. Am. Chem. Soc. 123, 5974–5982 (2001).
Jo, H. H. et al. Application of a high-throughput enantiomeric excess optical assay involving a dynamic covalent assembly: parallel asymmetric allylation and ee sensing of homoallylic alcohols. Chem. Sci. 6, 6747–6753 (2015).
Borovkov, V. V., Hembury, G. A. & Inoue, Y. Origin, Control, and Application of Supramolecular Chirogenesis in Bisporphyrin-Based Systems. Acc. Chem. Res. 37, 449–459 (2004).
Hembury, G. A., Borovkov, V. V. & Inoue, Y. Chirality-Sensing Supramolecular Systems. Chem. Rev. 108, 1–73 (2008).
Berova, N., Pescitelli, G., Petrovic, A. G. & Proni, G. Probing molecular chirality by CD-sensitive dimeric metalloporphyrin hosts. Chem. Commun. 5958–5980 (2009).
Liu, M. H., Zhang, L. & Wang, T. Y. Supramolecular Chirality in Self-Assembled Systems. Chem. Rev. 115, 7304–7397 (2015).
Lintuluoto, J. M., Borovkov, V. V. & Inoue, Y. Direct determination of absolute configuration of monoalcohols by bis(magnesium porphyrin). J. Am. Chem. Soc. 124, 13676–13677 (2002).
Borovkov, V. V., Lintuluoto, J. M., Fujiki, M. & Inoue, Y. Temperature Effect on Supramolecular Chirality Induction in Bis(zinc porphyrin). J. Am. Chem. Soc. 122, 4403–4407 (2000).
Hayashi, S., Yotsukura, M., Noji, M. & Takanami, T. Bis(zinc porphyrin) as a CD-sensitive bidentate host molecule: direct determination of absolute configuration of mono-alcohols. Chem. Commun. 51, 11068–11071 (2015).
Han, Z. et al. Crystallographic and Spectroscopic Studies of a Host-Guest Complex Consisting of a Novel Zinc Trisporphyrinate and a Chiral Monoamine. Inorg. Chem. 55, 3730–3737 (2016).
Li, L., Hu, C. J., Shi, B. & Wang, Y. Rationalization of chirality induction and inversion in a zinc trisporphyrinate by a chiral monoalcohol. Dalton Trans. 45, 8073–8080 (2016).
Borovkov, V. V., Harada, T., Inoue, Y., Kuroda, R. Angew. Chem. 114, 1436–1439 (2002).
Padula, D. et al. Chirality 26, 462–470 (2014).
Leggett, D. J. Computational Methods for the Determination of Formation Constants. Plenum: New York (1985).
Jiang, J., Fang, X., Liu, B. & Hu, C. m-Phthalic Diamide-Linked Zinc Bisporphyrinate: Spontaneous Resolution of Its Crystals and Its Application in Chiral Recognition of Amino Acid Esters. Inorg. Chem. 53, 3298–3306 (2014).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C 71, 3–8 (2015).
Spek, A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr., Sect. C 71, 9–18 (2015).
Acknowledgements
This work was financially supported by the National Nature Science Foundation of China for financial support (No. 21271133, 21305098 and 21531006), the Natural Science Foundation of Jiangsu Province (BK2016127502), and State and Local Joint Engineering Laboratory for Novel Functional Polymeric Materials and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Contributions
C.-J. Hu conceived the study and wrote the manuscript. J.-P. Lang contributed to the single crystal X-ray analysis, analyzed the results and revised the manuscript. C.-C. Zhuo and L. Li carried out all the experimental tests. All authors read and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41598_2017_3441_MOESM1_ESM.pdf
Supporting information for Host-guest assembly for highly sensitive probing of a chiral mono-alcohol with a zinc trisporphyrinate
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhuo, CC., Li, L., Hu, CJ. et al. Host-guest assembly for highly sensitive probing of a chiral mono-alcohol with a zinc trisporphyrinate. Sci Rep 7, 3829 (2017). https://doi.org/10.1038/s41598-017-03441-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03441-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.