Abstract
Among the three major food crops (rice, wheat and maize), wheat is unique in accumulating gluten proteins in its grains. Of these proteins, the high and low molecular weight glutenin subunits (HMW-GSs and LMW-GSs) form glutenin macropolymers that are vital for the diverse end-uses of wheat grains. In this work, we developed a new series of deletion mutants lacking one or two of the three Glu-1 loci (Glu-A1, -B1 and -D1) specifying HMW-GSs. Comparative analysis of single and double deletion mutants reinforced the suggestion that Glu-D1 (encoding the HMW-GSs 1Dx2 and 1Dy12) has the largest effects on the parameters related to gluten and dough functionalities and breadmaking quality. Consistent with this suggestion, the deletion mutants lacking Glu-D1 or its combination with Glu-A1 or Glu-B1 generally exhibited strong decreases in functional glutenin macropolymers (FGMPs) and in the incorporation of HMW-GSs and LMW-GSs into FGMPs. Further examination of two knockout mutants missing 1Dx2 or 1Dy12 showed that 1Dx2 was clearly more effective than 1Dy12 in promoting FGMPs by enabling the incorporation of more HMW-GSs and LMW-GSs into FGMPs. The new insight obtained and the mutants developed by us may aid further research on the control of wheat end-use quality by glutenin proteins.
Similar content being viewed by others
Introduction
Wheat is one of the major food crops in the world. Compared with other major cereals like rice and maize, wheat has unique end-use traits that are important for making a variety of globally consumed foods, such as various types of bread and noodles1. Two main families of gluten proteins, i.e., glutenins and gliadins, are involved in the formation of wheat end-use quality. The glutenins can be further divided into two subfamilies, high molecular weight glutenin subunits (HMW-GSs) and low molecular weight glutenin subunits (LMW-GSs), while gliadins contain four subfamilies, α/β-, γ-, δ- and ω-gliadins2,3,4. During dough processing, these proteins form a complex network (i.e., gluten), which confers viscoelasticity to the dough. The viscoelastic property of a dough determines its suitability for making a particular type of wheat food2, 5. Thus, variations in the relative amount and composition of glutenins and gliadins have important effects on gluten functionality, dough viscoelasticity, and end-use quality6, 7.
In common wheat (Triticum aestivum, 2n = 6x = 42), HMW-GSs are encoded by three homoeologous loci (Glu-A1, Glu-B1 and Glu-D1) on the long arms of group 1 chromosomes8. In each locus, there exist two HMW-GS genes, encoding a x- and a y-type subunits, respectively7, 9. Because of gene silencing and allelic variation, three to five HMW-GSs are usually expressed in common wheat, with HMW-GS composition often differing among different cultivars2, 8. The LMW-GSs are encoded by Glu-A3, Glu-B3 and Glu-D3 loci on the short arms of group 1 chromosomes10. In general, each Glu-3 locus contain several LMW-GS genes highly similar in nucleotide sequence and expression pattern, and each LMW-GS gene member frequently has two or more alleles11, 12. Gliadin genes are located in six major chromosomal loci, with Gli-A1, -B1, -D1 on the short arms of group 1 chromosomes and Gli-A2, -B2 and -D2 on the short arms of group 6 chromosomes13, 14. Some minor gliadin loci, e.g., Gli-3 and Gli-5, have also been reported on the short arms of group 1 chromosomes15, 16. Although the precise number of genes specifying gliadins is still unknown at present, it is generally accepted that the genes expressing gliadins are substantially more than those producing glutenins. Consequently, in bread wheat, HMW-GSs, LMW-GSs and total gliadins account for 7–15%, 20–35% and 40–50% of the gluten proteins, respectively1, 6, 17.
In the gluten complex, HMW-GSs and LMW-GSs covalently interact with each other by inter-molecular disulfide bonds, thus exist as glutenin macropolymers (GMPs)18,19,20,21. Gliadins exist mainly as monomers, and interact non-covalently with GMPs7, 17, 22. However, the gliadin carrying an odd number of cysteine residues can also take part in the formation of GMPs via inter-molecular disulfide bond23. Previous studies have shown that GMPs, especially those with a molecular mass greater than 250 kD, are the key determinant of gluten and dough functionalities and end-use quality6, 17, 24, 25. Gliadins may act as plasticizer to modify the extensibility of gluten and dough and thus the end-use traits14, 26, 27. The GMPs with larger molecular mass, i.e., functional GMPs (FGMPs), are insoluble in the protein extraction buffers without reductant, and can be separated from the smaller polymers and monomers and measured quantitatively. The sodium dodecyl sulfate (SDS) unextractable polymeric protein (UPP) fraction28,29,30 and the 50% (v/v) 1-propanol insoluble glutenin (IG) preparation31,32,33 are reliable indicators of FGMPs because both have been proved to be significantly and strongly related to wheat end-use quality. The protocol for IG preparation also allows the fractionation of soluble glutenin (SG) polymers that have comparatively smaller molecular mass31. Since both IG and SG consist of HMW-GSs and LMW-GSs31, the relative amounts of the two fractions reflect the behavior of glutenin subunits during their polymerization into GMPs.
Many studies aimed to dissect the structure of GMP have been carried out. Regarding HMW-GSs, only x-x and x-y dimers were found, but not those consisting of solely y-type HMW-GSs34,35,36,37. LMW-GSs could aggregate solely to form smaller polymers, and these polymers interacted covalently with y-type HMW-GSs by disulfide bonds18, 38. Mapping the disulfide bonds formed between glutenin subunits using mass spectrometry confirmed the existence of x-x and x-y HMW-GS interactions23, 39,40,41,42, and verified the disulfide bond formed by LMW-GSs and y-type HMW-GSs41. Therefore, in the current model on the main structure of GMP, HMW-GSs act as the backbone, with LMW-GSs as branches through bonding to y-type HMW-GSs43. This structural model is consistent with the dominant effects of HMW-GSs over LMW-GSs on wheat end-use quality. It has been widely observed that HMW-GSs, though occupying only 7–15% of the total gluten proteins, can explain 45–70% of the variations of breadmaking performance5, 44, 45.
Despite the studies described above, there remain important gaps in the understanding of the action of HMW-GSs and LMW-GSs in controlling wheat end-use quality. Although it is known that the importance of three Glu-1 loci in end-use quality control is Glu-D1 > Glu-B1 > Glu-A1 46,47,48,49, the mechanism causing such difference is still not very clear. Since Glu-D1 specifies one x- and one y-type HMW-GSs, it is logic to ask if the two subunits may differ in their effects on wheat end-use quality. And if so, what is the underlying mechanism? Answering these questions will deepen our understanding of the genetic and molecular basis of wheat end-use quality, thus increasing the effectiveness in improving related processing traits. Therefore, the main objective of this study was to gather new information on the function of HMW-GSs and LMW-GSs in controlling important gluten, dough and breadmaking quality parameters using two sets of genetic mutants. The first set lacked one or two of the three Glu-1 loci, with the double mutants being developed using the single mutants that we published in a previous study49. The second set included two ethyl methanesulfonate (EMS) induced knockout mutants lacking Glu-D1 specified HMW-GSs 1Dx2 and 1Dy12, respectively45. Considering that the gluten, dough and end-use quality traits of common wheat are generally controlled by polygenes, and affected by the environments22, 28, 50, 51, the mutants and their wild type (WT) progenitors were cultivated in multiple environments. The use of the resultant grain samples facilitated a more objective assessment of the effects of different genetic mutations on representative gluten, dough and breadmaking parameters. The observed effects were then related to the changes in GMP, UPP, IG and SG in order to explore the mechanisms involved. Finally, the new insight gained and its implications for understanding the gluten protein interactions in wheat end-use quality control are discussed.
Results
Development of double mutant deletion lines
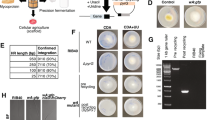
In our previous work, three ion beam induced deletion lines (DLGluA1, DLGluB1 and DLGluD1) missing Glu-A1, -B1 and -D1, respectively, were created49. Here, by conducting appropriate crossing among DLGluA1, DLGluB1 and DLGluD1, three double mutant deletion lines, DLGluA1B1 (lacking Glu-A1 and -B1), DLGluA1D1 (missing Glu-A1 and -D1) and DLGluB1D1 (devoid of Glu-B1 and -D1) were developed. SDS-PAGE analysis of seed proteins confirmed that DLGluA1B1, DLGluA1D1 and DLGluB1D1 lacked three (1Ax1, 1Bx14 and 1By15), three (1Ax1, 1Dx2 and 1Dy12) and four (1Bx14, 1By15, 1Dx2 and 1Dy12) HMW-GSs, respectively, whereas the five subunits were all normally accumulated in the wild type (WT) progenitor Xiaoyan 81 (Fig. 1). In general, the six deletion lines did not differ substantially from the WT control (Xiaoyan 81) in plant height, tiller number, grain number per spike, grain length, grain weight, and thousand kernel weight under field conditions (Table S1).
Composition of HMW-GSs in Xiaoyan 81 and six derivative deletion mutants. Xiaoyan 81 is the wild type progenitor, has all three Glu-1 loci (Glu-A1, -B1 and -D1), and expresses five HMW-GSs (1Ax1, 1Dx2, 1Bx14, 1By15 and 1Dy12). DLGluA1, DLGluB1 and DLGluD1 are single deletion mutants lacking Glu-A1, -B1 and -D1, respectively. DLGluA1B1, DLGluA1D1 and DLGluB1D1 are double deletion mutants lacking Glu-A1/Glu-B1, Glu-A1/Glu-D1 and Glu-B1/Glu-D1, respectively. The HMW-GSs missed in the six mutants are 1Ax1 (DLGluA1), 1Bx14 + 1By15 (DLGluB1), 1Dx2 + 1Dy12 (DLGluD1), 1Ax1 + 1Bx14 + 1By15 (DLGluA1B1), 1Ax1 + 1Dx2 + 1Dy12 (DLGluA1D1), and 1Bx14 + 1By15 + 1Dx2 + 1Dy12 (DLGluB1D1), respectively.
Changes of gluten, dough and end-use quality parameters
Xiaoyan 81 and the six deletion lines were cultivated in five field environments in two wheat crop seasons (2013/2014 and 2014/2015). The grains were harvested and milled, and the resultant flour samples were examined for representative gluten, dough and end-use quality parameters. In the two environments (ZX and XX) in 2013/2014, Zeleny sedimentation value (ZSV) and three Mixograph parameters, i.e., midline peak time (MPT), midline peak height (MPH) and midline peak width (MPW), were measured. ZSV is a reliable and commonly used indicator of gluten strength, with a higher ZSV indicating stronger gluten52, 53. MPT, MPH and MPW reflect dough property, with stronger and more elastic dough having higher values in the three parameters54, 55. Relative to Xiaoyan 81, the four parameters were all significantly decreased in the six deletion lines in both environments (Table 1). Generally, the decrease was strongest in DLGluD1, DLGluA1D1 and DLGluB1D1, intermediate in DLGluA1B1, and relatively low in DLGluA1 and DLGluB1 (Table 1).
In the three environments (BJ, ZX and XX) in 2014/2015, ZSV and two Farinograph parameters, i.e., dough development time (DDT) and dough stability time (DST), were measured. Concomitantly, a key breadmaking quality parameter, loaf volume (LV), was also examined. Higher DDT and DST values are generally associated with stronger dough56, 57, while a larger LV indicates better breadmaking quality58. From Table 2, it is apparent that the ZSV and the DDT and DST values of the six deletion lines were all significantly reduced when compared with those of Xiaoyan 81 in all three environments. Again, the strongest reduction was observed in DLGluD1, DLGluA1D1 and DLGluB1D1, with the decrease shown by DLGluA1B1 being intermediate and that by DLGluA1 and DLGluB1 being relatively low (Table 2). The changes in LV exhibited by the six deletion lines were more complex. Nevertheless, the LV values of DLGluD1, DLGluA1D1 and DLGluB1D1 were significantly and consistently lower than that of Xiaoyan 81 in all three environments (Table 2). On the other hand, significant decrease in LV was observed in only two environments for DLGluB1 and DLGluA1B1 and merely one environment for DLGluA1 (Table 2).
Among DLGluD1, DLGluA1D1 and DLGluB1D1, DLGluB1D1 generally exhibited the largest decreases in the measured parameters, the reductions displayed by DLGluA1D1 were intermediate, and those by DLGluD1 were comparatively smaller (Tables 1 and 2). Despite that there was only one HMW-GS (i.e., 1Ax1) left and that the examined gluten and dough functionality parameters were all severely lowered in DLGluB1D1, the LV value of this mutant was still 74.3–82.0% of that of WT control in the three environments in 2014/2015 (Table 2).
Changes of UPP and IG
The levels of UPP and IG, which indicate FGMPs28,29,30,31,32,33, were analyzed for Xiaoyan 81 and the six deletion lines cultivated in five environments using SE-HPLC and RP-HPLC, respectively (see Methods). Relative to Xiaoyan 81, UPP was significantly decreased in the six deletion lines in all five environments, with the magnitude of the decrease being high in DLGluD1, DLGluA1D1 and DLGluB1D1, intermediate in DLGluA1B1 and comparatively low in DLGluA1 and DLGluB1 (Fig. 2A). A similar finding was made with respect to IG for Xiaoyan 81 and the six deletion lines (Fig. 2B). Among DLGluD1, DLGluA1D1 and DLGluB1D1, DLGluB1D1 frequently exhibited the largest decreases in UPP and IG, with the reduction shown by DLGluD1 being comparatively smaller (Fig. 2). Nevertheless, there were still considerable amounts of UPP (20–30% of that of WT control, Fig. 2A) or IG (30–40% of that of WT control, Fig. 2B) present in DLGluB1D1.
Decrease of UPP and IG in the six deletion mutants cultivated in five environments. The five environments were created by growing Xiaoyan 81 and the six deletion lines (DLGluA1, DLGluB1, DLGluD1, DLGluA1B1, DLGluA1D1 and DLGluB1D1) in two locations (ZX and XX) in 2013/2014 and three locations (BJ, ZX and XX) in 2014/2015. The values presented are means ± SE of three separate tests, with the UPP and IG levels of Xiaoyan 81 being set as 1 to facilitate comparisons. In each environment, the means marked by different letters are statistically significant (P < 0.05). (A) Relative UPP levels in Xiaoyan 81 and the six deletion mutants. (B) Relative IG levels in Xiaoyan 81 and the six deletion mutants. BJ, Beijing; IG, insoluble glutenin; UPP, unextractable polymeric protein; XX, Xinxiang; ZX, Zhaoxian.
A Pearson correlation analysis was conducted to investigate relationships between the levels of UPP and IG and the gluten, dough and breadmaking quality parameters presented in Tables 1 and 2. The results showed that both UPP and IG were highly significantly and positively correlated with ZSV, MPT, MPH, MPW, DDT, DST and LV, with the coefficients varying from 0.783 to 0.988 (Table 3). These data suggested that UPP and IG were equally effective in representing FGMPs, and that the different degrees of reductions in gluten, dough and breadmaking quality parameters exhibited by the six deletion lines were mainly caused by differential decreases in FGMPs.
Investigation of HMW-GSs in IG
The level of HMW-GSs in IG was investigated for Xiaoyan 81 and the six deletion lines. As anticipated, the presence of HMW-GSs in IG was significantly reduced in all six deletion lines relative to that of Xiaoyan 81 in all five environments (Fig. 3). The reductions shown by DLGluD1, DLGluA1D1 and DLGluB1D1 were generally high, which was followed by DLGluA1B1; the decreases exhibited by DLGluA1 and DLGluB1 were comparatively low. In general, DLGluB1D1 exhibited the largest decrease, with only a minor amount of HMW-GSs (3.85–7.51% of that WT control, Fig. 3) present in IG.
Reduction of HMW-GSs in IG in the six deletion mutants cultivated in five environments. The five environments were formed by growing Xiaoyan 81 and the six deletion lines (DLGluA1, DLGluB1, DLGluD1, DLGluA1B1, DLGluA1D1 and DLGluB1D1) in two locations (ZX and XX) in 2013/2014 and three locations (BJ, ZX and XX) in 2014/2015. The values presented are means ± SE of three separate tests, with that of Xiaoyan 81 being set as 1 to facilitate comparisons. In each environment, the means marked by different letters are statistically significant (P < 0.05). BJ, Beijing; HMW-GSs, high-molecular-weight glutenin subunits; IG, insoluble glutenin; XX, Xinxiang; ZX, Zhaoxian.
The effects of lacking one or more HMW-GSs in the six deletion lines on the incorporation of the remaining HMW-GSs into IG were also examined. Generally, the lack of one or more HMW-GSs in the six deletion lines decreased the incorporation of the remaining HMW-GSs into IG (Figure S1). Such effects were most pronounced in DLGluD1, DLGluA1D1 and DLGluB1D1, with the reductions ranging from 31.9% to 74.35%. These effects were lessened in DLGluA1B1 (reductions varying from 18.8% to 32.6%), and became relatively weak in DLGluA1 and DLGluB1 (reductions ranging from 3.65% to 13.2%) (Figure S1).
Investigation of LMW-GSs in IG and SG
Relative to WT control, the presence of LMW-GSs in IG was consistently and most severely decreased in DLGluD1, DLGluA1D1 and DLGluB1D1 in all five environments, but this decrease was less severe in DLGluA1B1, and relatively low in DLGluA1 and DLGluB1 (Fig. 4A). Remarkably, substantial LMW-GSs (47.3–55.7% of that of WT control, Fig. 4A) were detected in the IG of DLGluB1D1, despite that there was only one HMW-GS (1Ax1) expressed in this mutant (Fig. 1).
Changes in the amount of LMW-GSs in IG and SG in the six deletion mutants cultivated in five environments. Xiaoyan 81 and the six deletion lines (DLGluA1, DLGluB1, DLGluD1, DLGluA1B1, DLGluA1D1 and DLGluB1D1) were grown in two locations (ZX and XX) in 2013/2014 and three locations (BJ, ZX and XX) in 2014/2015. The resultant grain samples were analyzed for the amounts of LMW-GSs in the IG and SG fractions, respectively. The values shown are means ± SE of three separate tests, with that of Xiaoyan 81 being set as 1 to facilitate comparisons. In each environment, the means marked by different letters are statistically significant (P < 0.05). The amounts of LMW-GSs in IG were generally decreased in the six deletion lines compared to that in Xiaoyan 81 (A). On the contrary, the amounts of LMW-GSs in SG were generally increased in the six deletion lines relative to that in Xiaoyan 81 (B). BJ, Beijing; IG, insoluble glutenin; LMW-GSs, low-molecular-weight glutenin subunits; SG, soluble glutenin; XX, Xinxiang; ZX, Zhaoxian.
On the contrary, the existence of LMW-GSs in SG was generally and strongly enhanced in DLGluD1, DLGluA1D1 and DLGluB1D1 in all five environments, with presence of LMW-GSs in SG increased by 57–106% in the three mutants relative to WT control (Fig. 4B). This enhancement was, however, less pronounced for DLGluA1, DLGluB1 and DLGluA1B1 (increased by 1–26% relative to WT control, Fig. 4B).
Relative abundance of different HMW-GSs in IG
As shown in Table 4, the percentages of IG occupied by the HMW-GSs encoded by three Glu-1 loci differed significantly, 14.79–16.38% by the Glu-D1 subunits 1Dx2 + 1Dy12, 10.86–12.96% by the Glu-B1 subunits 1Bx14 + 1By15, and 4.26–7.02% by the Glu-A1 subunit 1Ax1. Obviously, the abundance of 1Dx2 + 1Dy12 in IG was substantially higher than that of 1Bx14 + 1By15 or 1Ax1, with the amount of 1Ax1 being the lowest. In line with this difference, the lack of 1Dx2 and 1Dy12 together caused the largest reduction in IG (by 47.93–53.52%), the strongest decrease of HMW-GSs in IG (69.51–72.08%), and the most severe reduction of LMW-GSs in IG (37.19–45.37%) (Table 4). The three effects were lessened when 1Bx14 and 1By15 were missed, and tended to be small when 1Ax1 was absent (Table 4).
Effects of 1Dx2 or 1Dy12 alone on gluten, dough and end-use quality parameters
The foregoing experiments highlighted the functional dominance of Glu-D1 over Glu-A1 and -B1. Because Glu-D1 encodes both 1Dx2 and 1Dy12, it became necessary and important to examine if the two subunits may act similarly or differently in wheat end-use quality control. To this end, we compared two EMS knockout mutants (md2-1 and md12-1) lacking the expression of 1Dx2 and 1Dy12, respectively (Figure S2). The two mutants were developed using the common wheat cultivar Xiaoyan 5445, and their genetic backgrounds were made near-identical to that of Xiaoyan 54 through six rounds of backcrossing (see Methods). Xiaoyan 54 is one of the two parents of Xiaoyan 81, and expresses an identical set of HMW-GSs as Xiaoyan 81 (Figure S2).
Xiaoyan 54 and the two knockout mutants were cultivated in two crop seasons (environments) (2014/2015 and 2015/2016), with the grains harvested being used for measuring gluten, dough and end-use quality parameters. In the two environments, ZSV and the DDT and DST values of the two knockout mutants were generally and significantly decreased relative to those of Xiaoyan 54, and in four of the six cases, the reduction exhibited by md2-1 was significantly more severe than that by md12-1 (Table 5). In agreement with these results, the loaf volume values of the two knockout mutants were significantly lower than those of Xiaoyan 54 in both environments (Table 5). Moreover, the loaf volume of md2-1 tended to be smaller than that of md12-1 (Figure S3), with the difference reached to a significant level (P < 0.05) in 2014/2015 (Table 5).
Alterations in IG content and composition caused by knocking out 1Dx2 or 1Dy12
The potential consequences of lacking 1Dx2 or 1Dy12 on IG content and the incorporations of HMW-GSs and LMW-GSs into IG were investigated as described above. The results obtained for the grain samples harvested in 2014/2015 are displayed in Fig. 5. Compared with Xiaoyan 54, IG content was significantly decreased in both md2-1 and md12-1, but the scale of the decrease was much higher in md2-1 (Fig. 5A). The knockout of 1Dx2 reduced the incorporation of the remaining HMW-GSs into IG, with the percentage of the reduction being 38.7%, 47.0%, 48.2% and 45.4% for 1Ax1, 1Bx14, 1By15 and 1Dy12, respectively (Fig. 5B). The knockout of 1Dy12 also decreased the incorporation of other HMW-GSs into IG, but the percentages of the reduction observed (21.5% for 1Ax1, 33.8% for 1Bx14, 44.4% for 1By15 and 32.8% for 1Dx2) were generally lower than those caused by the lacking of 1Dx2 (compare Fig. 5B and C). Lastly, the lack of 1Dx2 decreased the incorporation of LMW-GSs in IG by 39.3%, whereas the absence of 1Dy12 reduced the incorporation of LMW-GSs in IG by only 18.7% (Fig. 5D). The results gathered for the grain samples harvested in 2015/2016 (Figure S4) were similar to those shown in Fig. 5, although the scales of the decreases in IG, LMW-GSs in IG, and the percentages of reduction of different HMW-GSs in IG tended to be smaller. These variations may be caused by differences in the growth environment between the two seasons.
Comparative analysis of Xiaoyan 54 and two derivative knockout mutants (md2-1 and md12-1) lacking the high-molecular-weight glutenin subunits (HMW-GSs) 1Dx2 and 1Dy12, respectively. The three lines were cultivated in Beijing in 2014/2015, and the resultant grain samples were collected for this set of analysis. (A) The amount of insoluble glutenin (IG) was decreased in md2-1 and md12-1 relative to that of Xiaoyan 54 (set as 1), with the decrease exhibited by md2-1 being substantially stronger. The values shown are means ± SE of three separate tests, and those labeled by different letters are statistically significant (P < 0.05). (B) Reduction of the remaining four HMW-GSs (1Ax1, 1Bx14, 1By15 and 1Dy12) in the IG of md2-1. The percentages of reduction were calculated by setting the amounts of the four subunits in the IG of Xiaoyan 54 as 100%. The values shown are means ± SE of three different tests. (C) Reduction of the remaining four HMW-GSs (1Ax1, 1Bx14, 1By15 and 1Dx2) in the IG of md12-1. The percentages of reductions presented were calculated as described in (C). (D) The amount of low-molecular-weight glutenin subunits (LMW-GSs) was reduced in md2-1 and md12-1 relative to that of Xiaoyan 54 (set as 1), with the reduction shown by md2-1 being considerably stronger. The values shown are means ± SE of three different tests, and those labeled by different letters are statistically significant (P < 0.05). The same set of analysis was also conducted using the grain samples of the three lines harvested in 2015/2016 with similar results obtained (Figure S4).
Discussion
In this work, we investigated the function of glutenin proteins in wheat end-use quality control and the mechanism involved through analyzing two series of well-defined genetic mutants. Complementary sets of data were obtained using the grain samples harvested from multiple environments, which permitted an objective assessment of the genetic effects of lacking one or two Glu-1 loci on the examined gluten, dough and breadmaking quality parameters. The new insight obtained is discussed below.
Comparative analysis of single and double mutants of Glu-1 loci reinforces the dominance of Glu-D1 in wheat end-use quality control
Previously, we found that the contribution of three Glu-1 loci to wheat gluten and GMP parameters can be ranked as Glu-D1 > Glu-B1 > Glu-A1 through analyzing single deletion mutants lacking individual Glu-1 loci49. Here, we substantially extended our investigation by including both single and double deletion mutants of Glu-1 loci, using the grain samples from multiple field environments, and testing more gluten, dough and end-use quality parameters. We consistently observed that the mutants lacking Glu-D1 (DLGluD1) or its combination with Glu-A1 (DLGluA1D1) or Glu-B1 (DLGluB1D1) showed the strongest reductions in the examined gluten, dough and breadmaking quality parameters (Tables 1 and 2). These observations reinforce the dominance of Glu-D1 in wheat end-use quality control. In most cases, the reductions displayed by DLGluA1D1 were larger than those by DLGluD1 but smaller than those by DLGluB1D1. This is consistent with the fact that the number of HMW-GSs lacked in DLGluB1D1 (i.e., 4) was more than that in DLGluA1D1 (3) or DLGluD1 (2) (Fig. 1). Clearly, there exist positive and additive interactions among the three Glu-1 loci studied in this work, with the functional effects of the interactions between Glu-A1 and Glu-D1 being comparatively weaker than those between Glu-B1 and Glu-D1. Past studies have also detected positive and additive interactions among the three Glu-1 loci47, 48, 59, 60.
While comparing the doughs of Xiaoyan 81 and the single and double mutants using Farinograph test (Table 1), we focused on only two major parameters (DDT and DST) owing to the large number of samples needing to be assayed. However, other parameters of this test, i.e., width of Farinograph curve at peak consistency and rapidity of decline of Farinograph curve after peak consistency, also provide useful information on dough elasticity and cohesiveness61, 62. Upon closer examination of the Farinograph curves (Figure S5), the width of the curve in DLGluA1, DLGluB1 and DLGluA1B1 was not reduced as severely as that in DLGluD1, DLGluA1D1 and DLGluB1D1, and in general, the Farinograph curves of DLGluA1, DLGluB1 and DLGluA1B1 were declined less rapidly than those of DLGluD1, DLGluA1D1 and DLGluB1D1. Since DLGluA1, DLGluB1 and DLGluA1B1 all possessed a functional Glu-D1, these observations suggest that Glu-D1 is more important than Glu-A1 and Glu-B1 in maintaining the width of Farinograph curve at peak consistency and for slowing down the decline of Farinograph curve after peak consistency. Because of the presence of Glu-D1 in DLGluA1, DLGluB1 and DLGluA1B1, the functionality of the doughs of the three lines was not lowered as drastically as that of DLGluD1, DLGluA1D1 and DLGluB1D1 (all lacking Glu-D1). This may help to explain the less consistent decreases in LV observed for DLGluA1, DLGluB1 and DLGluA1B1 in different environments despite extensive reductions in their ZSV, DDT and DST values (Table 2).
The functional dominance of Glu-D1 over Glu-A1 and Glu-B1 had also been suggested by prior studies using recombinant wheat lines differing in the composition of Glu-1 loci and in genetic background46,47,48, 63. For example, Lawrence and coauthors demonstrated that Glu-D1d (a different Glu-D1 allele encoding 1Dx5 and 1Dy10 subunits) was functionally superior to Glu-A1 and Glu-B1 46. In contrast, our data were obtained by using six mutant lines with highly similar genetic background. Therefore, our work validated previous observation by more robust genetic data. The Glu-D1 allele studied by us is Glu-D1a, which is predominant in worldwide common wheat varieties64, 65. Apart from Glu-D1a and Glu-D1d, there are several minor Glu-D1 alleles (Glu-D1b, -D1c, -D1e and -D1f)64. It will be interesting to investigate if these minor Glu-D1 alleles may also be functionally dominant over Glu-A1 and Glu-B1 in the future.
Glu-D1 has the strongest potency to promote the incorporation of HMW-GSs and LMW-GSs into FGMPs
FGMPs play pivotal roles in gluten and dough functionality and end-use quality20, 66. Their amount and polymerization characteristics are strongly affected by both the quantity and structural features of different HMW-GSs and LMW-GSs. Based on the changes in UPP, IG and the amount of HMW-GSs and LMW-GSs in IG among WT control and the six deletion mutants observed in this work (Figs 2–4), we suggest that the three Glu-1 loci differ significantly in the ability to control the accumulation of FGMPs through promoting the incorporation of HMW-GSs and LMW-GSs into FGMPs. Specifically, Glu-D1 has the strongest potency to promote the incorporation of HMW-GSs and LMW-GSs into FGMPs, and thus makes the largest contribution to FGMP accumulation. In contrast, Glu-B1 is less effective than Glu-D1, and Glu-A1 is weaker than Glu-B1 in these processes. From this suggestion and the existence of highly significant correlations between the changes in gluten, dough and breadmaking quality parameters and those in UPP and IG content (Table 3), we further propose that, for individual Glu-1 loci, the higher the potency to promote the incorporation of HMW-GSs and LMW-GSs into FGMPs, the stronger the contributions to FGMPs, gluten and dough functionality, and end-use quality performance.
The high potency of Glu-D1 in promoting the incorporation of HMW-GSs and LMW-GSs into FGMPs is also supported by two additional lines of evidence. First, the absence of Glu-D1 or its combination with Glu-A1 or Glu-B1 reduced the incorporation of the remaining HMW-GSs into IG (by 31.9–74.35%) much more strongly than that (18.8–32.6%) caused by lacking Glu-A1, Glu-B1 or both (Figure S1). Second, in the absence of Glu-D1 or its combination with Glu-A1 or Glu-B1, the presence of LMW-GSs in SG was greatly enhanced (by 57–106%) relative to that (1–26%) due to the mutation of Glu-A1, Glu-B1 or both (Fig. 4B).
The reason(s) underlying the enhanced potency of Glu-D1 to promote the incorporation of HMW-GSs and LMW-GSs into FGMPs may be complex, because the subunits encoded by Glu-D1 (1Dx2 and 1Dy12) differ from those encoded by Glu-B1 (1Bx14 and 1By15) and Glu-A1 (1Ax1) in multiple aspects. Nevertheless, we noticed that the abundance in IG of 1Dx2 + 1Dy12 was significantly higher than that of 1Bx14 + 1By15 or 1Ax1, and nearly equaled to the amount of 1Bx14 + 1By15 + 1Ax1 in all five environments (Table 4). Furthermore, the reduction of IG and the decreases of HMW-GSs and LMW-GSs in IG brought about by lacking 1Dx2 + 1Dy12 were always more severe than those caused by missing 1Bx14 + 1By15 or 1Ax1 (Table 4). Therefore, the high abundance of 1Dx2 + 1Dy12 in IG (relative to that of 1Bx14 + 1By15 or 1Ax1) is likely an important factor for the functional dominance of Glu-D1 (over that of Glu-B1 or Glu-A1). Because of the existence of many amino acid substitutions among the deduced proteins of 1Dx2, 1Ax1 and 1Bx14 and between those of 1Dy12 and 1By1567, 68, the structural differences of these subunits may also contribute to the functional dominance of Glu-D1. Further work is needed to validate this possibility.
1Dx2 has a stronger function than 1Dy12
In common wheat, Glu-B1, Glu-D1 and their different alleles usually express two different HMW-GSs (one x- and one y-type)2, 8. Consequently, uncovering functional difference between the two subunits is essential for more comprehensively understanding the action of HMW-GSs in controlling wheat end-use quality. Some information has been gained on the function of certain HMW-GSs (e.g., 1Dx5 and 1Dy10) in controlling wheat end-use quality through studying variety population differing in HMW-GS composition, transgenic overexpression or RNA interference69,70,71,72. However, there is still no report on the use of knockout mutants with a near identical genetic background in investigating functional difference between the two subunits encoded by a Glu-1 locus. In this work, we examined functional difference between the Glu-D1 encoded subunits 1Dx2 and 1Dy12 by comparing two knockout mutants, md2-1 (lacking 1Dx2 expression) and md12-1 (without 1Dy12 accumulation), with their WT progenitor Xiaoyan 54. Judging from the data presented in Table 5, the function of 1Dx2 is generally and considerably stronger than that of 1Dy12 with respect to the control of the examined gluten, dough and breadmaking quality parameters. The stronger function of 1Dx2 (relative to that of 1Dy12) is most likely caused by its higher contribution to FGMPs through promoting the incorporation of more HMW-GSs and LMW-GSs into FGMPs (Fig. 5). Thus, the ability to promote the incorporation of more HMW-GSs and LMW-GSs into FGMPs is a common reason for the functional superiority of both Glu-D1 and the 1Dx2 subunit encoded by it.
In line with our finding, earlier studies also revealed that x-type subunits had greater effects on dough functionality parameters than y-type subunits by analyzing transgenic lines and variety population71, 73 or through artificial incorporation of HMW-GSs into developing dough74. Thus, the function of x-type HMW-GSs may be generally stronger than that of y-type HMW-GSs in the control of wheat end-use quality. This raises the question what is the mechanism behind the stronger function of x-type HMW-GSs. In the current model on the structure of GMPs, y-type HMW-GSs interact covalently with LMW-GSs, with the resultant units linked by x-type HMW-GSs43. Although x-y and x-x linkages have been found among HMW-GSs, it is still uncertain if covalent interactions may happen between x-type HMW-GSs and LMW-GSs23, 39, 40, 42. We speculate that x-type HMW-GSs may interact with LMW-GSs and form FGMPs. This speculation is based on the gluten, dough and breadmaking quality parameters obtained in this work for the double mutant DLGluB1D1. Although this mutant had only one x-type HMW-GS (i.e., 1Ax1) accumulated in the grains (Fig. 1), its bread volume was still 74.3–82.0% of that of WT control (Table 2), and its UPP and IG contents were still 20–30% and 30–40% of those of WT control, respectively (Fig. 2). Moreover, a substantial amount of LMW-GSs was present in the IG of DLGluB1D1 (47.3–55.7% of that of WT control, Fig. 4). Considering that there was no y-type HMW-GS present in DLGluB1D1, and the level of 1Ax1 in its grains was rather low (Fig. 3 and Figure S1), the interactions between 1Ax1 and LMW-GSs, if existed, may be fairly effective. Therefore, the actions of x-type HMW-GSs in FGMP formation are likely more extensive than currently thought. A better elucidation of these actions may help to explain the functional superiority of x-type HMW-GSs (over their y-type counterparts) in wheat-end use quality control.
In summary, we have generated new information on the functional difference among three Glu-1 loci and between two HMW-GSs (1Dx2 and 1Dy12). The three loci, as well as the two subunits, differ significantly in the efficacy to promote the incorporation of HMW-GSs and LMW-GSs into FGMPs, and these differences are largely responsible for the functional dominance of Glu-D1 over Glu-A1 and Glu-B1 and the functional superiority of 1Dx2 to 1Dy12. This insight increases our understanding of the function of HMW-GSs in controlling important gluten and dough properties and breadmaking performance. Moreover, the data from this work and our previous study45 confirm that the Glu-1 locus deletion mutants and the EMS mutants lacking individual or combinations of HMW-GSs are valid materials for further research on wheat end-use quality. Continued analysis of these mutants with functional genomics approaches (e.g., using transcriptomic, proteomic and/or metabolic methods) may shed new light on the genetic and molecular basis of gluten and dough functionalities and lead to valuable strategies for improving wheat end-use traits.
Methods
Plant materials and growth conditions
Genetic crosses were conducted in between DLGluA1, DLGluB1 and DLGluD1, which have Glu-A1, -B1 and -D1 deleted, respectively49. Homozygous plants missing two Glu-1 loci (Glu-A1 and -B1, Glu-A1 and -D1 or Glu-B1 and -D1) were identified by checking HMW-GS composition in F2 seeds, and used to develop the three double deletion mutants DLGluA1B1, DLGluA1D1 and DLGluB1D1. Xiaoyan 81 (WT progenitor) and the six deletion mutants were grown in five field environments with normal supplies of irrigation water and chemical fertilizers75. The five environments were created by growing the materials in two locations (Zhaoxian and Xinxiang) in 2013/2014 and three locations (Beijing, Zhaoxian and Xinxiang) in 2014/2015. The knockout mutants md2-1 and md12-1, lacking the expression of 1Dx2 and 1Dy12, respectively, were backcrossed six times using their WT progenitor Xiaoyan 54 as recurrent parent45. In this study, the three isogenic lines (Xiaoyan 54, md2-1 and md12-1) were cultivated in Beijing in two wheat crop cycles (2014/2015 and 2015/2016) as described above. The deletion mutants and their grains were checked for agronomic traits using standard methods49, 75. Flour samples were prepared for the different lines as reported before49.
SDS-PAGE
HMW-GSs accumulated in the different experimental lines were extracted using 20 mg flour, and separated with 10% SDS-PAGE according to the method describe previoulsy76.
RP-HPLC
IG and SG levels in the flour samples were assayed using RP-HPLC. The majority of the analysis steps were carried out at room temperature (RT, approximately 25 °C) except where noted. IG and SG were extracted following the method detailed previously31. Briefly, for each line (WT control or deletion mutant), the flour sample (50 mg) was extracted twice with 0.5 ml of 50% (v/v) 1-propanol. After each extraction, the sample was centrifuged at 2,200 g for 3 min. The pellet was washed with 0.5 ml 50% (v/v) 1-propanol for 1 min and centrifuged at 15,000 g for 3 min. The pellet was further extracted with 0.5 ml 50% (v/v) 1-propanol containing 1% (w/v) dithiothreitol (DTT) at 65 °C for 1 h, followed by the addition of 1.4% (v/v) 4-vinylpyridine for 30 min at 65 °C. The mixture was then centrifuged at 15,000 g for 10 min, with the supernatant retained as IG fraction. The three supernatants after the extraction with 50% (v/v) 1-propanol in the preceding steps were combined, and 1-propanol was added to 70%. After centrifugation at 12,000 g for 3 min, the precipitated proteins were dissolved in 0.5 ml 50% (v/v) 1-propanol containing 1% (w/v) DTT by incubating at 65 °C for 1 h. Subsequently, 4-vinylpyridine was added to 1.4% (v/v), and the mixture was maintained at 65 °C for another 30 min. Lastly, the mixture was centrifuged at 15,000 g for 10 min, with the supernatant kept as SG fraction. The SG and IG fractions were all filtered through 0.45 μm nylon filter before being analyzed by RP-HPLC. RP-HPLC analysis was accomplished with the Agilent 1260 infinity Quaternary LC System using a C18 column. The elution conditions were essentially those described by González-Torralba and coauthors77. For each RP-HPLC run, a volume of 15 μl of the filtered IG (SG) was analyzed. The amount of HMW-GSs and LMW-GSs were calculated by integrating the areas under the corresponding protein peaks of the chromatogram.
SE-HPLC
The assay of UPP content by SE-HPLC was conducted at RT following the method described in a previous study78. Briefly, for each sample to be assayed for UPP, 10 mg flour was suspended in 1 ml extraction buffer (50 mM sodium phosphate containing 0.5% SDS, pH 6.9), and vortexed for 10 min. The mixture was centrifuged at 17,000 g for 15 min, and the resultant pellet was resuspended in 1 ml extraction buffer, followed by sonication in a SCIENTZ-IID sonicator (Scientz Biotechnology Co., Ningbo, China). The condition of sonication was 20% output power for 30 s using a 3 mm sonicator probe, with the probe placed at 1/3 distance from the bottom of the microfuge tube. Afterwards, the mixture was centrifuged at 17,000 g for 15 min, with the supernatant retained as UPP. It was filtered through 0.45 μm nylon filter, and analyzed by SE-HPLC with the Agilent 1260 infinity Quaternary LC System using a Biosep SEC-4000 column (Phenomenex, Torrence, CA, USA). For each filtered UPP sample, an aliquot of 15 μl was assayed by SE-HPLC, with UPP content calculated by integrating the areas under the corresponding peaks of the chromatogram.
Evaluation of ZSV and Mixograph, Farinograph and loaf volume parameters
ZSV was measured following the method described previously52. Mixograph parameters (MPT, MPH and MPW) were determined with a 10 g mixograph system (National Manufacturing Co., Lincoln, NE, USA) using the AACCI method 54–40.0279. Farinograph parameters (DDT and DST) and loaf volume were measured according to the AACCI methods 54–21.02 and 10–10.03, respectively79.
Statistical analysis
For the experiments described above, three separate tests were carried out for each sample. The data obtained were subjected to one-way analysis of variance (ANOVA) using IBM SPSS Statistics 19 software (IBM, New York, USA), followed by the least significant difference multiple comparison test. Pearson’s correlation coefficients between IG, UPP and the gluten, dough and breadmaking quality parameters were calculated using the IBM SPSS Statistics 19 software.
References
Rasheed, A. et al. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J Cereal Sci 60, 11–24 (2014).
Veraverbeke, W. S. & Delcour, J. A. Wheat protein composition and properties of wheat glutenin in relation to breadmaking functionality. Crit Rev Food Sci 42, 179–208 (2002).
Anderson, O. D., Dong, L., Huo, N. & Gu, Y. Q. A New Class of Wheat Gliadin Genes and Proteins. PLoS One 7, e52139 (2012).
Wan, Y., Shewry, P. R. & Hawkesford, M. J. A novel family of γ-gliadin genes are highly regulated by nitrogen supply in developing wheat grain. J Exp Bot 64, 161–168 (2013).
Shewry, P. R. et al. in Advances in Food and Nutrition Research, Vol. 45 (ed. Taylor, S.L.) 219–302 (Academic Press, 2003).
Wrigley, C. et al. In Wheat Science and Trade (ed. Carver, B.F.) 495-520 (Wiley-Blackwell, 2009).
Delcour, J. A. et al. Wheat gluten functionality as a quality determinant in cereal-based food products. Annu Rev Food Sci Technol 3, 469–492 (2012).
Payne, P. I., Law, C. N. & Mudd, E. E. Control by homoeologous group 1 chromosomes of the high-molecular-weight subunits of glutenin, a major protein of wheat endosperm. Theor Appl Genet 58, 113–120 (1980).
Payne, P. I., Holt, L. M. & Law, C. N. Structural and genetical studies on the high-molecular-weight subunits of wheat glutenin. Theor Appl Genet 60, 229–236 (1981).
Singh, N. K. & Shepherd, K. W. Linkage mapping of genes controlling endosperm storage proteins in wheat. Theor Appl Genet 75, 628–641 (1988).
Dong, L. et al. New insights into the organization, recombination, expression and functional mechanism of low molecular weight glutenin subunit genes in bread wheat. PLoS One 5, e13548 (2010).
Zhang, X. et al. Novel insights into the composition, variation, organization, and expression of the low-molecular-weight glutenin subunit gene family in common wheat. J Exp Bot 64, 2027–2040 (2013).
Qi, P. F., Wei, Y. M., Yue, Y. W., Yan, Z. H. & Zheng, Y. L. Biochemical and molecular characterization of gliadins. Mol Biol 40, 713–723 (2006).
Barak, S., Mudgil, D. & Khatkar, B. S. Biochemical and functional properties of wheat gliadins: a review. Crit Rev Food Sci 55, 357–368 (2015).
Payne, P. I., Holt, L. M. & Lister, P. G. In Proceedings of the 7th International Wheat Genetics symposium (eds. Miller, T. E. & Koebner, R. M. D.) 999–1002 (Bath Press, 1988).
Pogna, N. E., Metakovsky, E. V., Redaelli, R., Raineri, F. & Dachkevitch, T. Recombination mapping of Gli-5, a new gliadin-coding locus on chromosomes 1A and 1B in common wheat. Theor Appl Genet 87, 113–121 (1993).
Juhász, A., Békés, F. & Wrigley, C. W. In Applied Food Protein Chemistry (ed Ustunol, Z.) 219–303 (John Wiley & Sons, Ltd, 2014).
Graveland, A., Bosveld, P., Lichtendonk, W. J., Moonen, H. E. H. & Scheepstra, A. Extraction and fractionation of wheat flour proteins. J Sci Food Agr 33, 1117–1128 (1982).
Don, C., Lichtendonk, W., Plijter, J. J. & Hamer, R. J. Glutenin macropolymer: a gel formed by glutenin particles. J Cereal Sci 37, 1–7 (2003).
Don, C., Lichtendonk, W. J., Plijter, J. J. & Hamer, R. J. Understanding the link between GMP and dough: from glutenin particles in flour towards developed dough. J Cereal Sci 38, 157–165 (2003).
Don, C., Mann, G., Bekes, F. & Hamer, R. J. HMW-GS affect the properties of glutenin particles in GMP and thus flour quality. J Cereal Sci 44, 127–136 (2006).
Békés, F. New aspects in quality related wheat research: 1. Challenges and achievements. Cereal Res Commun 40, 159–184 (2012).
Köhler, P., Belitz, H. D. & Wieser, H. Disulphide bonds in wheat gluten: further cystine peptides from high molecular weight (HMW) and low molecular weight (LMW) subunits of glutenin and from gamma-gliadins. Z Lebensm Unters Forsch 196, 239–247 (1993).
Bangur, R., Batey, I. L., McKenzie, E. & MacRitchie, F. Dependence of extensograph parameters on wheat protein composition measured by SE-HPLC. J Cereal Sci 25, 237–241 (1997).
MacRitchie, F. Theories of glutenin/dough systems. J Cereal Sci 60, 4–6 (2014).
Uthayakumaran, S. et al. Effects of gliadin fractions on functional properties of wheat dough depending on molecular size and hydrophobicity. Cereal Chem 78, 138–141 (2001).
Gómez, A., Ferrero, C., Calvelo, A., Añón, M. C. & Puppo, M. C. Effect of mixing time on structural and rheological properties of wheat flour dough for breadmaking. Int J Food Prop 14, 583–598 (2011).
Moldestad, A. et al. Temperature variations during grain filling obtained in growth tunnel experiments and its influence on protein content, polymer build-up and gluten viscoelastic properties in wheat. J Cereal Sci 60, 406–413 (2014).
Koga, S. et al. Influence of temperature during grain filling on gluten viscoelastic properties and gluten protein composition. J Sci Food Agr 96, 122–130 (2016).
Simsek, S., Ohm, J.-B., Cariou, V. & Mergoum, M. Effect of flour polymeric proteins on dough thermal properties and breadmaking characteristics for hard red spring wheat genotypes. J Cereal Sci 68, 164–171 (2016).
Sapirstein, H. D. & Fu, B. X. Intercultivar variation in the quantity of monomeric proteins, soluble and insoluble glutenin, and residue protein in wheat flour and relationships to breadmaking quality. Cereal Chem 75, 500–507 (1998).
Hu, X. Z., Wei, Y. M., Wang, C. & Kovacs, M. I. P. Quantitative assessment of protein fractions of Chinese wheat flours and their contribution to white salted noodle quality. Food Res Int 40, 1–6 (2007).
Jin, H. et al. Genetic analysis of chromosomal loci affecting the content of insoluble glutenin in common wheat. J Genet Genomics 42, 495–505 (2015).
Lawrence, G. J. & Payne, P. I. Detection by gel electrophoresis of oligomers formed by the association of high-molecular-weight glutenin protein subunits of wheat endosperm. J Exp Bot 34, 254–267 (1983).
Lindsay, M. P. & Skerritt, J. H. Examination of the structure of the glutenin macropolymer in wheat flour and doughs by stepwise reduction. J Agr Food Chem 46, 3447–3457 (1998).
Veraverbeke, W. S., Larroque, O. R., Békés, F. & Delcour, J. A. In vitro polymerization of wheat glutenin subunits with inorganic oxidizing agents. I. Comparison of single-step and stepwise oxidations of high molecular weight glutenin subunits. Cereal Chem 77, 582–588 (2000).
Veraverbeke, W. S., Larroque, O. R., Békés, F. & Delcour, J. A. In vitro polymerization of wheat glutenin subunits with inorganic oxidizing agents. II. Stepwise oxidation of low molecular weight glutenin subunits and a mixture of high and low molecular weight glutenin subunits. Cereal Chem 77, 589–594 (2000).
Graveland, A. et al. A model for the molecular structure of the glutenins from wheat flour. J Cereal Sci 3, 1–16 (1985).
Köhler, P., Belitz, H.-D. & Wieser, H. Disulphide bonds in wheat gluten: isolation of a cystine peptide from glutenin. Z Lebensm Unters Forch 192, 234–239 (1991).
Tao, H. P., Adalsteins, A. E. & Kasarda, D. D. Intermolecular disulfide bonds link specific high-molecular-weight glutenin subunits in wheat endosperm. Biochim Biophys Acta 1159, 13–21 (1992).
Keck, B., Kohler, P. & Wieser, H. Disulphide bonds in wheat gluten: cystine peptides derived from gluten proteins following peptic and thermolytic digestion. Z Lebensm Unters Forsch 200, 432–439 (1995).
Lutz, E., Wieser, H. & Koehler, P. Identification of disulfide bonds in wheat gluten proteins by means of mass spectrometry/electron transfer dissociation. J Agr Food Chem 60, 3708–3716 (2012).
Wieser, H. Chemistry of gluten proteins. Food Microbiol 24, 115–119 (2007).
Payne, P. I., Holt, L. M., Krattiger, A. F. & Carrillo, J. M. Relationships between seed quality characteristics and HMW glutenin subunit composition determined using wheats grown in Spain. J Cereal Sci 7, 229–235 (1988).
Li, Y. et al. Dissecting and enhancing the contributions of high-molecular-weight glutenin subunits to dough functionality and bread quality. Mol Plant 8, 332–334 (2015).
Lawrence, G. J., MacRitchie, F. & Wrigley, C. W. Dough and baking quality of wheat lines deficient in glutenin subunits controlled by the Glu-A1, Glu-B1 and Glu-D1 loci. J Cereal Sci 7, 109–112 (1988).
Beasley, H. L. et al. Synergistic and additive effects of three high molecular weight glutenin subunit loci. II. Effects on wheat dough functionality and end-use quality. Cereal Chem 79, 301–307 (2002).
Uthayakumaran, S. et al. Synergistic and additive effects of three high molecular weight glutenin subunit loci. I. Effects on wheat dough rheology. Cereal Chem 79, 294–300 (2002).
Yang, Y. et al. Efficient isolation of ion beam-induced mutants for homoeologous loci in common wheat and comparison of the contributions of Glu-1 loci to gluten functionality. Theor Appl Genet 127, 359–372 (2014).
Malik, A. H., Kuktaite, R. & Johansson, E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to bread-making quality. J Cereal Sci 57, 170–174 (2013).
Rozbicki, J. et al. Influence of the cultivar, environment and management on the grain yield and bread-making quality in winter wheat. J Cereal Sci 61, 126–132 (2015).
Zeleny, L., Greenaway, W. T., Gurney, G. M., Fifield, C. C. & Lebsock, K. Sedimentation value as an index of dough-mixing characteristics in early-generation wheat selections. Cereal Chemistry 37, 673–678 (1960).
Wang, C. & Kovacs, M. Swelling index of glutenin test. I. Method and comparison with sedimentation, gel-protein, and insoluble glutenin tests. Cereal Chem 79, 183–189 (2002).
Chung, O. K., Ohm, J. B., Caley, M. S. & Seabourn, B. W. Prediction of baking characteristics of hard winter wheat flours using computer-analyzed mixograph parameters. Cereal Chem 78, 493–497 (2001).
Mao, X. et al. The interactive effects of transgenically overexpressed 1Ax1 with various HMW-GS combinations on dough quality by introgression of exogenous subunits into an elite Chinese wheat variety. PLoS One 8, e78451 (2013).
Dowell, F. E. et al. Relationship of bread quality to kernel, flour, and dough properties. Cereal Chem 85, 82–91 (2008).
Wang, Y. et al. Low molecular weight glutenin subunit gene Glu-B3h confers superior dough strength and breadmaking quality in wheat (Triticum aestivum L.). Sci Rep 6, 27182 (2016).
Ross, A. S. & Bettge, A. D. In Wheat Science and Trade (ed. Carver, B. F.) 455-493 (Wiley-Blackwell, 2009).
Kolster, P., van Eeuwijk, F. A. & van Gelder, W. M. J. Additive and epistatic effects of allelic variation at the high molecular weight glutenin subunit loci in determining the bread-making quality of breeding lines of wheat. Euphytica 55, 277–285 (1991).
He, Z. H., Liu, L., Xia, X. C., Liu, J. J. & Peña, R. J. Composition of HMW and LMW glutenin subunits and their effects on dough properties, pan bread, and noodle quality of Chinese bread wheats. Cereal Chem 82, 345–350 (2005).
Mann, G. et al. Comparison of small-scale and large-scale mixing characteristics: Correlations between small-scale and large-scale mixing and extensional characteristics of wheat flour dough. J Cereal Sci 47, 90–100 (2008).
Stojceska, V. & Butler, F. Digitization of farinogram plots and estimation of mixing stability. J Cereal Sci 48, 729–733 (2008).
Zhang, Y. et al. The gluten protein and interactions between components determine mixograph properties in an F6 recombinant inbred line population in bread wheat. J Cereal Sci 50, 219–226 (2009).
Payne, P. I. & Lawrence, G. J. Catalogue of alleles for the complex gene loci, Glu-A1, Glu-B1, and Glu-D1 which code for high-molecular-weight subunits of glutenin in hexaploid wheat. Cereal Res Commun 11, 29–35 (1983).
Dong, Z. et al. Haplotype variation of Glu-D1 locus and the origin of Glu-D1d allele conferring superior end-use qualities in common wheat. PLoS One 8, e74859 (2013).
Weegels, P. L., Hamer, R. J. & Schofield, J. D. Functional properties of wheat glutenin. J Cereal Sci 23, 1–17 (1996).
Li, W. et al. Molecular characterization of HMW glutenin subunit allele 1Bx14: further insights in to the evolution of Glu-B1-1 alleles in wheat and related species. Theor Appl Genet 109, 1093–1104 (2004).
Pang, B. S. & Zhang, X. Y. Isolation and molecular characterization of high molecular weight glutenin subunit genes 1Bx13 and 1By16 from hexaploid wheat. J Integr Plant Biol 50, 329–337 (2008).
Zhang, P., Jondiko, T. O., Tilley, M. & Awika, J. M. Effect of high molecular weight glutenin subunit composition in common wheat on dough properties and steamed bread quality. J Sci Food Agr 94, 2801–2806 (2014).
León, E. et al. Mixing properties and dough functionality of transgenic lines of a commercial wheat cultivar expressing the 1Ax1, 1Dx5 and 1Dy10 HMW glutenin subunit genes. J Cereal Sci 49, 148–156 (2009).
Blechl, A. et al. Transgenic wheats with elevated levels of Dx5 and/or Dy10 high-molecular-weight glutenin subunits yield doughs with increased mixing strength and tolerance. J Cereal Sci 45, 172–183 (2007).
Yue, S. J. et al. Generation of transgenic wheat lines with altered expression levels of 1Dx5 high-molecular weight glutenin subunit by RNA interference. J Cereal Sci 47, 153–161 (2008).
Wieser, H. & Kieffer, R. Correlations of the amount of gluten protein types to the technological properties of wheat flours determined on a micro-scale. J Cereal Sci 34, 19–27 (2001).
Uthayakumaran, S., Stoddard, F. L., Gras, P. W. & Bekes, F. Effects of incorporated glutenins on functional properties of wheat dough. Cereal Chem 77, 737–743 (2000).
Zhang, K. et al. Association analysis of genomic loci important for grain weight control in elite common wheat varieties cultivated with variable water and fertiliser supply. PLoS One 8, e57853 (2013).
Wan, Y., Liu, K., Wang, D. & Shewry, R. P. High-molecular-weight glutenin subunits in the Cylindropyrum and Vertebrata sections of the Aegilops genus and identification of subunits related to those encoded by the Dx alleles of common wheat. Theor Appl Genet 101, 879–884 (2000).
González-Torralba, J., Arazuri, S., Jarén, C. & Arregui, L. M. Stable quality traits of soft winter wheat under nonlimiting nitrogen conditions. Crop Sci 51, 2820–2828 (2011).
Sroan, B. S., Bean, S. R. & MacRitchie, F. Mechanism of gas cell stabilization in bread making. I. The primary gluten–starch matrix. J Cereal Sci 49, 32–40 (2009).
American Association of Cereal Chemists International (AACCI). Approved Methods of Analysis (11th Edition), http://methods.aaccnet.org/search.aspx (2014–2016).
Acknowledgements
This research was financially supported by the Ministry of Science and Technology of China (2016YFD0100500), the National Natural Science Foundation of China (31300280), and Chinese Academy of Sciences (XDA08010302). We thank Professor Jichun Tian (Shandong Agricultural University, Taian, China) for constructive suggestions on the evaluation of gluten, dough and breadmaking quality parameters.
Author information
Authors and Affiliations
Contributions
D.W., K.Z. and Z.W. designed the research. Z.W., Y.L. and Y.Y. performed the experiments. X.L., H.Q., Z.D. and S.Z. contributed reagents and farming facility to the work. Z.W., D.W., K.Z. and Z.D. analyzed the data. Z.W., Y.L. and D.W. wrote the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Z., Li, Y., Yang, Y. et al. New insight into the function of wheat glutenin proteins as investigated with two series of genetic mutants. Sci Rep 7, 3428 (2017). https://doi.org/10.1038/s41598-017-03393-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03393-6
This article is cited by
-
Quantitative traits loci mapping and molecular marker development for total glutenin and glutenin fraction contents in wheat
BMC Plant Biology (2021)
-
Genetic diversity and selective sweeps in historical and modern Canadian spring wheat cultivars using the 90K SNP array
Scientific Reports (2021)
-
High molecular weight glutenin gene diversity in Aegilops tauschii demonstrates unique origin of superior wheat quality
Communications Biology (2021)
-
Analyzing the action of evolutionarily conserved modules on HMW-GS 1Ax1 promoter activity
Plant Molecular Biology (2020)
-
Allelic variation of high molecular weight glutenin subunits of bread wheat in Hebei province of China
Journal of Genetics (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.