Abstract
Significant individual susceptibility to intravenous anesthetic propofol exists. The etiology of individual variability in the response to propofol may be influenced by genetic polymorphisms in metabolic and functional pathways. With current pharmacogenetics and modern molecular biology technologies, it is possible to study the influence of genetic polymorphisms on susceptibility to propofol. When inducing general anesthesia with intravenous propofol, high individual susceptibility to propofol was found. Using Sequenom MassARRAY single-nucleotide polymorphism (SNP) genotyping, we identified a mutation (rs6313) in the 5HT2A gene that was correlated to individual susceptibility to propofol effect-site concentration (Cep) and onset time of propofol induction. Carriers of the minor allele (G) of 5HT2A rs6313 required less propofol (20% decrease in Cep) and less time (40% decrease in onset time) to induce anesthesia. Moreover, associations were found between the gamma-aminobutyric acid (GABA) receptor SNP rs2279020 and the SCN9A SNP rs6746030 and the susceptibility of bispectral index (BIS) after propofol-induced anesthesia. In addition, dominant mutations in GABAA1 rs2279020, GABAA2 rs11503014, and CHRM2 rs1824024 were putatively associated with cardiovascular susceptibility to propofol anesthesia. No gene-gene interactions were found through a standardized measure of linkage disequilibrium and a multifactor dimensionality reduction analysis. Our results suggest that genetic polymorphisms related to mechanisms of propofol anesthesia are involved in propofol susceptibility.
Similar content being viewed by others
Introduction
Anesthetic and analgesic drugs are widely used in various clinical settings. Although different methods have been developed for the management of applied anesthetics, drug susceptibility is inevitable and difficult to control. Propofol was introduced into clinical practice as a general anesthetic agent in 1977 and has become the agent of choice for rapid intravenous induction. However, susceptibility to propofol anesthesia has been shown to be remarkably variable based on clinical observations of responses of patients of the same ethnic origin, and this variability is reflected in different dose requirements and the amount of required recovery time1, 2.
Deep sedation with propofol, which inhibits the stress response3, is followed by hypotension; sedation-related complications, or even brain injury4, sometimes subsequently deteriorate the outcome of patients. Likewise, light propofol sedation, defined as inadequate anesthesia, would induce hypertension, tachycardia, or patient movement and, more seriously, lead to intraoperative awareness5. However, based simply on the traditional dose calculation algorithm of propofol, it is very hard for patients to receive accurate and comfortable anesthesia because of propofol susceptibility. Thus, particular anesthetic measures are needed to provide the most appropriate anesthesia to patients.
The etiology of susceptibility to propofol is complex, involving complicated associations among drugs, biological and psychological factors6. More importantly, genetic components could play an essential role in the pathogenesis of susceptibility to propofol7.
Studies have demonstrated that individual differences in genetic factors (polymorphisms in selected genes responsible for pharmacokinetics and pharmacodynamics) and nongenetic factors (sex, weight and height) contributed to the variability in dose requirements of propofol8, 9. In addition, the anesthesia mechanism of propofol can’t be ignored or even more important for propofol susceptibility, which determined the reaction of loss of consciousness to propofol. Furthermore, mutations in genes involved in the molecular targets and molecular binding sites of propofol may be associated with propofol susceptibility. Therefore, we speculated that genetic polymorphisms in metabolic or functional pathways or receptors might have an influence on individual variability in response to propofol susceptibility.
Previous researches have indicated that the main targets for propofol’s actions are the genes of gamma-aminobutyric acid (GABA) systems; nACR; dopaminergic, serotoninergic, or noradrenergic pathways or associated voltage-dependent ion channels; or enzymes associated with metabolism and mechanisms. Cytochrome P450 family (CYP450), ATP-binding cassette (ABCB1), serine/threonine-protein kinase 3 (TAOK3), family with sequence similarity 53 member B (FAM53B), and the cannabinoid receptor (CNR1) are postulated to be involved in propofol pharmacokinetics; opioid receptors (OPRM1 and OPRD1), β-adrenoceptor (ADRB1), Catechol-O-methyltransferase (COMT), and ligand-gated ion channel (P2RX7) are postulated to be directly or indirectly involved in the pharmacodynamic response to propofol; nitric oxide synthase (NOS3), GABA type A (GABAA) receptor, NMDA receptors (GR1N3A and GR1N2B), Galanin (GAL), fatty acid amide hydrolase (FAAH), 5-hydroxytryptamine receptor (5HT2A), cholinergic receptors (CHRM2 and CHRNA5), dopamine transporters (DAT and DRD2), casein kinase (CSNK1E), calcium channels, potassium channels (KCNS1 and GIRK) and sodium channels (SCN9A) are also likely involved in the action of propofol7, 10,11,12,13,14,15,16,17,18,19,20,21,22,23. However, evidence for an association between single-nucleotide polymorphisms (SNPs) in the genes mentioned above and propofol susceptibility in patients is still lacking. Thus, the major objective of this study was to test whether the SNPs in these genes associated with propofol’s metabolism and actions contribute to the variability in individual susceptibility to propofol.
Results
Sociodemographic and clinical characteristics of the study subjects
Demographic parameters of study groups
In total, 179 of the 192 recruited patients were included in the study. One patient was excluded due to hypertension, and 13 patients were excluded for other reasons (e.g., no continuous data monitoring, no blood provided, or lack of phenotypic data). The key sociodemographic data are summarized in Table 1. All the study participants were Han Chinese. Age, sex and body mass index (BMI) were recorded for all patients. Overall, 83 males and 96 females participated in our study, and the mean age and BMI were 43.33 ± 8.76 years and 23.86 ± 2.38 kg/m2, respectively.
Clinical characteristics
Under standard total intravenous anesthesia (TIVA), the effect-site concentration (Cep), onset time and total amount of propofol anesthesia, bispectral index (BIS) and hemodynamics of patients were recorded when the patients reached different stages of sedation as determined by the Observer’s Assessment of Alertness/Sedation Scale (OAAS) score.
Trends in clinical data regarding anesthesia induction and susceptibility to propofol anesthesia
During anesthesia induction, the intravenous anesthetic propofol induced time- and dose-dependent sedative effects on patients. As shown in Fig. 1, while propofol-induced anesthesia gradually deepened, 0.80 ± 0.41 min, 1.16 ± 0.77 min, 1.58 ± 1.18 min, 2.04 ± 1.54 min, and 2.60 ± 1.99 min (Fig. 1C, left) were required to produce the effects for OAA/S scores 4, 3, 2, 1, 0 respectively. A total amount of propofol required for OAA/S scores of 4, 3, 2, 1, and 0 were 58.90 ± 20.34 mg, 67.21 ± 19.51 mg, 74.91 ± 19.89 mg, 82.75 ± 22.60 mg, and 92.48 ± 27.70 mg, respectively (Fig. 1D, left). The propofol Cep increased from 0.68 ± 0.35 μg/ml, 0.93 ± 0.51 μg/ml, 1.20 ± 0.64 μg/ml, 1.44 ± 0.74 μg/ml, to 1.68 ± 0.83 μg/ml (Fig. 1A, left) and BIS decreased from 86.04 ± 5.94, 80.06 ± 6.72, 73.99 ± 7.50, 67.86 ± 8.67 to 59.79 ± 11.23 (Fig. 1B, left) at OAA/S scores of 4, 3, 2, 1, and 0, respectively. All these clinical data seemed to be well correlated with different levels of sedation, as indicated by different OAA/S scores (Fig. 1, left). However, the results revealed high individual diversity (Fig. 1, right) in dose response under propofol anesthesia. There was a 20-fold difference between the fastest and slowest onset times of propofol anesthesia (Fig. 1C, right), which ranged from 0.55 to 10.97 min. In Fig. 1D, right, when the patients lost consciousness, the total propofol infusions were significantly different and ranged from 48 to 221 mg. The Cep of propofol was also highly variable and ranged from 0.40 to 3.80 μg/ml (Fig. 1A, right). The Cep value was 0.4–1.0 μg/ml in 48 patients, 1.0–3.0 μg/kg/min in 115 patients, and 3.0–3.8 μg/ml 16 patients. A nine-fold difference existed between the maximum and minimum Cep values. Figure 1B, right shows that when patients lost consciousness, the BIS varied significantly from 40 to 84, ranging from 40 to 60 in 108 patients, 60 to 80 in 64 patients, and 80 to 84 in 7 patients.
Clinical characteristics of patients during anesthesia induction. Anesthesia was induced with propofol via target-controlled-infusion (TCI) at 4 µg/ml. At OAA/S scores of 5, 4, 3, 2, 1, and 0, the effect-site concentration (Cep), bispectral index (BIS), onset time and total propofol were recorded. (A,B,C and D), left, reflect the Cep, BIS, onset time and total propofol recorded at OAA/S scores of 5, 4, 3, 2, 1, and 0. (A,B,C and D), right, reflect the distribution of Cep, BIS, onset time and total propofol at the score of 0, when patients lost consciousness. The different colors in Fig. 1, right side, obviously reflect the wide distribution of Cep, BIS, onset time and total propofol values at the score of 0, when patients lost consciousness. Variance between different groups was analyzed by one-way ANOVA. *P < 0.05 vs. Cep and BIS at an OAA/S score of 5 in (A and B); *P < 0.05 vs. onset time and total propofol at an OAA/S score of 4 in (C and D).
Propofol also produced significant effects on hemodynamics, including changes in blood pressure and heart rate during anesthesia induction24. As shown in Fig. 2A, with increased sedation from propofol, there was a significant decline in the mean arterial pressure (MAP). The percent changes in MAP were −3.42 ± 5.85%, −7.73 ± 6.94%, −9.32 ± 7.13%, −11.13 ± 7.00%, and −12.05 ± 7.56% at OAA/S scores of 4, 3, 2, 1, and 0 (Fig. 2A, left). As shown in Fig. 2B, except for a slight increase in heart rate (HR) seen at the beginning, propofol induced a significant decline in HR. The HR values changed by 4.92 ± 8.23%, −0.53 ± 8.05%, −3.84 ± 8.68%, −7.29 ± 8.42% and −10.27% ± 7.28% at OAA/S scores of 4, 3, 2, 1, and 0 (Fig. 2B, left), respectively.
High individual diversity of cardiovascular responses to propofol anesthesia under the condition of unconsciousness in patients. The MAP and HR values of 179 participants at OAA/S scores of 5, 4, 3, 2, 1, and 0 are shown in (A and B), left. (A and B), right, show the distribution of MAP and HR changes under the condition of unconsciousness. The different colors in Fig. 1, right side, obviously reflect the wide distribution of MAP and HR changes at the score of 0, when patients lost consciousness. The variance between different groups was analyzed by one-way ANOVA. *P < 0.05 vs. changes in MAP and HR at an OAA/S score of 4 in (A and B).
As shown in Fig. 2A, right and Fig. 2B, right, the hemodynamic effects of propofol anesthesia also showed significant variability. The MAP values ranged from 60.67 to 98.00 mmHg, and HR ranged from 50 to 98 bpm. The changes in MAP and HR ranged from −37.24% to 21.41% and from −28.87% to 10.61%, respectively, when the patients lost consciousness.
Genotyping results
SNP information
In the present study, 10,919 SNPs (58 SNPs in 179 individuals) were genotyped. The genotype distributions of the 58 SNPs and the minor allele frequency (MAF) of each SNP are shown in Table 2. The test of ADRB1 rs1801253 and DRD3 rs6454674 didn’t show any useful results. In addition, no mutations in ADH4 rs1042363, DRD2 rs9288993 and DRD3 rs6454674 were observed in the patients, all patients carried the major alleles. Except for CNR1 rs324419, ADH4 rs1126671 and COMT rs174696, the frequencies of all the other polymorphisms were in Hardy-Weinberg equilibrium (HWE) (P > 0.05).
Correlation between genotype and susceptibility to propofol
The Cep, onset time, BIS, MAP and HR values at OAA/S scores of 0 were selected to evaluate susceptibility to propofol.
Based on the SNPs, the patients were divided into two groups: 1. homozygous for the major allele; and 2. a combination of heterozygous and homozygous for the minor allele. Differences in Cep, onset time, BIS, MAP and HR were analyzed between the two groups. When different patient indexes were compared based on different genotypes, the genes showed significant differences in each index, as shown in Table 3. Unfortunately, the SNPs in the remaining genes, including CYP450, ABCB1, TAOK3, FAM53B, CNR1, OPRM1, OPRD1, ADRB1, COMT, P2RX7, NOS3, GR1N3A, GR1N2B, GAL, FAAH, CHRNA5, DAT, DRD2, CSNK1E, calcium channels, and potassium channels, showed no differences in each index, as shown in Table 4.
As shown in Table 3, the following SNPs predicted susceptibility to propofol anesthesia, as indicated by differences in Cep, onset time, BIS, MAP and HR.
5HT2A rs6313
5-HT (serotonin) is an important amino acid that acts as both a neurotransmitter and a neuromodulator25. The 5-HT receptors are located in the central nervous system, and polymorphisms affecting the serotonergic system, such as those in the serotonin transporter (5-HTT) and 5-HT genes, have been linked to regulation of sleep and alertness26. In our study, the 31.5% (46/146) of patients who were homozygous for the major allele (AA genotype) showed higher Cep values (1.85 ± 0.96 μg/ml) than the 68.5% (100/146) of patients who were either heterozygous or homozygous for the minor allele (GA + GG) (1.53 ± 0.76 μg/ml) (P = 0.03, df = 1, F = 4.82).
Interestingly, the AA genotype of 5HT2A showed not only higher Cep values but also longer onset times of propofol induction (3.12 ± 2.68 min) than the GA + GG genotypes (2.19 ± 1.53 min) (P = 0.01, df = 1, F = 6.893). Carriers of the minor allele (G) required less propofol and less time for propofol to induce anesthesia.
GABA receptors
The GABAA receptor is an important target site of propofol anesthesia27. Propofol acts via a wide range of sites, including GABA receptors, and the action of propofol involves the positive modulation of the inhibitory function of the neurotransmitter GABA via GABAA receptors28.
rs2279020 in GABAA1
In our study, homozygous carriers (28.99%, 40/138) of the major allele (GG) had significantly lower BIS values than those either heterozygous (AG) or homozygous for the minor allele (AA) (57.15 ± 13.29 vs. 61.43 ± 10.51, P = 0.047, df = 1, F = 4.019). This result indicated that carriers of the homozygous major allele (GG) for the GABAA1 receptor (SNP rs2279020) were more susceptible to propofol anesthesia.
In addition, mutation of the GABAA1 receptor (rs2279020) also contributed to the different effects of propofol on blood pressure. The 28.99% (40/138) homozygous carriers of the major allele (GG) had significantly less change in MAP after propofol anesthesia than the 71.01% (98/138) carriers of either heterozygous (AG) or homozygous for the minor allele (AA) (−9.44% ± 5.28% vs. −12.16% ± 7.92, P = 0.048, df = 1, F = 3.967).
rs11503014 in GABAA2
In total, homozygous carriers(83.45%,116/139) of the major allele (CC) had significantly lower changes in HR after propofol anesthesia than those either heterozygous (CG) or homozygous for the minor allele (GG) (−9.44% ± 6.94% vs. −12.85% ± 8.54, P = 0.040, df = 1, F = 4.297).
To further analyze the relationship between different GABA receptors and the effects of propofol anesthesia, the SNPs in the GABAA receptor were also evaluated as 2-locus genotype patterns (Table 5) and analyzed for linkage disequilibrium (LD). LD is the non-random association of alleles at different loci; if there is no LD between two alleles at different loci, they are said to be in linkage equilibrium.
To analyze the LD of GABAA receptor SNP pairs, the standardized LD, as measured by Lewontin’s coefficient (LC: D′ = 0.2005, r2: 0.0043, P = 0.8), was estimated using the software 2LD29. No strong LD was observed in the GABAA receptor SNP pairs (Table 5).
SCN9A rs6746030
Duan et al.30 demonstrated that SCN9A is related to pain sensitivity and that sodium channels are the target of some anesthetics. Previous studies have provided evidence that clinically relevant concentrations of propofol alter the functions of voltage-dependent sodium channels, which inhibits the synaptic release of glutamate31. Regarding the SNP rs6746030 in SCN9A, we recorded a significantly lower BIS value under propofol anesthesia in the 11.76% (16/136) of patients who were either heterozygous or homozygous for the minor allele (GA + AA), compared to the 88.24% (120/136) of patients who were homozygous carriers of the major allele (GG) (51.13 ± 15.37 vs. 61.30 ± 10.39, P = 0.001, df = 1, F = 11.9531). Thus, the minor allele (A) of rs6746030 in SCN9A is involved in susceptibility to propofol anesthesia.
CHRM2 rs2283265
The cholinergic muscarinic 2 receptor (CHRM2) is a cholinergic neurotransmitter, and cholinergic stimulation through inhibition of the release of acetylcholinesterase has been shown to have a role in autonomic cardiac control32. In our study, propofol produced a significantly less change in HR in the 34.56% (47/136) of homozygous carriers of the major allele (CC) of rs2283265 in CHRM2, compared to the 65.44% (89/136) of patients who were heterozygous or homozygous for the minor allele (CA + AA) (−8.25% ± 7.15 mmHg vs. −10.86% ± 7.32, P = 0.048, df = 1, F = 3.965). Thus, our results indicated that the major allele (C) of CHRM2 may be associated with cardiovascular susceptibility to propofol anesthesia.
To rule out the effects of age, weight and sex in our pharmacogenetic analysis, we further assessed the correlation between the genotype frequencies of the significant SNPs and clinical features of the patients. The results of our statistical analysis are shown in Table 6. There were no significant differences in age or BMI between individuals who were homozygous for the major allele and individuals who were either heterozygous or homozygous for the minor allele for each of the tested SNPs.
SNP-SNP interaction reveals moderate synergistic effects
A multifactor dimensionality reduction method (MDR) analysis was used to examine gene-gene interactions and the predictive power of the combined variants33. The MDR software provided a number of output parameters for each genetic model. The cross-validation consistency (CVC) score is a measure of the degree of consistency with which the selected model is identified as the best model among all possibilities considered. Testing balanced accuracy (TBA) is a measure of the degree to which the interaction accurately predicts case-control status, with scores between 0.50 (indicating that the model prediction was no better than chance) and 1.00 (indicating a perfect prediction).
Our results showed that rs2279020 in GABAA1 and rs6746030 in SCN9A were involved in susceptibility to propofol anesthesia according to the BIS value; and rs1150314 in GABAA2 and rs2283265 in CHRM2 participated in the hemodynamic response to propofol anesthesia according to the HR results. We performed an MDR analysis to examine the significant SNP-SNP interactions. Compared to rs6746030 in SCN9A (TBA 0.5443, CVC 6, P = 0.9325) alone, the interaction between rs2279020 in GABAA1 and rs6746030 in SCN9A (TBA 0.5723, CVC 10, P = 0.6142) was highly significant. Likewise, compared to rs2283265 in CHRM2 (TBA 0.525, CVC 6, P = 0.4850), the interaction between rs2279020 in GABAA1 and rs6746030 in SCN9A (TBA 0.650, CVC 10, P = 0.2617) was also highly significant. As shown in Table 7, despite the high TBA and CVC of rs2279020 in combination with rs6746030 compared to that of rs6746030 alone, there was no interaction between these two SNPs, indicating that the GABA receptor and sodium channels did not produce synergistic effects on propofol susceptibility. Similarly, the GABA receptor and CHRM2 did not produce synergistic effects on cardiovascular susceptibility to propofol anesthesia.
Discussion
In this study, we systemically investigated the roles of multiple polymorphisms in susceptibility to propofol anesthesia. Our results demonstrated that there were significant differences in individual susceptibility to propofol anesthesia and that different patients required different times and different Cep to produce different BIS values and different hemodynamic responses at the same level of propofol anesthesia. Several important molecular targets, including the 5HT receptor, GABA receptor and sodium channels, were shown to contribute to susceptibility to propofol anesthesia.
Previous experimental and clinical studies have demonstrated that individual susceptibility to propofol anesthesia is dependent on factors governing drug susceptibility and drug disposition34. Moreover, great inter-patient variability exists in the dose of propofol required to achieve a BIS value <701. Although potential susceptibility to propofol has been reported in vitro35, traditional methods of clinical anesthesia render it difficult to judge susceptibility to propofol4, 5. It is difficult to maintain appropriate propofol anesthesia because of individual susceptibility.
Target controlled infusion (TCI) systems were used to precisely deliver propofol in our study. The device provides precise control of the propofol level (the target concentration) at the Cep. BIS is used to measure the depth of anesthesia. In our study, when the patients lost consciousness, their BIS scores were approximately 60, which is similar to scores recorded after induction by propofol in other studies36. After the loss of patient consciousness, the Cep, onset time and BIS of propofol anesthesia can be used to evaluate susceptibility to propofol. In our study, there was a 20-fold difference between the fastest and slowest onset times for anesthesia induction when patients were anesthetized with propofol to reach a steady unconscious state. The Cep values of propofol were highly variable, showing a 9-fold difference between the most and least sensitive patients. The BIS of propofol anesthesia in patients also ranged from 40 to 84 at the same level of sedation.
Genetic components play an essential role in susceptibility to propofol. Early studies evaluating susceptibility to propofol focused on only the CYP450 gene, which is involved in the metabolism of propofol. Mourão et al.37 found that a polymorphism in the CYP2B6 gene (rs3745274) affected susceptibility to propofol anesthesia, showing that the total propofol doses based on the GG or GT/TT genotypes were 151.5 mg and 129.3 mg. Mastrogianni et al.38 also indicated that the c.516G> T polymorphism in CYP2B6 was associated with high blood propofol concentrations. In addition, Lian et al.39 demonstrated that the impact of a polymorphism in CYP2C9 contributed to susceptibility to propofol. All these studies indicated that enzymes involved in the metabolism of propofol affect susceptibility to propofol and that the total propofol consumption can be disrupted by other anesthetics used during surgery. Our study didn’t only pay attention to the genes involved in the pharmacokinetics and pharmacodynamics of propofol anesthesia, but also aimed to test whether the genes associated with the mechanism of action of propofol affect susceptibility to propofol. Unfortunately, unlike the above studies demonstrating that the metabolism of propofol influenced susceptibility to propofol, our study showed that no SNPs involved in the metabolism of propofol were associated with susceptibility to propofol anesthesia. This trend may result from our research design, which was focused only on propofol-induced anesthesia to avoid interference by other anesthetics. The short time may have limited the functions of SNPs involved in the metabolism of propofol.
In this study, using a candidate gene approach, significant associations between the pharmacogenetic variants of these genes and susceptibility to propofol were identified. We found that polymorphisms in the 5-HT receptor, GABA receptor and sodium channel were associated with susceptibility to propofol anesthesia. Moreover, due to the observation of wide ranges of HR and MAP values, differences in HR or MAP between patients were compared based on the SNP results. Interestingly, polymorphisms in GABA receptors and the cholinergic receptor were associated with hemodynamic responses to propofol.
The 5-HT receptor is involved in the regulation of brain activity and function, and it participates in sensory processing, sleep and alertness40. Highly effective modulation is completed by releasing 5-HT to targeted areas, in which several pre- and postsynaptic receptors are implicated20. Networks are important for developing cortical neural circuits41, and some areas in the networks are associated with anesthesia; these areas of the network include the sleep-promoting ventrolateral preoptic area (VLPO) neurons that contribute to anesthetic hypnosis42. Notably, Okamoto et al.43 confirmed that 5HT2A was involved in antinociceptive actions.
We found that carriers of the minor allele (G) of rs6313 showed lower Cep values and shorter onset times for propofol anesthesia than patients who were homozygous for the major allele (AA). Several reports have suggested that anesthetic doses of propofol increase serotonergic activity44, and propofol may facilitate 5-HT release in the brain by increasing serotonergic metabolism. The variation of rs1636 in 5-HT2A from A to G may change the metabolism of 5-HT under propofol anesthesia, and the release of 5-HT increases in carriers of the minor allele of the rs6313 SNP in the 5-HT2A receptor compared with patients who are homozygous for the major allele (AA). Thus, carriers of the minor allele of rs6313 may show stronger activation of the sleep-promoting VLPO neurons that contribute to anesthetic hypnosis. This result may significantly contribute to elucidating the role of 5HT2A in susceptibility to propofol anesthesia.
GABA is the primary inhibitory neurotransmitter in the mammalian brain, and GABA-mediated transmission is involved in adjusting three interactive states: sleep, anesthesia, and pain45, 46. GABA regulates these processes by activating GABAA receptors and GABAB receptors. GABAA receptors permit chloride ions to access the center of the pentamer and the binding sites of GABA and modulatory drugs; these sites include binding sites for various anesthetics47, 48. Binding to the agonist sites of GABAA is believed to contribute to the hypnotic effects of propofol.
Based on our study, the G-to-A mutation in rs2279020 in GABAA1 may change the pharmacological properties of the receptor by varying the composition and arrangement of subunits49. Under propofol anesthesia, the minor A allele of rs2279020 in GABAA1 may induce a stronger inhibition in the brain, as shown by the higher BIS in those patients after the loss of consciousness. This result significantly supports the role of GABAA1 in susceptibility to propofol anesthesia.
Sodium channels are an important regulator of neuronal action potentials, and previous studies have provided evidence that clinically relevant concentrations of propofol alter the functions of voltage-dependent sodium channels, thereby inhibiting the synaptic release of glutamate31. Thus, sodium channels were considered an important target of anesthetics50. The SCN9A gene, which encodes the Nav1.7 voltage-gated sodium channel, is associated with different abnormal pathophysiological conditions, such as human pain sensitivity51, 52. In addition, recent studies have indicated that propofol can act directly on sodium channels to modulate intrinsic ionic conductance53.
In our study, the significant role of SCN9A in susceptibility to propofol was confirmed. In patients who were heterozygous or homozygous for the minor allele (A) of the rs6746030 SNP in SCN9A, we recorded significantly lower BIS values. Previous reports have suggested that anesthetic doses of propofol inhibit glutamate release; the decrease in the excitatory neurotransmitter may remodel brain activity, followed by a state of anesthesia. The variation in rs6746030 in SCN9A from G to A may change the function of the sodium channel; the changes in glutamate release and intrinsic ionic conductance resulted in greater susceptibility to propofol, which induced significantly lower BIS values after propofol-induced loss of consciousness. This result may significantly contribute to elucidating the role of SCN9A in susceptibility to propofol anesthesia.
Previous studies have demonstrated that propofol produces inhibitory effects on the cardiovascular system, including reductions in blood pressure and HR54; these effects were also found in our current results. In the present study, MAP tended to decrease following anesthesia induced by the TCI of propofol. HR tended to increase at first, followed by a decrease. Our study also showed significant variability in the hemodynamic effects induced by propofol anesthesia, with MAP values ranging from 60.67 to 98.00 mmHg and HRs ranging from 50 to 98 bpm when the patients lost consciousness.
The effects of propofol on cardiovascular systems include depressing the activity of the cardiovascular nervous center, reducing cardiac function and influencing the vascular system55. Because the initial purpose of our study was to explore polymorphisms in genes involved in the molecular mechanism of propofol anesthesia, this study analyzed only the relationship between polymorphisms in central nervous system targets and hemodynamic susceptibility to propofol. Interestingly, we found that polymorphisms in the GABAA receptor and the CHRM2 receptor contributed to hemodynamic susceptibility to propofol.
Our current study showed that propofol significantly lowered the HR upon loss of consciousness in patients who were carriers of the minor allele G of rs11503014 in GABAA2 compared with that in patients without the G allele. Moreover, the variation in the C-to-A polymorphism of rs2283265 in CHRM2 resulted in lower HR values. The variation from G to A of rs2279020 in GABAA1 resulted in lower MAP after propofol anesthesia. These results were somewhat surprising because little is known about the effects of anesthetics on the central regulation of the cardiovascular system.
Actually, several targets in the central nervous system play important roles in the regulation of the cardiovascular system. GABAAs play an important role in hemodynamic balance: accumulating evidence suggests that GABAergic inhibition in the rostral ventrolateral medulla (RVLM) and hypothalamic paraventricular nucleus (PVN) significantly contributes to the sympatho-excitation associated with cardiovascular-related disorders, such as hypertension and heart failure56. In our study, the polymorphisms rs2279020 in GABAA1 and rs11503014 in GABAA2 may have changed the structure of the GABA receptor, thereby influencing the binding of propofol. These changes may have affected the control of cardiovascular and sympathetic functions in the RVLM and PVN.
The CHRM2 receptor is a cholinergic neurotransmitter, and cholinergic stimulation via the inhibition of acetylcholinesterase release has demonstrated the role of the cholinergic system in autonomic cardiac control32. Evidence from animal models and clinical trials suggests that modulating cholinergic activity and restoring sympathovagal balance has salutary effects on hemodynamics57. Gamou et al.58 demonstrated that a microinjection of propofol into the perifornical area of rats induced a decrease in the cortical release of acetylcholine. We speculate that CHRM2 may be a target of propofol and that the activation of CHRM2 by propofol may decrease the HR via decreasing release of acetylcholine. Variations in rs2283265 in CHRM2 may change the structure and function of CHRM2, resulting in a decrease in the inhibitory effects of propofol on HR.
In summary, our study showed that the GABAA receptor and cholinergic receptors contributed to cardiovascular susceptibility to propofol anesthesia. Interestingly, only the GABAA1 receptor was implicated in genetic susceptibility to both propofol-induced anesthesia and the hemodynamic response to propofol. This suggested that the intravenous anesthetic propofol most likely produced anesthesia and cardiovascular action by different mechanisms. These results indicated that evaluating the anesthesia level of propofol through a simple hemodynamic response is insufficient.
To investigate whether other factors influenced the differences in Cep, onset time, BIS and hemodynamics, age and BMI were compared between individuals who were homozygous for the major allele and individuals who were heterozygous or homozygous for the minor allele for each of the tested SNPs. As shown in Table 6, there were no significant differences in age or BMI between the two groups, which demonstrate that the differences in Cep, onset time, BIS and hemodynamics were likely not influenced by other factors.
Based on the LD and MDR analyses, none of the genes were found to interact with each other. This result was somewhat disappointing because we originally expected that these molecular targets might interact with each other to synergistically contribute to both anesthesia and the hemodynamic response to propofol. This prediction was based on recent studies of the networks in the central nervous system related to anesthetic actions59.
The current study demonstrated that patients with different pharmacogenetic polymorphisms had individualized susceptibility to propofol anesthesia. Thus, based simply on the traditional dose calculation algorithm for propofol, patients with the minor allele (G) for rs6313 in 5HT2A, homozygous carriers of the major allele (GG) of rs2279020 in GABAA1, and carriers of the minor allele (A) of rs6746030 in SCN9A may be more likely to experience an overdose of propofol. This result may allow us to implement a preoperative genetic screening to identify individuals with a high risk of experiencing a propofol-induced anesthetic overdose. In addition, propofol induced lower HR in patients carrying the minor allele (G) of rs11503014 in GABAA2 and the minor allele (A) of rs2283265 in CHRM2 as well as lower MAP in carriers of the minor allele (A) of rs2279020 in GABAA1. This outcome should remind us to provide better preoperative screening for individuals at high risk of cardiovascular susceptibility to propofol anesthesia.
The results of this study also demonstrated that propofol susceptibility is not determined by a single factor and that some genes associated with the mechanisms of propofol action are involved in propofol susceptibility. There are some limitations in our study. The study sample size was relatively small; we did not measure plasma concentrations of propofol but, rather, forecasted concentrations of propofol8; the blood- and effect-site concentrations may not have been consistent; the genes we selected were based only on existing polymorphisms, while many genes with undiscovered polymorphisms may have effects on the action of propofol. The findings of genetic susceptibility targets in this study may contribute to the understanding of the neurobiological mechanism underlying the propofol susceptibility and aid in the development of novel anesthetic options.
Materials and Methods
Study participants
For the current candidate gene association study, 192 individuals undergoing thyroid gland resection were recruited at the operating theater of the Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, China. The following inclusion criteria were applied: (1) written informed consent; (2) patients were undergoing elective surgery, were 25–55 years of age, had a BMI of 20–30 kg/m2, and were ASA I or II; (3) no history of surgery; and (4) no history of drug addiction. Patients were not included if they met one of the following exclusion criteria: (1) allergies or a history of drug dependence; (2) pregnant or lactating; and (3) ASA III or IV. These criteria were confirmed in a structured personal interview and by a self-designed questionnaire for data collection. All the interviews were conducted by the same physician.
Assessment of sedation by propofol anesthesia
The state of sedation was assessed using the BIS60 and OAA/S scale (score 5 = awake and responds readily to name spoken in normal tone; 4 = lethargic response to name in normal tone; 3 = response only after name is called loudly or repeatedly; 2 = response only after name is called loudly and after mild shaking; 1 = does not respond when name is called and after mild shaking)61.
Study procedures
Patients did not receive medication prior to the procedure. Standard monitoring procedures, including non-invasive arterial pressure (NIAP), electrocardiography, pulse oximetry and BIS measurements, were conducted upon patient arrival in the operating room. The MAP, HR and BIS values were recorded.
Anesthesia was induced with propofol via TCI at 4 µg/ml with the pharmacokinetic and pharmacodynamic (PK-PD) model introduced by Schnider et al.8. Another investigator then recorded the OAA/S score every 30 s until the patient was sedated to an OAA/S score of 0. At OAA/S scores of 4, 3, 2, 1, and 0, the Cep, onset time, BIS, MAP and HR values were recorded.
Skilled anesthetists inserted a tracheal catheter for airway management, and mechanical ventilation was started with intravenous remifentanil and cis-atracurium injections after the patient lost consciousness. Anesthesia was maintained with propofol TCI (3 μg/ml), and the dose of remifentanil was maintained to ensure that the patient maintained stable vital signs.
Assessment of susceptibility to propofol
Cep, onset time, and BIS at an OAA/S score of 0 were selected to demonstrate susceptibility to propofol.
Assessment of cardiovascular susceptibility to propofol
MAP and HR at an OAA/S score of 0 were selected to demonstrate cardiovascular susceptibility to propofol.
Genotyping
Selection of polymorphic loci
This was a candidate gene association study. Based on knowledge of metabolic pathways, transporters, targets and the mechanism of action of propofol, the genes and polymorphic loci were selected after an extensive literature study. The main criterion for the selection of genes and polymorphic loci was evidence speculating or confirming a functional correlation. In total, 58 SNPs located in 35 different genes were selected (Table 2). Five of the 35 investigated genes were postulated to be involved in propofol pharmacokinetics (CYP1A2, ABCB1, TAOK3, FAM53B, and ADH4), ten genes were postulated to be directly or indirectly involved in the pharmacodynamics of the response to propofol (OPRM1, GR1N3A, GR1N2B, OPRD1, GAL, ADRB1, COMT, CNR1, P2RX7, and FAAH), and twenty genes are known to participate in the mechanism of action of propofol (NOS3, GABAB2, GABAA6, GABAA1, GABAG2, GABAA2, GABA, 5HT2A, 5-HTTLPR, CHRNA5, CHRM2, DAT, DRD2, DRD3, CSNK1E, ANKK1, calcium channel, potassium channel, GIRK, and sodium channel).
DNA sample collection and DNA extraction
One milliliter of arterial blood was sampled from the femoral artery of each patient after anesthesia induction. Fresh blood was stored at −80 °C and was subsequently extracted using a standard phenol-chloroform procedure. Genotypes were determined using a Sequenom MassARRAY SNP genotyping system, which was based on detection through MALDI-TOF MS (Sequenom Inc., San Diego, CA, USA).
Statistical analysis
Statistical analyses were performed with SPSS 20.0 (SPSS Inc., Chicago, IL, USA). All values were expressed as the means ± SD. Differences in clinical characteristics among different OAA/S scores were assessed using ANOVA and Wilcoxon signed-rank tests. Furthermore, Chi-square testing was applied to assess the HWE of each of the tested SNPs, and P < 0.05 indicated deviation from equilibrium. The genotypes of each tested SNP were divided into two groups: homozygous for the major allele and a combination of heterozygous and homozygous for the minor allele. In this study, dichotomization of genotypes was necessary because the number of subjects with the minor allele for some SNPs was <5. Furthermore, the statistical power would not have been sufficiently high (<60%) if the genotypes were trichotomized and comparisons were made between homozygotes for the major allele and homozygotes for the minor allele for some SNPs. For data with a normal distribution, differences in Cep, MAP, HR, induction time, total infusion and BIS among SNPs were determined using an ANOVA test. Data with an abnormal distribution were compared using a Wilcoxon signed-rank test, and a P value of <0.05 was considered significant. The standardized measure of LD was calculated with the 2LD and SPSS 20.0 software programs for each pair of markers and was indicated by a coefficient of LD (D′) and r 2. D′ >0.7 was considered eligible for LD.
The MDR method, which is described in detail elsewhere, was used to examine gene-gene interactions and the predictive power of combined variants. MDR was used to assess all possible genetic models by reducing the dimensionality of the genotype determinants and providing the best genetic model for predicting outcomes. The MDR software provided a number of output parameters for each genetic model. The cross-validation consistency score was a measure of the degree of consistency with which the selected model was identified as the best model among all possibilities considered. Testing balanced accuracy is a measure of the degree to which the interaction accurately predicts case-control status, with scores between 0.50 (indicating that the model prediction was no better than chance) and 1.00 (indicating a perfect prediction).
Study approval
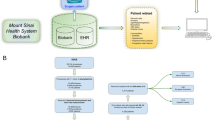
The present study was approved by the Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, China. All methods were performed in accordance with the relevant guidelines and regulations. All participants signed an informed consent after receiving a complete description of the study and were given the chance to discuss any questions or issues. The procedures performed in the research are depicted in Fig. 3.
Flow chart. Among the 192 participants enrolled in our study, one participant showed hypertension at the induction of anesthesia, and the remaining 13 early terminations were due to other reasons (no continuous monitoring data, no blood provided, or lack of phenotypic data). Based on the SNP results, the patients were divided into two groups. By comparing the clinical characteristics between the two groups, susceptibility to propofol was determined.
References
Iohom, G. et al. An investigation of potential genetic determinants of propofol requirements and recovery from anaesthesia. Eur. J. Anaesthesiol. 24, 912–919, doi:10.1017/S0265021507000476 (2007).
Ypsilantis, P. et al. Attenuation of propofol tolerance conferred by remifentanil co-administration does not reduce propofol toxicity in rabbits under prolonged mechanical ventilation. J. Surg. Res. 168, 253–261, doi:10.1016/j.jss.2009.08.020 (2011).
Yang, L. Q., Li, J. J., Chen, S. Q. & Wang, Y. W. Effect of different depths of anesthesia on perioperative stress response in children undergoing adenoidectomy and tonsillectomy. CNS Neurosci. Ther. 19, 134–135, doi:10.1111/cns.2013.19.issue-2 (2013).
Cremer, O. L. et al. Long-term propofol infusion and cardiac failure in adult head-injured patients. Lancet 357, 117–118, doi:10.1016/S0140-6736(00)03547-9 (2001).
Kam, P. C. & Cardone, D. Propofol infusion syndrome. Anaesthesia 62, 690–701, doi:10.1111/j.1365-2044.2007.05055.x (2007).
Jung, Y. S. et al. The optimal anesthetic depth for interventional neuroradiology: comparisons between light anesthesia and deep anesthesia. Korean J. Anesthesiol. 68, 148–152, doi:10.4097/kjae.2015.68.2.148 (2015).
Iohom, G., Fitzgerald, D. & Cunningham, A. Principles of pharmacogenetics—implications for the anaesthetist. Br. J. Anaesth. 93, 440–450, doi:10.1093/bja/aeh200 (2004).
Schnider, T. W. et al. The influence of method of administration and covariates on the pharmacokinetics of propofol in adult volunteers. Anesthesiology 88, 1170–1182, doi:10.1097/00000542-199805000-00006 (1998).
Loryan, I. et al. Influence of sex on propofol metabolism, a pilot study: implications for propofol anesthesia. Eur. J. Clin. Pharmacol. 68, 397–406, doi:10.1007/s00228-011-1132-2 (2012).
Beer, B. et al. Association of polymorphisms in pharmacogenetic candidate genes (OPRD1, GAL, ABCB1, OPRM1) with opioid dependence in European population: a case-control study. PLoS One 8, e75359, doi:10.1371/journal.pone.0075359 (2013).
Weiser, B. P., Woll, K. A., Dailey, W. P. & Eckenhoff, R. G. Mechanisms revealed through general anesthetic photolabeling. Curr. Anesthesiol. Rep. 4, 57–66, doi:10.1007/s40140-013-0040-7 (2014).
Cook-Sather, S. D. et al. TAOK3, a novel genome-wide association study locus associated with morphine requirement and postoperative pain in a retrospective pediatric day surgery population. Pain 155, 1773–1783, doi:10.1016/j.pain.2014.05.032 (2014).
Thermes, V. et al. Medaka simplet (FAM53B) belongs to a family of novel vertebrate genes controlling cell proliferation. Development 133, 1881–1890, doi:10.1242/dev.02350 (2006).
Campos, S. P. et al. Expression of CYP1A1 and CYP1A2 in the liver and kidney of rabbits after prolonged infusion of propofol. Exp. Toxicol. Pathol. 68, 521–531, doi:10.1016/j.etp.2016.07.006 (2016).
Hauer, D. et al. Propofol enhances memory formation via an interaction with the endocannabinoid system. Anesthesiology 114, 1380–1388, doi:10.1097/ALN.0b013e31821c120e (2011).
Moriyama, A. et al. Association between genetic polymorphisms of the beta1-adrenergic receptor and sensitivity to pain and fentanyl in patients undergoing painful cosmetic surgery. J. Pharmacol. Sci. 121, 48–57, doi:10.1254/jphs.12159FP (2013).
Martignoni, E. et al. Two patients with COMT inhibitor-induced hepatic dysfunction and UGT1A9 genetic polymorphism. Neurology 65, 1820–1822, doi:10.1212/01.wnl.0000187066.81162.70 (2005).
Nakanishi, M. et al. The effects of general anesthetics on P2X7 and P2Y receptors in a rat microglial cell line. Anesth. Analg. 104, 1136–1144, doi:10.1213/01.ane.0000260615.12553.4e (2007).
Nagakawa, T., Yamazaki, M., Hatakeyama, N. & Stekiel, T. A. The mechanisms of propofol-mediated hyperpolarization of in situ rat mesenteric vascular smooth muscle. Anesth. Analg. 97, 1639–1645, doi:10.1213/01.ANE.0000087043.61777.1F (2003).
Xu, X., Zheng, C., An, L., Wang, R. & Zhang, T. Effects of dopamine and serotonin systems on modulating neural oscillations in hippocampus-prefrontal cortex pathway in rats. Brain Topogr. 29, 539–551, doi:10.1007/s10548-016-0485-3 (2016).
Zhang, Y., Yu, T., Liu, Y., Qian, K. & Yu, B. W. Muscarinic M1 receptors regulate propofol modulation of GABAergic transmission in rat ventrolateral preoptic neurons. J. Mol. Neurosci. 55, 830–835, doi:10.1007/s12031-014-0435-z (2015).
Zhou, L. et al. The circadian clock gene Csnk1e regulates rapid eye movement sleep amount, and nonrapid eye movement sleep architecture in mice. Sleep 37, 785–793, doi:10.5665/sleep.3590 (2014).
Kinde, M. N. et al. Common anesthetic-binding site for inhibition of pentameric ligand-gated ion channels. Anesthesiology 124, 664–673, doi:10.1097/ALN.0000000000001005 (2016).
Han, L. et al. Propofol-induced inhibition of catecholamine release is reversed by maintaining calcium influx. Anesthesiology 124, 878–884, doi:10.1097/ALN.0000000000001015 (2016).
Moe, A. A. K. et al. Risperidone induces long-lasting changes in the conditioned avoidance response and accumbal gene expression selectively in animals treated as adolescents. Neuropharmacology 108, 264–274, doi:10.1016/j.neuropharm.2016.04.035 (2016).
van Laar, M., Volkerts, E. & Verbaten, M. Subchronic effects of the GABA-agonist lorazepam and the 5-HT2A/2C antagonist ritanserin on driving performance, slow wave sleep and daytime sleepiness in healthy volunteers. Psychopharmacology 154, 189–197, doi:10.1007/s002130000633 (2001).
Forman, S. A. & Miller, K. W. Mapping general anesthetic sites in heteromeric γ-aminobutyric acid type A receptors reveals a potential for targeting receptor subtypes. Anesth. Analg. 123, 1263–1273, doi:10.1213/ANE.0000000000001368 (2016).
Trapani, G., Altomare, C., Liso, G., Sanna, E. & Biggio, G. Propofol in anesthesia. Mechanism of action, structure-activity relationships, and drug delivery. Curr. Med. Chem. 7, 249–271, doi:10.2174/0929867003375335 (2000).
Corradin, O. et al. Modeling disease risk through analysis of physical interactions between genetic variants within chromatin regulatory circuitry. Nat. Genet. 48, 1313–1320, doi:10.1038/ng.3674 (2016).
Duan, G. et al. A single-nucleotide polymorphism in SCN9A may decrease postoperative pain sensitivity in the general population. Anesthesiology 118, 436–442, doi:10.1097/ALN.0b013e31827dde74 (2013).
Lingamaneni, R., Birch, M. L. & Hemmings, H. C. Jr. Widespread inhibition of sodium channel-dependent glutamate release from isolated nerve terminals by isoflurane and propofol. Anesthesiology 95, 1460–1466, doi:10.1097/00000542-200112000-00027 (2001).
Blanco, J. H. et al. Chronic cholinergic stimulation promotes changes in cardiovascular autonomic control in spontaneously hypertensive rats. Auton. Neurosci. 193, 97–103, doi:10.1016/j.autneu.2015.09.002 (2015).
Hahn, L. W., Ritchie, M. D. & Moore, J. H. Multifactor dimensionality reduction software for detecting gene-gene and gene-environment interactions. Bioinformatics 19, 376–382, doi:10.1093/bioinformatics/btf869 (2003).
Hachenberg, T. Perioperative management with short-acting intravenous anesthetics. Anaesthesiol. Reanim. 25, 144–150 (2000).
Court, M. H., Duan, S. X., Hesse, L. M., Venkatakrishnan, K. & Greenblatt, D. J. Cytochrome P-450 2B6 is responsible for interindividual variability of propofol hydroxylation by human liver microsomes. Anesthesiology 94, 110–119, doi:10.1097/00000542-200101000-00021 (2001).
Restoux, A. et al. Pilot study of closed-loop anaesthesia for liver transplantation. Br. J. Anaesth. 117, 332–340, doi:10.1093/bja/aew262 (2016).
Mourão, A. L., de Abreu, F. G. & Fiegenbaum, M. Impact of the cytochrome P450 2B6 (CYP2B6) gene polymorphism c. 516G> T (rs3745274) on propofol dose variability. Eur. J. Drug Metab. Pharmacokinet. 41, 511–515, doi:10.1007/s13318-015-0289-y (2015).
Mastrogianni, O. et al. Association of the CYP2B6 c. 516G> T polymorphism with high blood propofol concentrations in women from northern Greece. Drug Metab. Pharmacokinet. 29, 215–218, doi:10.2133/dmpk.DMPK-13-NT-092 (2014).
Lian, Q.-Q. et al. Impact of CYP2C9 polymorphism found in the Chinese population on the metabolism of propofol in vitro. Biol. Pharm. Bull. 38, 531–535, doi:10.1248/bpb.b14-00671 (2015).
Puig, M. V. & Gener, T. Serotonin modulation of prefronto-hippocampal rhythms in health and disease. ACS Chem. Neurosci. 6, 1017–1025, doi:10.1021/cn500350e (2015).
Peinado, A. Immature neocortical neurons exist as extensive syncitial networks linked by dendrodendritic electrical connections. J. Neurophysiol. 85, 620–629 (2001).
Moore, J. T. et al. Direct activation of sleep-promoting VLPO neurons by volatile anesthetics contributes to anesthetic hypnosis. Curr. Biol. 22, 2008–2016, doi:10.1016/j.cub.2012.08.042 (2012).
Okamoto, K. et al. Activation of central 5HT2A receptors reduces the craniofacial nociception of rats. Neuroscience 147, 1090–1102, doi:10.1016/j.neuroscience.2007.05.012 (2007).
Shyr, M.-H. et al. Propofol anesthesia increases dopamine and serotonin activities at the somatosensory cortex in rats: a microdialysis study. Anesth. Analg. 84, 1344–1348, doi:10.1213/00000539-199706000-00031 (1997).
Brown, R. E., Basheer, R., McKenna, J. T., Strecker, R. E. & McCarley, R. W. Control of sleep and wakefulness. Physiol. Rev. 92, 1087–1187, doi:10.1152/physrev.00032.2011 (2012).
Enna, S. & McCarson, K. E. The role of GABA in the mediation and perception of pain. Adv. Pharmacol. 54, 1–27, doi:10.1016/S1054-3589(06)54001-3 (2005).
Brown, E. N., Lydic, R. & Schiff, N. D. General anesthesia, sleep, and coma. N. Engl. J. Med. 363, 2638–2650, doi:10.1056/NEJMra0808281 (2010).
Franks, N. P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386, doi:10.1038/nrn2372 (2008).
Minier, F. & Sigel, E. Positioning of the α-subunit isoforms confers a functional signature to γ-aminobutyric acid type A receptors. Proc. Natl. Acad. Sci. USA 101, 7769–7774, doi:10.1073/pnas.0400220101 (2004).
Franks, N. P. Molecular targets underlying general anaesthesia. Br. J. Pharmacol. 147, S72–S81, doi:10.1038/sj.bjp.0706441 (2006).
Dib-Hajj, S. D., Yang, Y., Black, J. A. & Waxman, S. G. The NaV1. 7 sodium channel: from molecule to man. Nat. Rev. Neurosci. 14, 49–62, doi:10.1038/nrn3404 (2013).
Bennett, D. L. & Woods, C. G. Painful and painless channelopathies. Lancet Neurol. 13, 587–599, doi:10.1016/S1474-4422(14)70024-9 (2014).
Rehberg, B. & Duch, D. S. Suppression of central nervous system sodium channels by propofol. Anesthesiology 91, 512–520, doi:10.1097/00000542-199908000-00026 (1999).
Shirasaka, T., Yoshimura, Y., Qiu, D.-L. & Takasaki, M. The effects of propofol on hypothalamic paraventricular nucleus neurons in the rat. Anesth. Analg. 98, 1017–1023, doi:10.1213/01.ANE.0000107960.89818.35 (2004).
El Beheiry, H. & Mak, P. Effects of aging and propofol on the cardiovascular component of the autonomic nervous system. J. Clin. Anesth. 25, 637–643, doi:10.1016/j.jclinane.2013.07.004 (2013).
Stocker, S. D., Lang, S. M., Simmonds, S. S., Wenner, M. M. & Farquhar, W. B. Cerebrospinal fluid hypernatremia elevates sympathetic nerve activity and blood pressure via the rostral ventrolateral medulla. Hypertension 66, 1184–1190, doi:10.1161/HYPERTENSIONAHA.115.05936 (2015).
Dewland, T. A., Androne, A. S., Lee, F. A., Lampert, R. J. & Katz, S. D. Effect of acetylcholinesterase inhibition with pyridostigmine on cardiac parasympathetic function in sedentary adults and trained athletes. Am. J. Physiol. Heart Circ. Physiol. 293, H86–H92, doi:10.1152/ajpheart.01339.2006 (2007).
Gamou, S., Fukuda, S., Ogura, M., Sakamoto, H. & Morita, S. Microinjection of propofol into the perifornical area induces sedation with decreasing cortical acetylcholine release in rats. Anesth. Analg. 111, 395–402, doi:10.1213/ANE.0b013e3181e24776 (2010).
McCormick, D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 39, 337–388, doi:10.1016/0301-0082(92)90012-4 (1992).
Ge, D. J., Qi, B., Tang, G. & Li, J. Y. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci. Rep. 6, 21514, doi:10.1038/srep21514 (2016).
Chernik, D. A. et al. Validity and reliability of the Observer’s Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J. Clin. Psychopharmacol. 10, 244–251 (1990).
Acknowledgements
We thank all the patients for being part of the study and the staff at Union Hospital, Tongji Medical School, Huazhong University of Science and Technology for their support and undertaking of trial implementation. We gratefully acknowledge the contribution of Dr. Carl Lynch, who critically reviewed the manuscript. This work was supported by Grants 81171274 and 81571075 (to Dr. Xiangdong Chen) from the National Natural Science Foundation of China (Beijing, China).
Author information
Authors and Affiliations
Contributions
Qi Zhong and Xiangdong Chen designed the experiments. Qi Zhong and Yan Zhao performed the experiments. Qi Zhong and Yan Zhao collected blood samples. Qi Zhong, Xiangdong Chen, and Ru Liu conducted the statistical analysis. Xiangdong Chen, Shanglong Yao and Bayliss D.A. contributed advice. Qi Zhong and Xiangdong Chen wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, Q., Chen, X., Zhao, Y. et al. Association of Polymorphisms in Pharmacogenetic Candidate Genes with Propofol Susceptibility. Sci Rep 7, 3343 (2017). https://doi.org/10.1038/s41598-017-03229-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03229-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.