Abstract
Recently, more and more attention has been paid to the development of a new generation of injectable bone cements that are bioactive, biodegradable and are able to have appropriate mechanical properties for treatment of vertebral compression fractures (VCFs). In this study, a novel PSC/CS composite cement with high content of PSC (a phytic acid-derived bioactive glass) was prepared and evaluated in both vitro and vivo. The PSC/CS cement showed excellent injectability, good resistance to disintegration, radiopacity and suitable mechanical properties. The in vitro test showed that the cement was bioactive, biocompatible and could maintain its shape sustainably, which made it possible to provide a long-term mechanical support for bone regeneration. Radiography, microcomputed tomography and histology of critical sized rabbit femoral condyle defects implanted with the cements proved the resorption and osteoinductivity of the cement. Compared with the PMMA and CSPC, there were more osteocyte and trabeculae at the Bone-Cement interface in the group PSC/CS cement. The volume of the residual bone cement suggested that PSC/CS had certain ability of degradation and the resorption rate was much lower than that of the CSPC cement. Together, the results indicated that the cement was a promising bone cement to treat the VCFs.
Similar content being viewed by others
Introduction
Vertebral compression fracture (VCF) is probably the most common complication in patients with osteoporosis, with an estimated 1.4 million new fractures occurring every year worldwide1. The minimally invasive surgeries of percutaneous kyphoplasty (PKP) and percutaneous vertebroplasty (PVP) are safe and effective for VCFs. Both procedures involve percutaneous injection of the setting dough of an injectable bone cement either directly to the fractured vertebral body (PVP) or to a void created in it by an inflatable bone tamp (PKP). Thus, injectable bone cement is essential in both procedures. Currently available injectable bone cements in clinic mainly include poly(methyl methacrylate) (PMMA), calcium sulfate cement (CSC) and calcium phosphate cement (CPC)2.
PMMA is the most widely used injectable cement in PVP and PKP, because of its suitable curing behavior and easiness of handling. Although there have been great success, several drawbacks still need to be further improved. PMMA is not bio-resorbable, therefore cannot be replaced by new bone but forms an implant-host interface. PMMA also lacks active bonding with surrounding bone, thus the long-term mechanical stability of implant-host interface is still unsatisfactory3. Besides, the intensive exothermic effect in the polymerization process may lead to thermal necrosis in surrounding tissues4. Moreover, differences in mechanical strength between PMMA and the adjacent vertebral body are often found to cause adjacent vertebral fractures5.
CSC, with self-setting ability, has enjoyed a long history of clinical use as injectable bone augmentation6, 7. It is virtually complete resorbable in vivo, thus can overcome the long-term stability issue often faced by PMMA cement. These properties make it possible to serve as a delivery vehicle for drugs, growth factors or as a soluble additive to modulate the porosity or biodegradable rate when blended with other biomaterials6, 8. Despite these virtues, CSC has been criticized for its rapid resorption rate. Studies have shown that the degradation of CSC implant is much faster than bone ingrowth, which makes it fail to provide adequate mechanical support3, 6, 9.
Calcium phosphate has excellent biocompatibility, bone conductivity, and slower degradation rate than calcium sulfate. When mixed with calcium sulfate, injectable bone cements can still be formulated, which degrade slower and stimulate bone growth, thus are expected to solve the long-term mechanical support issue8. This strategy has obtained certain success and clinical products have been developed based on these combinations, for example Genex®, a widely used resorbable bone cement for bone defect filling. However, the resorption rate is still too fast and further collapse after treatment of vertebral compression fractures often takes place, thus the calcium phosphate-calcium sulfate combination only finds very limited success in PKP or PVP procedures10, 11. Further development should focus on slowing down degradation and improve osteogenic property of bone cement, in order to maintain a sufficient mechanical support until the fractured vertebrae recovers.
Bioactive glass (BG) represents one of the most promising artificial bone repair materials, due to their excellent biocompatibility, bioactivity and osteoinductivity12, 13. The ions released by BG, especially soluble silicon and calcium ions, were proved to stimulate osteoprogenitor cells at the genetic level and thus promote bone regeneration12. Up to date, BG has been successfully used in bone regeneration and dental applications13, 14. BG also degrades much slower than calcium phosphate15. Therefore, it is imaginable that when BG is blended with calcium sulfate, injectable bone cements with slower resorption and better osteogenic performance could be developed. Indeed, several studies have demonstrated that BG-contained calcium sulfate cements had better osteoinductivity and simulation of bone ingrowth9, 16, 17. However, because the conventional BG (i.e. 45S5) increases pH when contacting body fluid, which intervenes with the hydration reactions of calcium sulfate, the content of BG was often very low, i.e. only a few percent by weight. Consequently, it only slightly improved the biogenic property, while had little improvement or sometimes even deterioration on the long term mechanical support, because the curing of calcium sulfate became incomplete9, 16.
In this study, we aim to develop an injectable BG/calcium sulfate composite cement with high content of BG by using a novel phytic acid-derived bioactive glass. This bioactive glass with the composition of 10.8%P2O5-54.2%SiO2-35%CaO (mol. %; hereinafter referred to as PSC) can maintain a stable pH when reacting with physiological solution. The hypothesis was that, with higher content of BG, the PSC/calcium sulfate composite cement could show better osteogenic performance and have a slower biodegradation rate to match with the growth of new bone tissue. The physicochemical properties of the cement, such as setting time, injectability, disintegration resistance and mechanical properties were investigated. In vitro bioactivity, degradation behavior in simulated body fluid (SBF) and cytocompatibility were studied to evaluate its potential bone integration ability. Eventually, its’ in vivo performances were evaluated in a rabbit femoral condyle defect model.
Results
Characterization of the cements
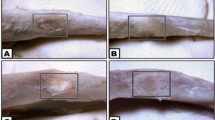
In the present study, a novel bioactive glass based injectable bone cement was developed. As mentioned earlier, the more content of BG, the higher bioactivity of the composite cement. In our preliminary experiments, it was found that when the content of PSC was above 55 wt.%, PSC/CS cement was difficult to set and the compressive strength of resultant material (<2 MPa) was lower than the requirements (2–12 MPa)18. Therefore, the PSC content was set to be 55 wt% in the PSC/CS cement through this study. Some physiochemical properties of PSC/CS cement are summarized in Table 1. The injectability of the PSC/CS cement remained above 90% during the first 6 minutes (Fig. S1, Supporting information) and no phase separation were observed when the cements were extruded from the syringe. Therefore, it is possible for them to be used for minimally invasive surgery. From Fig. 1a, it can be seen that the cement could almost remain its initial shape and no obvious decay was observed after immersed in PBS for 24 h. The disintegration resistance of the cements, as determined by Eq. (2), were ~94% (Table 1), which indicated a good resistance to disintegration in PBS. Some weight loss may be caused by the ion release when immersed in PBS (Fig. S2, Supporting information). The compressive strengths (Sc) and Young’s modulus (Ec) of the hardened PSC/CS cement were ~2.9 MPa and ~340 MPa, respectively, which were comparable to human trabecular bone (Sc:2–12 MPa; Ec:100–500 MPa)18, 19.
Figure 1b gives the in vitro degradation profile of the CSC, CSPC and PSC/CS cements after immersing in SBF. It is seen that the CSC degraded rapidly in SBF (Fig. 1d). The Mass loss of CSC was ~85.5% and the diameter reduced significantly after immersed in SBF for 4 weeks. The degradation rate of CSPC in SBF was even more rapid than CSC, probably due to the interruption of calcium sulfate hardening by calcium phosphate. It is generally accepted that a cement with a degradation rate faster than that of bone ingrowth, such as CSC, is not suitable because it does not provide long-term mechanical support and the space left after its degradation will restrict the growth of newly formed bone6, 20.
While for PSC/CS cement, its weight loss increased rapidly within the first 3 weeks, after which it only increases with a much slower rate and finally reaches a plateaued at ~52% weight loss (at about 7 ~ 8 weeks). Interestingly, the diameter of PSC/CS specimen had virtually no change and its shape remained almost the same in the whole experimental period (Fig. 1c), which might be caused by the formation of interconnected network of formed hydroxyapatite (HA) upon the reaction of PSC with SBF. Its weight loss was the comprehensive effect of calcium sulfate degradation, PSC degradation and HA formation. The disappearance of calcium sulfate peaks and the appearance of HA peaks on the FTIR spectra provide experimental evidence for the above conjecture (Fig. 2a).
(a) FTIR spectra of the PSC/CS cement after immersion in SBF for 0, 1, 2, 3 and 8 weeks. (b) SEM images of PSC/CS cement after immersion in SBF for 8 weeks. (c) MTT assay for proliferation of MG63 cells after culturing for 48 h. (d) The MG63 cell morphologies on PSC/CS cement after culturing for 24 h.
In Vitro Bioactivity and Cell Biocompatibility
After incubation in SBF, the peaks at 607 cm−1 and 567 cm−1 appeared on the FTIR spectra (Fig. 2a), which could be the evidence of crystalline phosphate, confirming the formation of HA. Figure 2b shows that dense hemispherical and needle like HA crystals was formed on the cement surface. The hemispherical and needle like HA crystals were consistent with previous reports21 and in agreement with the results of FTIR. It has been generally accepted that the bone-bonding ability of a material can be evaluated by examining the HA formation on its surface in SBF. Therefore, the PSC/CS cements are potentially bioactive.
The MG63 cell morphologies on the cement surfaces are illustrated in Fig. 2d. After culturing for 24 h, MG63 cells showed a spindle-like or polygonal morphology and spread well on the surfaces of PSC/CS cement, suggesting MG63 cells were able to grow and proliferate well on the samples. The MTT assay was adopted to quantitatively assesse the cell viability. Compared to the control groups (the blank groups, CSC and CSPC), the cell proliferations of PSC/CS cement were slightly higher and no significant difference was observed (p > 0.05). These results demonstrated the PSC/CS cement showed good cell compatibility and had no cytotoxic effect on MG63 cells.
Bone regeneration and resorption of cement in vivo
X-ray analysis
In Group A with PSC/CS, no significant change observed in the first 4 weeks after the operation. At the 8th week after the operation, the margin of the cement became unclear, indicating the resorption of the cement. At the 12th week, the PSC/CS cement further degraded, and there were some high density signals appeared at the edge of the implanted cement, indicating new bone was formed at the cement-bone interface. In the Group B with CSPC, most of the implanted cement had degraded within the first 4 weeks after the operation. At the 8th week, the implanted cement almost completely degraded and was replaced by new bone. No residual bone cement was observed at the 12th week and the bone defects were partially filled with the newly formed bone. However, in the Group C with PMMA, there was no visible change observed up to the 12th week after the operation (Fig. 3).
Micro-computed tomography analysis
Micro-CT images and the 3D reconstruction images of the residual cement as well as the newly formed bone tissue at the defect area were used for evaluation of the in vivo osteogenic capacity and the resorption of the cement. In Group A, the PSC/CS degraded partially and the residual material was surrounded by the newly formed bone tissue. In Group B with CSPC, the implanted bone cement almost degraded completely and was partially replaced by the new trabecular. However, in the Group C with PMMA, the bone cement showed few observable changes and only a few newly formed bones appeared at the bone-cement interface (Fig. 4).
The volume of the new bone and the residual cement within the defect area were calculated to precisely evaluate the bone growth and the resorption rate. As shown in the Fig. 5, at the 12th week, the BV/TV of the PSC/CS (7.7 ± 1.6%) is significantly higher than that of PMMA cement (0.9 ± 0.2%) and CSPC (5.6 ± 1.6%). In addition, the bone cement volume of PSC/CS (79.4 ± 5.2%) was significantly lower than that of the PMMA (96.9 ± 1.3%) at the 12th week after the implantation, and in the Group CSPC, there is no residual bone cement.
The results indicated that the PSC/CS had better capacity to stimulate bone regeneration compared with the CSPC cement and the PMMA cement. The volume of the residual bone cement suggested that compared with the PMMA, PSC/CS had certain ability of degradation. While the resorption rate of the PSC/CS was much lower than that of the CSPC cement. These characters empowered the PSC/CS to generate osteogenesis at the same time of providing biomechanical support in a long-term.
Histological evaluation
Histological analysis was also performed to get a more detailed analysis on the new bone formation. Figure 6 showed the photographs of H&E stained sections at the 12th week after surgery. In group PSC/CS, the material partially degraded and the residual materials were surrounded by areas of newly formed bone tissue (Fig. 6a). Compared with the other two groups, there were more osteocyte, trabeculae and vessels at the Bone-Cement interface. In addition, more cells gathered at the interface between the implant materials and the newly formed bone tissue, indicating further osteogenesis comes along with the degrading of the implanted materials. In group CSPC, the implant materials almost degraded completely (Fig. 6b). However, the spaces created by degradation of the materials were not fully replaced with bone tissue. In contrast, only a small amount of bone tissue was observed within the defects. In group PMMA, a thin layer of osteoid formed at the edge of the bone defect site (Fig. 6c). New trabeculae formed at the Bone-Cement interface but there was no bone tissue observed within the defects because of the occupied effect caused by the non-degradability of PMMA.
The result of H&E staining was consistent with the observation by the Micro-CT. It again was confirmed that PSC/CS had a better capacity to stimulate bone regeneration and had certain ability of degradation.
Discussions
PVP and PKP are now widely used for treating VCFs. For long-term sake, the injectable cements used in PVP and PKP should be biodegradable, thus enabling a total replace of implant by new bone tissue. The currently available degradable bone cements are blamed for the mismatch of bone regeneration rate and implants degradation rate, i.e. the implants disappear before new bone tissue fills the void, thus, insufficient mechanical support is expected. In this study, a new type of BG (PSC) was blended CS to form an injectable cement in order to address the above issue. As mentioned earlier, this new formulation is expected to degradation slower and stimulate bone regeneration better, thus sufficient mechanical support could be achieved during the healing process. All the related properties were evaluated under the context of using this new PSC/CS cement for potential application in PVP or PKP.
In clinic use, setting time, injectability and disintegration resistance are key characteristics for bone cements22, 23. According to the results showed in section 3.1, the PSC/CS cement showed excellent injectabiliy (>90%) and stayed homogeneous throughout the whole injection process. Its initial and final setting times were about 25 min and 31 min, respectively, which are longer than the other cements (5–15 mins)3. Although a relatively long setting time could offer the surgeons more time for the operation, it is risky because the paste may be washed out by the physiological liquids within this period2, 24. Fortunately, this PSC/CS cement showed good resistance to disintegration (D ~ 94%). Even immersed in PBS for 24 h, it can almost maintained the initial shape and no obvious disintegration was observed. These properties indicated that the PSC/CS cement was still a promising candidate for use in minimally invasive surgery.
Ideally, once implanted, the cement should be both bioactive and bioresorbable as well as provide required mechanical support to share the load with the surrounding bone tissues. The compressive strengths (Sc) and Young’s modulus (Ec) of the hardened PSC/CS cement were around 2.9 MPa and 340 MPa, respectively, which fulfilled the requirements for cancellous bone substitutes18, 19. Fig. 1b showed the in vitro degradation behaviors of CSC, CSPC and PSC/CS cements, which clearly highlighted the privilege of PSC/CS cement. The degradation behaviors of the PSC/CS cement seemed to meet the requirements for bone tissue regeneration and mechanical support during healing process.
It has been generally accepted that the formation of HA layer on a bioactive material is essential for the bonding between the material and surrounding tissues25. CSC has been criticized for the fact that no HA was deposited on its surface after immersion in SBF due to its non-bioactivity6, 9, 16. In comparison, when PSC/CS cement was incubated in SBF, both SEM observation and FTIR investigation revealed the rapid formation of HA on surface, indicating their excellent bioactivity. Furthermore, with the extension of immersion time, the intensity of HA became stronger (Fig. 2a), suggesting the further growth of HA. It should be noted that the deposited HA on surface not only played an important role on bioactivity but also slowed down the degradation rate compared with the pure calcium sulfate.
The implantation of the PSC/CS cement in the critical-sized rabbit femoral condyle defects helps to evaluate its potential in the eventual clinical application. Presently, the most commonly used filling material in PKP and PVP is still the nondegradable PMMA. Because of the lack of osteoinductivity and bioactivity, the long-term stability of the interface between the cement and the bone is not guaranteed26, 27. In terms of biocompatibility and bone conductivity, bioresorbable cements such as CSC and CPC are better than PMMA. However, their rapid resorption often results in insufficient persistence of an osteocondutive scaffold to encourage bone apposition as well as the destabilization of early bony apposition28, and eventually leads to the recollapse of the fractured vertebra10. In the treatment of VCFs, spinal deformity reduction is as important as pain relief29, hence requesting the implanted cement to have the ability of osteogenesis while having a reabsorbed rate matched to the rate of new bone formation in order to provide a long-term maintenance of vertebral height restoration. Based on our in vivo test, PMMA did not degrade at all and new bone tissue was only found at the bone-cement interface (Fig. 6c). It can stabilize the fracture fragment, thus leading to a rapid pain relief, however, the bone-cement may be loosen in long term due to the lack of bony bonding. CSPC showed a certain ability of osteogenesis, however, because of the rapid resorption, the newly formed bone tissue cannot fully fill the spaces created by degradation of the CSPC (Fig. 6b), which will result in recollapse and fail to maintain the vertebral height restoration. This new PSC/CS cement can significantly enhance the osteogenesis at the interfacial areas and stimulate more bone formation, superior to both PMMA and CSPC cements (Fig. 6a). Besides, PSC/CS resorbed slower than CSPC, thus can provide sufficient mechanical support in the healing process.
The appropriate mechanical properties, better osteoinductivity and suitable resorption rate would have a positive effect on the long-term maintenance of the vertebral height while concurrently promote the fracture healing and reduce the postoperative complications such as new fracture of adjacent vertebral body and recollapse of the fracture vertebral body. Based on the results of the properties determined in vitro as well as the femoral condyle defect model in a rabbit, this new PSC/CS cement is a potential substitute of commercial bone cements for application in PVP and PKP. In the future, further investigations will focus on larger animal models.
Materials and Methods
Materials
PSC powers with the composition of 10.8%P2O5-54.2%SiO2-35%CaO (mol. %) were prepared by a sol–gel method according to our previous work30. The obtained powders were ground to form particles of size <38 μm and stored in a desiccator until usage. α-Calcium sulfate hemihydrate (CSH, ≥97.0%) and Tricalcium phosphate (TCP) were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. PMMA cement was purchased from Medtronic Inc.
Preparation of PSC/CS cements
The PSC/calcium sulfate composite cements (PSC/CS cements) were prepared by combining a solid and a liquid phase. The solid phase was prepared by mixing the obtained PSC and CSH powers with PSC content of 55 wt %. The setting-liquid phase was a chitosan solution that prepared according to the literature31. Then, the solid powders were mixed with setting-liquid phases for 1 min with a spatula at the L/P ratio of 0.5 ml/g to get a homogeneous cement pastes.
CSC and calcium sulfate/calcium phosphate composite cement (CSPC, CSH: TCP = 1:1) samples were also prepared as a comparison following the same procedure for PSC/CSC cements.
Characterization of PSC/CS cement
Injectability
The injectability of composite cements was tested according to the literatures32, 33. Briefly, approximately 4 g of the homogeneous cement pastes prepared as described above were put into a 5 ml syringe with an opening nozzle diameter of 2 mm. After 3 min since the beginning of mixing the composite powders and liquid, the cement was extruded by hand until it was too hard to push the syringe. The percentage of cement that could be extruded from the syringe is used to evaluate the injectability coefficient (J), as (1):
where M 0 is the mass of the empty syringe, M 1 is the total mass of syringe and cements, and M 2 is the remaining mass of syringe and cements after extrusion.
The disintegration resistance
The degree of cohesion or the disintegration resistance of cements in liquid was tested by injecting 1 g newly prepared cement into phosphate-buffered saline (PBS, pH = 7.2 ~ 7.4) at 37 °C. After soaking in PBS for 24 h, the integrity of cement was observed by naked eyes and the amount of non-decayed cement was carefully collected, dried and weighed (W 1 ). Another 1 g newly prepared cement were also dried and weighed (W 2 ) as a control. Each measurement was performed in triplicate. The disintegration resistance (D) was determined using the equation (2):
The setting time and Mechanical Properties
The setting time of the composite cements were measured with a Vicat apparatus, according to ISO 9597:2008(E). The mechanical properties of PSC/CS cements (Ø4.5 × 9.0 mm) were performed on a universal testing machine (Instron3365, Instron Co., Canton, MA, USA) with a 5kN load cell at a loading rate of 0.5 mm/min until sample failure. At least 5 samples of each cement were tested, and the results were expressed as a mean ± SD.
In Vitro Bioactivity and degradability
The in vitro bioactivity and degradability of cements were evaluated by soaking in simulated body fluid (SBF)33. The hardened cylinder samples (Ø 4.5 × 4.0 mm) were immersed in SBF with a surface area-to-volume ratio of 0.1 cm−1 and kept at 37.0 °C. The surface morphology and chemical structures of the PSC/CS before and after SBF immersion were measured using scanning electron microscopy (SEM, JEOL-6700) and Fourier transform infrared (FTIR, Bruker Equinox 55 instrument) to examining the ability of apatite formation on their surfaces.
The initial weight (W 0 ) of each sample was recorded. After soaking in the SBF for different periods, the specimen were taken out, washed and dried at 60 °C until constant weight. The final weight (W t ) of the dried specimen was monitored. The degradation was determined by weight loss according to the following equation (3):
Cell Toxicity and Attachment
MG63 cells were used to investigate the biocompatibility of cements.
4.5.1 Cell toxicity
The specimen of each cement were previously sterilized by autoclaving at 180 °C for 2 h and then soaked in α-MEM, preheated to 37 °C (extracting vehicle ratio = 12.5 mg/mL), for 3 days. Under sterile conditions, α-MEM was filtered to eliminate solid cements and these extracts were used as culture medium after adding 10 vol. % fetal bovine serum (FBS) and 1 vol. % penicillin/streptomycin antibiotics. MG63 cells with a density of 2000 cells/plate were seeded onto 96-well culture plates and incubated in the cell incubator. After cell adhesion was verified, the culture medium was replaced by the extracts of different cements. The cells incubated in a-MEM without extract were used as a control. After culturing for 48 h, cell viability on the samples was assessed quantitatively by the thiazolyl blue (MTT) assay. The optical density (O.D. value) of each sample was measured at 490 nm. Five specimens in each group were tested.
Cell attachment
In a parallel experiment, the samples (Ø 12 × 2 mm) were sterilized by autoclaving at 180 °C for 2 h and then put in each well of the 24-well plate. MG63 cells with a density of 5 × 104 cells/sample were seeded onto the samples, and incubated in the cell incubator. At 24 h, the cell-cements were gently rinsed with PBS three times, and fixed with 2.5% glutaraldehyde at 4 °C for 12 h. The cell-cements were sequentially dehydrated in graded ethanol solutions (50, 75, 95, and 100 wt %), dried and the cell attachment and morphology were directly observed by SEM.
In vivo evaluation of cement in a rabbit femoral condyle defect model
Surgical procedure
All the animal experimental procedures were approved by the Animal Research Committee of Peking University (Permit number: LA201415) and we conformed that all methods were performed in accordance with the relevant guidelines and regulations. In total there were 12 adolescent male New Zealand white rabbits weighing 3.0–3.5 kg used and all of them were anesthetized using an ketamine hydrochloride (50 mg/kg, IM) and fentanile (0.17 mg/kg, IM). The distal femoral epiphysis exposure was performed using lateral approach, and a critical-sized condyle defect (5 mm in diameter and 10 mm in depth)34, 35 was created using an electric trephine (Johnson & Johnson, USA) under irrigation with 0.9% sterile saline solution. The defect model was treated using three kinds of injectable bone cement: the novel PSC/CS and two commercially available bone cements: PMMA cement (Medtronic, America) and CSPC cement (CSH: TCP = 1:1). The left femoral condyle defect of all the rabbits were implanted with the PSC/CS (Group A), while the right femoral condyle defect of six rabbits were treated with the CSPC (Group B), and the right femoral condyle defect of the other six were treated with the PMMA (Group C). The wounds were sutured well and the prophylactic antibiotic together with the analgesics was given for three days.
Radiographic examination
After the surgery, rabbits were fastened in the lateral position under anesthesia for the X-ray radiographs of the bone defect after 0, 4, 8 and 12 weeks. In order to precisely evaluate the defect healing process, the radiographs of each rabbit were inspected by at least two experienced orthopedic surgeons who did not participate in the study and were blinded from the detailed experimental process.
Micro CT evaluation
For microcomputed tomography evaluation, all the rabbits were sacrificed by overdose of pentobarbital at the 12th weeks after surgery. The rabbit femurs were examined by the Micro-CT (Inveon Scanners, SIEMENS, German) after removing the soft tissue attached to the femur. To evaluate the in vivo resorption of the implanted bone cement, the residual bone cement volume fraction was calculated as the ratio between the volume of residual cement and the defined VOI (volume of interest). And the amount of newly formed bone was quantified as the bone volume (BV) within the defined VOI (volume of interest) in each defect site by using a CT analyzing software (Inveon Research Workplace, SIEMENS, German).
Histologic analysis
After the radiographic examination and Micro CT evaluation, the harvested femur were washed with saline thoroughly, then fixed in 4% paraformaldehyde (10% neutral buffered formalin) for no more than 72 h and subsequently decalcified for up to 6 weeks in 10% EDTA, pH 7.0 at 4 °C. After complete decalcification and dehydration, the samples were embedded in paraffin wax. Then 5 mm serial slices were prepared using a microtome, subsequent surface staining was performed with haematoxylin and eosin (H&E) for microscopic observation. The slides were photographed using a digital camera (NanoZoomer-SQ, Hamamatsu Photonics K.K., Hamamatsu, Japan).
Statistical analysis
Statistical analysis was performed using one-way ANOVA and the Student’s t-test. All quantitative data were presented as mean ± SD. Differences were considered statistical significant at p < 0.05.
Data Availability
All data submitted in the manuscript are available.
Conclusion
In this study, a new PSC/CS composite cement with high content of PSC was developed and evaluated both in vitro and in vivo. Excellent injectability, good resistance to disintegration and radiopacity were observed from this composite cement, which all made it easier for operation. The in vitro test showed that the cement was bioactive and had suitable mechanical properties. More importantly, under physiological conditions, it could maintain its shape sustainably and possibly provide a long-term mechanical support for bone regeneration. In addition, this cement showed a better capacity than the PMMA and CSPC in terms of bone regeneration as well as the resorption rate observed in a critical-sized rabbit femoral condyle defect model. Based on these observations, this cement is a promising bone cement to treat the VCFs using minimally invasive surgery.
References
Johnell, O. & Kanis, J. A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporosis International 17, 1726–1733 (2006).
Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. Journal of biomedical materials research. Part B, Applied biomaterials 76, 456–468 (2006).
No, Y. J., Roohani-Esfahani, S. I. & Zreiqat, H. Nanomaterials: the next step in injectable bone cements. Nanomedicine (Lond) 9, 1745–1764 (2014).
Lv, Y. et al. A Novel Composite PMMA-based Bone Cement with Reduced Potential for Thermal Necrosis. ACS Appl Mater Interfaces 7, 11280–11285 (2015).
Masala, S. et al. Osteoporotic vertebral compression fracture augmentation by injectable partly resorbable ceramic bone substitute (Cerament (TM)|SPINESUPPORT): a prospective nonrandomized study. Neuroradiology 54, 1245–1251 (2012).
Thomas, M. V. & Puleo, D. A. Calcium sulfate: Properties and clinical applications. Journal of biomedical materials research. Part B, Applied biomaterials 88, 597–610 (2009).
Beuerlein, M. J. & Mckee, M. D. Calcium sulfates: what is the evidence? Journal of Orthopaedic Trauma 24(suppl 1), S46–51 (2010).
Hu, G. et al. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. Journal of materials science. Materials in medicine 21, 627–634 (2010).
Lin, M. et al. Novel highly bioactive and biodegradable gypsum/calcium silicate composite bone cements: from physicochemical characteristics to in vivo aspects. Journal of Materials Chemistry B 2, 2030 (2014).
Piazzolla, A., De Giorgi, G. & Solarino, G. Vertebral body recollapse without trauma after kyphoplasty with calcium phosphate cement. Musculoskeletal surgery 95, 141–145 (2011).
Ryu, K.-S., Shim, J.-H., Heo, H.-Y. & Park, C.-K. Therapeutic Efficacy of Injectable Calcium Phosphate Cement in Osteoporotic Vertebral Compression Fractures: Prospective Nonrandomized Controlled Study at 6-Month Follow-up. World Neurosurgery 73, 408–411 (2010).
Hench, L. L. The story of Bioglass. Journal of materials science. Materials in medicine 17, 967–978 (2006).
Jones, J. R. Review of bioactive glass: from Hench to hybrids. Acta Biomater 9, 4457–4486 (2013).
Polini, A., Bai, H. & Tomsia, A. P. Dental applications of nanostructured bioactive glass and its composites. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5, 399–410 (2013).
Zeimaran, E. et al. Bioactive glass reinforced elastomer composites for skeletal regeneration: A review. Mater Sci Eng C Mater Biol Appl 53, 175–188 (2015).
Ding, Y. T. et al. In vitro degradability, bioactivity and primary cell responses to bone cements containing mesoporous magnesium-calcium silicate and calcium sulfate for bone regeneration. Journal of the Royal Society Interface 12, 10 (2015).
Furlaneto, F. A. et al. Bone healing in critical-size defects treated with bioactive glass/calcium sulfate: a histologic and histometric study in rat calvaria. Clin Oral Implants Res 18, 311–318 (2007).
Fu, Q., Saiz, E., Rahaman, M. N. & Tomsia, A. P. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C Mater Biol Appl 31, 1245–1256 (2011).
Kokubo, T., Kim, H.-M. & Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 24, 2161–2175 (2003).
Kumar, C. Y., K, B. N., Menon, J., Patro, D. K. & B, H. B. Calcium sulfate as bone graft substitute in the treatment of osseous bone defects, a prospective study. J Clin Diagn Res 7, 2926–2928 (2013).
Vallet-Regı, A. J. S. A. M. A. Bioactive ceramics: from bone grafts to tissue engineering. RSC Adv. 3, 11116–11131 (2013).
El-Fiqi, A., Kim, J.-H., Perez, R. A. & Kim, H.-W. Novel bioactive nanocomposite cement formulations with potential properties: incorporation of the nanoparticle form of mesoporous bioactive glass into calcium phosphate cements. J. Mater. Chem. B 3, 1321–1334 (2015).
Zhang, Y. et al. Evaluation of injectable strontium-containing borate bioactive glass cement with enhanced osteogenic capacity in a critical-sized rabbit femoral condyle defect model. ACS Appl Mater Interfaces 7, 2393–2403 (2015).
Yu, L. et al. A novel injectable calcium phosphate cement-bioactive glass composite for bone regeneration. PLoS One 8, e62570 (2013).
Kokubo, T. & Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27, 2907–2915 (2006).
Yang, H. & Zou, J. Filling Materials Used in Kyphoplasty and Vertebroplasty for Vertebral Compression Fracture: A Literature Review. Artificial Cells Blood Substitutes and Biotechnology 39, 87–91 (2011).
Lewis, G. et al. Evaluation of two novel aluminum-free, zinc-based glass polyalkenoate cements as alternatives to PMMA bone cement for use in vertebroplasty and balloon kyphoplasty. J Mater Sci-Mater M 21, 59–66 (2010).
Hing, K. A., Wilson, L. F. & Buckland, T. Comparative performance of three ceramic bone graft substitutes. Spine Journal 7, 475–490 (2007).
Zhu, T. et al. Percutaneous kyphoplasty with or without temporary unipedicle screw reduction: A retrospective comparative study of osteoporotic vertebral fractures. Der Orthopäde 45, 607–615 (2016).
Li, A. L. & Qiu, D. Phytic acid derived bioactive CaO-P2O5-SiO2 gel-glasses. J Mater Sci-Mater M 22, 2685–2691 (2011).
Cui, X. et al. An injectable borate bioactive glass cement for bone repair: Preparation, bioactivity and setting mechanism. J Non-Cryst Solids 432, 150–157 (2016).
Khairoun, I., Boltong, M. G., Driessens, F. C. M. & Planell, J. A. Some factors controlling the injectability of calcium phosphate bone cements. J Mater Sci-Mater M 9, 425–428 (1998).
Liu, W., Wu, C., Liu, W., Zhai, W. & Chang, J. The effect of plaster (CaSO4. 1/2H2O) on the compressive strength, self-setting property, and in vitro bioactivity of silicate-based bone cement. Journal of biomedical materials research. Part B, Applied biomaterials 101, 279–286 (2013).
Ryan Dodde, I., Yavuzer, R., Bier, U. C., Alkadri, A. & Jackson, I. T. Spontaneous bone healing in the rabbit. Journal of Craniofacial Surgery 11, 346–349 (2000).
Miño-Fariña, N. et al. Quantitative analysis of the resorption and osteoconduction of a macroporous calcium phosphate bone cement for the repair of a critical size defect in the femoral condyle. The Veterinary Journal 179, 264–272 (2009).
Acknowledgements
This project was supported by the National Natural Science Foundation of China (Grant No. 81470101).
Author information
Authors and Affiliations
Contributions
Tengjiao Zhu as the joint first author who was highly involved in the surgeries mentioned in the article, all the experimental analysis (X-ray, Micro-CT, histological analysis) as well as the experimental results analysis and discussion. Huihui Ren as the joint first author who was involved in preparation of PCS/CS cements, in vitro and in vivo test (physical characterization, bioactivity, cell biocompatibility and degradability). These authors contributed equally to this work. Bingchuan Liu as the co-author, was involved in the surgeries and experimental analysis. Ailing Li, Caiyun cui and Yanmei Dong, as the co-authors, were involved in the test of the PCS/CS cement in vitro. Yun Tian, Dong Qiu, as the corresponding authors, were actively participated in the subject design and responsible for guiding the experimental results analysis and discussion. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
zhu, T., Ren, H., Li, A. et al. Novel bioactive glass based injectable bone cement with improved osteoinductivity and its in vivo evaluation. Sci Rep 7, 3622 (2017). https://doi.org/10.1038/s41598-017-03207-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03207-9
This article is cited by
-
Regulation of the antibiotic elution profile from tricalcium phosphate bone cement by addition of bioactive glass
Scientific Reports (2024)
-
Injectable bioactive polymethyl methacrylate–hydrogel hybrid bone cement loaded with BMP-2 to improve osteogenesis for percutaneous vertebroplasty and kyphoplasty
Bio-Design and Manufacturing (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.