Abstract
Radical prostatectomy (RP) is the gold standard for the treatment of localized PCa. A meta-analysis was conducted to evaluate the effect of different techniques of pelvic floor reconstruction on urinary continence. A comprehensive search was made for trials that evaluated the efficacy of pelvic floor reconstruction. Relevant databases included PubMed, Embase, Cochrane, Ovid, Web of Science databases and relevant trials from the references. Random-effects model was used to estimate risk ratios (RRs) statistics. Pooled results of patients treated with posterior reconstruction (PR) demonstrated complete urinary continence improved at 1–4, 28–42, 90, 180 and 360 days following catheter removal. Anterior suspension (AS) was associated with improvement only at 28–42 days. The anterior reconstruction (AR) + PR was associated with urinary continence at 1–4, 90 and 180 days. AS + PR was not associated with any benefit. And PR improved social urinary continence at 7–14 and 28–42 days. No benefit was associated with AS. AR + PR had better outcomes at 90 and 180 days. AS + PR was significant improved at 28–42 and 90 days. Patients who underwent RP and PR had the least urinary incontinence. No significant benefit was observed after AS. AR + PR and AS + PR had little benefit in the post-operative period.
Similar content being viewed by others
Introduction
Prostate cancer (PCa) is the most common cancer, with an incidence of approximately 21% in the general population. It is the second most common cause of male cancer death in the world, affecting about 8% of men1. By 2016 in the United States 180,890 new PCa cases and 26,120 deaths from PCa are predicted to occur1. Radical prostatectomy (RP) is the gold standard for the treatment of localized prostate cancer. Robot-assisted radical prostatectomy (RARP) and laparoscopic radical prostatectomy (LRP) are widely used, and have been associated with lower positive surgical margin rates, shorter hospitalizations, lower post-operative leakage rates, lower transfusion requirements and a shorter period of urinary catheterization2. Early urinary incontinence remains one of the most common complications after RP.
Post-operative urinary incontinence is severely bothersome3 and is associated with a decreased quality of life. Urinary incontinence is often perceived as more bothersome than erectile dysfunction4. Several methods of pelvic floor reconstruction have been introduced to reduce the risk of urinary incontinence. Posterior reconstruction (PR) of the rhabdosphincter was initially described by Walsh5 and later popularized by Rocoo et al.6, 7. It is still a popular technique for controlling urinary incontinence. Anterior reconstruction (AR) was introduced by Tewari et al.8 and later combined with PR to yield an incremental benefit (AR + PR)9,10,11. A simple anterior suspension (AS) technique using sutures anchored to the pubic bone was first described by Sugimura et al. to improve early urinary continence12. The effect of anterior suspension combined with posterior reconstruction (AS + PR) has also been examined.
Now the effect of different surgical techniques for improving urinary continence is not clear yet. Rocco et al.13 reported a meta-analysis of posterior reconstruction technique and several trials have been conducted to evaluate the time to urinary continence after LRP and RARP. However, the previous study didn’t evaluate other surgical techniques. The publication of new studies evaluating PR, AS, AR + PR, and AS + PR add to the power of a meta-analysis. We conducted a meta-analysis evaluating the continence rate at different time intervals after different surgical techniques.
Results
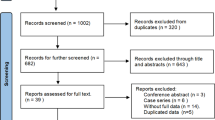
354 trials were identified by reviewing abstracts and articles. 159 duplicates were removed. Nine additional trials were excluded because there was no comparison group, outcome data was incomplete, it was a review article, or the article was not in English. The final set of trials eligible for analysis included 32 studies for the qualitative analysis7, 9,10,11,12, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40. The selection strategy is shown in Fig. 1. The characteristics of the included trials are outlined in Table 1. A total of 4697 patients were included in this meta-analysis. 19 trials7, 15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 evaluated the efficacy of PR, 7 trials12, 33,34,35,36,37,38 evaluated the efficacy of AS, 4 trials9,10,11, 14 evaluated the efficacy of PR + AR, and 2 trials39, 40 evaluated the efficacy of PR + AS. Seven of these trials were RCTs9, 15, 31, 32, 37, 38, 40. Six trials11, 18, 25, 29, 32, 33 evaluated IPSS and EPIC urinary domain scores.
Effect of surgical technique on complete urinary continence rate
Complete urinary continence rate was the primary outcome measure in this meta-analysis. Pooled analysis of data showed that the use of PR alone was associated with significantly better complete urinary continence at 1–4, 28–42, 90, 180 and 360 days following the catheter removal (RR = 3.7; 95%CI, 2.34–5.84; P < 0.001, Fig. 2A; RR = 1.63; 95%CI, 1.26–2.1, P < 0.001, Fig. 3A; RR = 1.28; 95% CI, 1.06–1.55; P = 0.009, Fig. 4A; RR = 1.14; 95% CI, 1.00–1.30; P = 0.044, Fig. 5A; RR = 1.23; 95% CI, 1.03–1.48; P = 0.021, Fig. 6A, respectively). The use of PR was not associated with better complete urinary continence at 7 -14 days following catheter removal (RR = 1.28.; 95% CI, 0.98–1.67; P = 0.073, Fig. 7A).
Forest plot of urinary continence across all studies at 1–4 days after catheter removal, (A) complete urinary continence; (B) social urinary continence; (C) complete urinary continence stratified by study design in studies including PR, AR + PR and AS + PR; (D) social urinary continence stratified by study design in studies including PR, AR + PR and AS + PR.
Forest plot of urinary continence across all studies at 28–42 days after catheter removal, (A) complete urinary continence; (B) social urinary continence; (C) complete urinary continence stratified by study design in studies including PR, AR + PR and AS + PR; (D) social urinary continence stratified by study design in studies including PR, AR + PR and AS + PR.
Forest plot of urinary continence across all studies at 90 days after catheter removal, (A) complete urinary continence; (B) social urinary continence; (C) complete urinary continence stratified by study design in studies including PR, AR + PR and AS + PR; (D) social urinary continence stratified by study design in studies including PR, AR + PR and AS + PR.
Forest plot of urinary continence across all studies at 180 days after catheter removal, (A) complete urinary continence; (B) social urinary continence; (C) complete urinary continence stratified by study design in studies including PR, AR + PR and AS + PR; (D) social urinary continence stratified by study design in studies including PR, AR + PR and AS + PR.
Forest plot of urinary continence across all studies at 7–14 days after catheter removal, (A) complete urinary continence; (B) social urinary continence; (C) complete urinary continence stratified by study design in studies including PR, AR + PR and AS + PR; (D) social urinary continence stratified by study design in studies including PR, AR + PR and AS + PR.
The use of AS was associated with significantly better complete urinary continence at 28–42 days following the catheter removal (RR = 2.11; 95% CI, 1.20–3.70; P = 0.009, Fig. 4A). No benefit was identified 1–4, 7–14, 90, 180 or 360 days (RR = 1.5.; 95% CI, 0.27–8.34; P = 0.643, Fig. 2A; RR = 1.37; 95% CI, 0.96–1.96; P = 0.081, Fig. 4A; RR = 1.13; 95% CI, 0.91–1.41; P = 0.266, Fig. 5A; RR = 1.02; 95% CI, 0.98–1.07; P = 0.247, Fig. 6A; RR = 5.1.; 95% CI, 0.73–35.6; P = 0.100, Fig. 7A, respectively).
The use of AR + PR was associated with significantly better complete urinary continence at 1–4, 90 and 180 days following the catheter removal (RR = 2.59; 95% CI, 1.15–5.82; P = 0.022, Fig. 2A; RR = 1.82; 95% CI, 1.58–2.10; P < 0.001, Fig. 4A; RR = 1.14; 95% CI, 1.00–1.30; P < 0.001, Fig. 5A, respectively). However, no benefit was seen from AR + PR at 7–14 and 28–42 days following the catheter removal (RR = 1.61; 95% CI, 0.82–3.13; P = 0.163, Fig. 3A; RR = 2.09; 95% CI, 0.94–4.64; P = 0.069, Fig. 7A, respectively).
Complete urinary continence was similar in patients with and without AS + PR at 7–14, 28–42, 90 and 180 days (RR = 3.71; 95% CI, 0.87–15.77; P = 0.076, Fig. 3A; RR = 1.65; 95% CI, 0.90–3.04; P = 0.107, Fig. 4A; RR = 1.13; 95% CI, 0.70–1.82; P = 0.615, Fig. 5A; RR = 1.69; 95% CI, 0.16–17.84; P = 0.076, Fig. 7A, respectively).
The subgroup analysis of randomized trials evaluating PR, AR + PR and AS + PR demonstrated no improvement of complete urinary continence at 7–14, 28–42, 90 and 180 days after catheter removal (RR = 1.22; 95% CI, 0.64–2.30; P = 0.548, Fig. 3C; RR = 0.96; 95% CI, 0.75–1.24; P = 0.769, Fig. 4C; RR = 1.16; 95% CI, 0.97–1.39; P = 0.108, Fig. 5C; RR = 1.68; 95% CI, 0.91–3.08; P = 0.096, Fig. 7C, respectively). There was a significant improvement at 1–4 days after catheter removal (RR = 2.59; 95% CI, 1.15–5.82; P = 0.022, Fig. 1C). Historical cohort studies demonstrated a significant improvement of complete urinary continence at 1–4, 28–42, 90 and 180 days (RR = 3.70; 95% CI, 2.34–5.84; P < 0.001, Fig. 2C; RR = 1.83; 95% CI, 1.41–2.37; P < 0.001, Fig. 3C; RR = 1.46; 95% CI, 1.14–1.86; P = 0.003, Fig. 4C; RR = 1.23; 95% CI, 1.01–1.50; P = 0.041, Fig. 5C, respectively). No benefit was found at 7–14 days (RR = 1.43; 95% CI, 0.93–2.19; P = 0.104, Fig. 7C).
Reports where a nerve-sparing technique was not used had better complete urinary continence at 28–42 days (RR = 2.03; 95% CI, 1.35–3.06; P = 0.001, Figure S1), but no improvement 90 and 180 days(RR = 1.43; 95% CI, 0.96–2.14; P = 0.134, RR = 1.39; 95% CI, 0.85–2.77; P = 0.324, Figure S1, respectively).
Effect of surgical technique on social urinary continence
Social urinary continence was a secondary outcome measure in this meta-analysis. Pooled analysis showed that the use of PR was associated with significantly improved social urinary continence at 7–14 and 28–42 days following catheter removal (RR = 1.54; 95% CI, 1.16–2.03; P = 0.003, Fig. 3B; RR = 2.31; 95% CI, 1.36–3.93; P = 0.002, Fig. 7B, respectively). No benefit was found at 1–4, 90 and 180 days (RR = 2.51; 95% CI, 0.71–8.92; P = 0.154, Fig. 2B; RR = 1.17; 95% CI, 0.98–1.40; P = 0.080, Fig. 4B; RR = 1.09; 95% CI, 0.95–1.26; P = 0.221, Fig. 5B, respectively).
Social urinary continence was not improved after AS at all time interval (1–4 days: RR = 1.78; 95% CI, 0.34–9.19; P = 0.493, Fig. 2B; 90 day: RR = 0.94; 95% CI, 0.73–1.21; P = 0.634, Fig. 4B; 180 day: RR = 1.29; 95% CI, 0.84–2.00; P = 0.247, Fig. 5B, respectively).
A significantly better outcome was observed after AR + PR at 90 and 180 days after catheter removal (RR = 1.75; 95% CI, 1.02–3.01; P = 0.043, Fig. 4B; RR = 1.53; 95% CI, 1.09–2.14; P = 0.014, Fig. 5B, respectively). No benefit was found at 1–4, 7–14 and 28–42 days (RR = 1.29; 95% CI, 0.73–2.26; P = 0.377, Fig. 2B; RR = 1.82; 95% CI, 0.40–8.20; P = 0.436, Fig. 3B; RR = 1.07; 95% CI, 0.75–1.51; P = 0.717, Fig. 7B, respectively).
Data was available evaluating the use of AS + PR at 28–42 and 90 days after catheter removal. The use of AS + PR significantly improved social urinary continence (28–42 days: RR = 2.80; 95% CI, 1.18–6.63; P = 0.019, Fig. 3B; 90 days: RR = 1.77; 95% CI, 1.30–2.42; P < 0.001, Fig. 4B, respectively).
Analysis of randomized trials evaluating PR, AR + PR and AS + PR demonstrated no improvement of social urinary continence at 1–4, 7–14, 28–42, 90 and 180 days after catheter removal (RR = 0.82; 95% CI, 0.29–2.31; P = 0.708, Fig. 2D; RR = 1.14; 95% CI, 0.89–1.46; P = 0.314, Fig. 3D; RR = 1.07; 95% CI, 0.75–1.53; P = 0.715, Fig. 4D; RR = 1.03; 95% CI, 0.95–1.10; P = 0.506, Fig. 5D; RR = 1.25; 95% CI, 0.87–1.78; P = 0.226, Fig. 7D, respectively). Historical cohort studies showed a significant benefit in social urinary continence at 1–4, 7–14, 28–42 and 90 days (RR = 4.26; 95% CI, 2.44–7.45; P < 0.001, Fig. 2D; RR = 1.92; 95% CI, 1.30–2.84; P = 0.001, Fig. 3D; RR = 1.38; 95% CI, 1.09–1.74; P = 0.007, Fig. 4D; RR = 3.06; 95% CI, 2.13–4.41; P < 0.001, Fig. 7D, respectively). No benefit was seen at 180 days (RR = 1.20; 95% CI, 0.95–1.52; P = 0.131, Fig. 5D).
Effect of surgical treatment on PSM and cystogram leakage
Thirteen trials evaluated PSM rate, including seven for PR, three for AS, one for AR + PR and two for AS + PR. No differences were observed in the PSM rates associated with each surgical technique (PR: RR = 0.93; 95% CI, 0.72–1.21; P = 0.604; AS: RR = 1.28; 95% CI, 0.80–2.05; P = 0.312; AR + PR: RR = 0.94; 95% CI, 0.42–2.11; P = 0.886; AS + PR: RR = 1.36; 95% CI, 0.58–3.19; P = 0.474, Fig. 8A, respectively).
PSM rates did not vary by surgical technique in patients with stage pT2 cancer (PR: RR = 1.01; 95% CI, 0.63–1.63; P = 0.951; AS: RR = 0.38; 95% CI, 0.04–3.31; P = 0.382; AR + PR: RR = 1.53; 95% CI, 0.43–5.43; P = 0.511, Fig. 8B, respectively). PSM rates also did not vary by surgical technique in patients with stage pT3 cancer (PR: RR = 0.90; 95% CI, 0.53–1.53; P = 0.693; AS: RR = 0.96; 95% CI, 0.70–1.31; P = 0.802; AR + PR: RR = 0.62; 95% CI, 0.26–1.47; P = 0.275, Fig. 8C, respectively).
Pooled data from 6 trials showed PR was associated with the least amount of cystogram leakage after surgery (RR = 0.37; 95% CI, 0.19–0.73; P = 0.004, Fig. 9). No significant benefit was detected in patients after AR + PR (RR = 0.78; 95% CI, 0.31–1.99; P = 0.610, Fig. 9).
Effect of surgical treatment on IPSS and EPIC urinary domain scores
IPSS and EPIC urinary domain scores were reported in six studies11, 18, 25, 29, 32, 33. Kojima et al.33 reported a median IPSS score before surgery of 12.5 in the AS group and 7.0 in the control group. These values were 11.0 and 16.0, respectively, 4 weeks after surgery (P < 0.05). No benefit was also seen at week 12 or week 24. Sutherland et al.32 reported that both the PR and control groups had a significantly improved IPSS score from postoperative week 6 to month 3 (P < 0.01). Krane et al.29 found no difference in the IPSS score of the AS and control groups (8.2 vs 8.1, P = 0.97).
“Urinary function” and “urinary bother” subscale score from the EPIC urinary domain were also reviewed. Hoshi et al.11 found that the proportion of recovery to baseline score was significantly improved in the “urinary function” subscale score at 12 months after surgery (P < 0.01) No significant improvement was found at other time points for the “urinary function” or at any time point for the “urinary bother” subscale score. Different outcomes were reported by Ito et al.18 and Brien et al.25. Both found “urinary function” and “urinary bother” subscale scores to be significantly higher in the PR treated group, compared to a control group, at 3 months after surgery. Ito et al.18 found a significant improvement in “urinary function” and “urinary bother” subscale scores at 6 months after surgery when PR was performed. In contrast, Brien et al. reported no benefit in these scores 6 months after catheter removal25.
Quality assessment of RCTs and historical cohort studies
The Jadad quality scores and methodological Newcastle-Ottawa scales are listed in Table 2. The quality of cohort studies was mostly high, but the level of evidence was low because of the nature of the study designs. Because of the lack of double blind for a surgery, the score for double blind in mostly studies was 0, expect one9. The quality of most RCTs was still high, and the level of evidence was stable expect one study38.
Publication bias
Funnel plots of urinary continence at six time intervals showed only one publication with bias, in the AS treated group at 28–42 days (Begger test P = 0.089, Egger test P = 0.002). This bias could be due to the small number of patients with follow-up. No evidence of publication bias was found at any time interval with the other surgical treatments used (Figs S2–S8) (Table 3).
Discussion
This meta-analysis included 7 randomized studies and 25 historical cohort studies of different urethral reconstruction methods after radical prostatectomy, including PR, AS, PR + AS and PR + AR. A quantitative synthesis of the evidence can be really helpful for urologist because urinary incontinence is the major problem after radical prostatectomy.
Urinary incontinence could be improved by many techniques, such as pelvic floor reconstruction, bladder neck preservation41 or intussusceptions42, preserving the fascia covering the levator ani muscle43 and preserving neurovascular bundles44. Among these techniques, pelvic floor reconstruction was reported most. The reconstruction prolonged a little surgery time and gained benefit in improving urinary continence. And the hot point for reconstruction is which layers to be sutured and how to suture. So many studies used different methods to improve the urinary continence compared to the common technique in this meta-analysis.
Patients were evaluated at a large number of time points for both complete and social continence, and a large number of surgical techniques were evaluated. Evaluation of pooled results demonstrated an improvement in urinary continence using these techniques. PR group outcomes in this meta-analysis were similar to the results in Rocco et al.13, but two different points should be noticed. First, we analyzed complete continence and social continence, respectively. Second, we used 1–4, 7–14, 28–42, 90, 180 and 360 day after catheter removal as cut-off point. Meanwhile, no differences in PSM and cystogram leakage were identified.
Treatment of patients with PR improved the complete urinary continence rate at 0–4, 28–42, 90, 180 and 360 days after catheter removal, but not at 7–14 days. These findings are similar to those reported by Grasso et al.8 and Rocco et al.13. Rocco et al.13 found no improvement in the urinary continence rate at 3 and 6 months after catheter removal. This finding was similar to the improvement in social urinary continence rate seen with the pooled data. The different inclusion criteria used and different number of trials evaluating different outcomes could have contributed to some of the different findings. AS provided no benefit of complete or social urinary continence, except at 28–42 days after catheter removal. AR + PR and AS + PR did not show significant benefit until 180 or more days after catheter removal.
There are some kinds of potential heterogeneity in this meta-analysis. First, surgical technical differences were reported in each of the surgical reconstructions, although these were felt to be minor. For example, Patel et al.34 anchored the anastomosis to the pubic bone, while Noguchi et al.36 anchored to the dorsal venous complex (DVC) and puboprostatic ligaments. Second, different methods were used to evaluate continence including a self-administrated questionnaire, EPIC questionnaire, valsalva leak-point pressure, and pad weighing. Third, different study designs including the variable use of a nerve-sparing technique, variations in reporting times, and differences in the historical cohorts used as control groups could have influenced the outcomes. We did not distinguish randomized studies from historical cohort studies because of the small number of reported trials. Finally, the difference in the number of patients treated in each study could introduce bias into our analysis. These potential effects make high heterogeneity of results. It’s impossible to control these differences in each trial.
Bias due to different study designs may be greater in subgroup analyses. Both complete and social urinary continence was present only at 1–4 days in RCTs, where heterogeneity was generally low. Complete urinary incontinence was observed at 7–14 days and social urinary incontinence at 180 days in historical studies. These differences could occur because RCTs better control patient related bias and also because there may be small differences in the surgical technique used in the two groups. The IPSS and EPIC urinary domain score was analyzed in this meta-analysis. Because the scale scores were not well described using RR, and so were individually described by report. This is another method to assess the postoperative urinary continence.
There were several limitations to this study. First, only publications reported in English were included because of the lack of a translator. Second, the individual patient data was not available for each study which is the gold standard for meta-analysis. Third, conference abstracts were also not included because of lack of available data. These factors could have reduced the number of trials evaluated in this meta-analysis. Fourth, heterogeneity and variation in study quality, as described above, could also have affected results. Lastly, different time intervals among the included studies also influenced the outcomes despite of grouping sections. These limitations may make the results unstable, so further studies are still needed to explore the effect of these surgical techniques in RP.
Conclusion
Patients with PCa who underwent RP with PR had the least urinary incontinence. PR is currently one of the most widely used surgical reconstructive techniques to improve the adverse effect of RP. No benefit was observed after AS. AR + PR, while AS + PR, might have little influence at early time points, but had the best outcomes at 180 or more days. More RCTs are needed to better assess the efficacy of different surgical reconstructions after RP.
Methods
Selection Criteria
Studies that were published in English were selected if they met the following criteria: (1) all patients were diagnosed with PCa by clinical examinations and prostate biopsy; (2) all patients underwent radical prostatectomy; and (3) the surgical modification was AS, AR, PR, AS + PR or AR + PR. Studies of patients who received neoadjuvant treatment were excluded.
Search Strategy
This meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement15. To identify studies that met the above selection criteria, we searched the PubMed, Embase, Cochrane Central Register of Controlled trials, Ovid and Web of Science databases for trials published before June 6, 2016. The search strategy was followed using all possible combinations of the medical subject headings (MeSH) or non-MeSH terms including prostate neoplasm, prostatic neoplasm, and prostatic cancer; posterior reconstruction, anterior reconstruction, anterior suspension, pelvic floor reconstruction and total reconstruction; urinary incontinence and incontinence or urinary continence and continence. Each search strategy was conducted in each database. We also manually searched for potentially relevant trials from the references of studies identified by the above search.
Data extraction
Two reviewers (JF Cui and Hu Guo) independently assessed all eligible publications. Any discrepancies were settled by discussion with a third reviewer (BK Shi). Data that met the selection criteria were collected on a standardized form by two independent reviewers. Data extracted from the studies included the author’s name, publication year, country, study period, study design, surgical technique, definition of continence, method for evaluation of continence, use of nerve sparing techniques, number of patients and results, including risk ratios [RRs], 95% confidence intervals [CIs] and P values.
Outcome Measures
The primary outcome measure in this meta-analysis was complete urinary continence rate. Complete urinary continence was defined as using 0 pad per day. The secondary outcome measure was social urinary continence. Social urinary continence was defined as using 0–1 pads per day. The study group was defined as the group with one kind of reconstruction which not mentioned in the control group. The control group was defined as the group without the reconstruction which mentioned in study group. Continence rates were determined at 1–4, 7–14, 28–42, 90, 180 and 360 days after catheter removal. Positive surgical margin (PSM) rate, leakage on cystogram, international prostate symptoms scores (IPSS) and expanded prostate cancer index composite (EPIC) urinary domain score were also determined.
Statistical Analysis
RRs with 95% CIs were used to evaluate the primary outcome and secondary outcome. A RR > 1 indicated an advantage of reconstruction over non-reconstruction (NR). Heterogeneity across studies was quantified using the I2 statistic and the Chi-square (Cochrane Q statistic) test. Studies with an I2 statistic greater than 40% and a P value less than 0.1 for the Chi-square test had a high level of heterogeneity. A random-effects model was used to pool estimates regardless of high or low levels of heterogeneity in order to better deal with the heterogeneous nature of the different surgical modifications. Study designs, surgical modifications and other confounding factors were not consistent between studies. Therefore, there was a significant advantage of a random-effects model compared with a fixed-effects model in accounting for heterogeneity between studies16. A p value less than 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 13.0 (College Station, Texas, USA).
Quality Assessment
The methodological quality of each randomized controlled trial (RCT) was evaluated using the Jadad scale17. Quality was assessed using presence of randomization (0–2 points), used of double blind (0–2 points) and presence of patient withdrawals and dropouts (0–1 point). The 2 reviewers classified studies into two quality grades: low (0–2 points) and high (3–5 points).
The methodological quality of each cohort study was evaluated according to the Newcastle-Ottawa Scale (NOS)18. Method of selection of the study groups (0–4 points), comparability of cohorts (0–2 points) and ascertainment of the outcome (0–3 points) were the three major aspects used for calculating the quality score of included reports. The studies were classified into three quality grades: low (0–3 points), moderate (4–6 points) or high (7–9 points). All studies were evaluated using the level of evidence (LOE) defined by Phillips et al.19, 45,46,47,48,49. Two independent reviewers evaluated each study. Disagreements were resolved through discussion.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer Statistics, 2016. CA: A Cancer Journal for Clinicians. 66, 7–30, doi:10.3322/caac.21332 (2016).
Tewari, A. et al. Positive Surgical Margin and Perioperative Complication Rates of Primary Surgical Treatments for Prostate Cancer: A Systematic Review and Meta-Analysis Comparing Retropubic, Laparoscopic, and Robotic Prostatectomy. Eur Urol. 62, 1–15, doi:10.1016/j.eururo.2012.02.029 (2012).
Litwin, M. S. et al. Quality-Of-Life Outcomes in Men Treated for Localized Prostate Cancer. JAMA. 273, 129–135, doi:10.1001/jama.1995.03520260051032 (1995).
Gao, X., Wang, K. B., Pu, X. Y., Zhou, X. F. & Qiu, J. G. Modified Apical Dissection of the Prostate Improves Early Continence in Laparoscopic Radical Prostatectomy: Technique and Initial Results. J Cancer Res Clin Oncol. 136, 511–516, doi:10.1007/s00432-009-0683-4 (2010).
Walsh, P. C., Quinlan, D. M., Morton, R. A. & Steiner, M. S. Radical Retropubic Prostatectomy. Improved Anastomosis and Urinary Continence. Urol Clin North Am. 17, 679–684 (1990).
Rocco, F. et al. Personal Research: Reconstruction of the Urethral Striated Sphincter. Arch Ital Urol Androl. 73, 127–137 (2001).
Rocco, F. et al. Restoration of Posterior Aspect of Rhabdosphincter Shortens Continence Time After Radical Retropubic Prostatectomy. J Urol. 175, 2201–2206, doi:10.1016/S0022-5347(06)00262-X (2006).
Grasso, A. A. C. et al. Posterior Musculofascial Reconstruction After Radical Prostatectomy: An Updated Systematic Review and a Meta-Analysis. Bju Int. 118, 20–34, doi:10.1111/bju.13480 (2016).
Menon, M., Muhletaler, F., Campos, M. & Peabody, J. O. Assessment of Early Continence After Reconstruction of the Periprostatic Tissues in Patients Undergoing Computer Assisted (Robotic) Prostatectomy: Results of a 2 Group Parallel Randomized Controlled Trial. The Journal of Urology. 180, 1018–1023, doi:10.1016/j.juro.2008.05.046 (2008).
Koliakos, N. et al. Posterior and Anterior Fixation of the Urethra During Robotic Prostatectomy Improves Early Continence Rates. Scand J Urol Nephrol. 44, 5–10, doi:10.3109/00365590903413627 (2010).
Hoshi, A. et al. Total Pelvic Floor Reconstruction During Non-Nerve-Sparing Laparoscopic Radical Prostatectomy: Impact On Early Recovery of Urinary Continence. Int J Urol. 21, 1132–1137, doi:10.1111/iju.2014.21.issue-11 (2014).
Sugimura, Y., Hioki, T., Yamada, Y., Fumino, M. & Inoue, T. An Anterior Urethral Stitch Improves Urinary Incontinence Following Radical Prostatectomy. Int J Urol. 8, 153–157, doi:10.1046/j.1442-2042.2001.00273.x (2001).
Rocco, B. et al. Posterior Musculofascial Reconstruction After Radical Prostatectomy: A Systematic Review of the Literature. Eur Urol. 62, 779–790, doi:10.1016/j.eururo.2012.05.041 (2012).
Tewari, A. et al. Total Reconstruction of the Vesico-Urethral Junction. Bju Int. 101, 871–877, doi:10.1111/j.1464-410X.2008.07424.x (2008).
Anceschi, U., Gaffi, M. & Molinari, C. & Anceschi, C. Posterior Reconstruction and Outcomes of Laparoscopic Radical Prostatectomy in a High-Risk Setting. JSLS: Journal of the Society of Laparoendoscopic Surgeons. 17, 535–542, doi:10.4293/108680813X13794522666365 (2013).
Coelho, R. F. et al. Influence of Modified Posterior Reconstruction of the Rhabdosphincter on Early Recovery of Continence and Anastomotic Leakage Rates after Robot-Assisted Radical Prostatectomy. Eur Urol. 59, 72–80, doi:10.1016/j.eururo.2010.08.025 (2011).
Daouacher, G. & Waldén, M. A Simple Reconstruction of the Posterior Aspect of Rhabdosphincter and Sparing of Puboprostatic Collar Reduces the Time to Early Continence After Laparoscopic Radical Prostatectomy. J Endourol. 28, 481–486, doi:10.1089/end.2013.0633 (2014).
Ito, K. et al. Modified Posterior Musculofascial Plate Reconstruction Decreases the Posterior Vesicourethral Angle and Improves Urinary Continence Recovery in Patients Undergoing Laparoscopic Radical Prostatectomy. Mol Clin Oncol 1, 970–976, doi:10.3892/mco.2013.182 (2013).
Jeong, C. W. et al. Novel Posterior Reconstruction Technique During Robot-Assisted Laparoscopic Prostatectomy: Description and Comparative Outcomes. Int J Urol. 19, 683–687, doi:10.1111/j.1442-2042.2012.02988.x (2012).
Kim, I. Y., Hwang, E. A., Mmeje, C., Ercolani, M. & Lee, D. H. Impact of Posterior Urethral Plate Repair on Continence Following Robot-Assisted Laparoscopic Radical Prostatectomy. Yonsei Med J. 51, 427–31, doi:10.3349/ymj.2010.51.3.427 (2010).
Nguyen, M. M. et al. Early Continence Outcomes of Posterior Musculofascial Plate Reconstruction During Robotic and Laparoscopic Prostatectomy. Bju Int. 101, 1135–1139, doi:10.1111/j.1464-410X.2007.07425.x (2008).
Rocco, F. et al. Early Continence Recovery after Open Radical Prostatectomy with Restoration of the Posterior Aspect of the Rhabdosphincter. Eur Urol. 52, 376–383, doi:10.1016/j.eururo.2007.01.109 (2007).
Sano, T., Nakashima, M., Haitani, T., Kajita, Y. & Shichiri, Y. Posterior Musculofascial Plate Reconstruction Promotes Early Restoration of Continence and Prevents Severe Incontinence in Patients Undergoing Laparoscopic Radical Prostatectomy. Int J Urol. 19, 475–479, doi:10.1111/j.1442-2042.2011.02954.x (2012).
You, Y. C., Kim, T. H. & Sung, G. T. Effect of Bladder Neck Preservation and Posterior Urethral Reconstruction during Robot-Assisted Laparoscopic Radical Prostatectomy for Urinary Continence. Korean Journal of Urology 53, 29–33, doi:10.4111/kju.2012.53.1.29 (2012).
Brien, J. C., Barone, B., Fabrizio, M. & Given, R. Posterior Reconstruction Before Vesicourethral Anastomosis in Patients Undergoing Robot-Assisted Laparoscopic Prostatectomy Leads to Earlier Return to Baseline Continence. J Endourol. 25, 441–445, doi:10.1089/end.2010.0251 (2011).
Gondo, T. et al. The Powerful Impact of Double-Layered Posterior Rhabdosphincter Reconstruction on Early Recovery of Urinary Continence After Robot-Assisted Radical Prostatectomy. J Endourol. 26, 1159–1164, doi:10.1089/end.2012.0067 (2012).
Woo, J. R., Shikanov, S., Zorn, K. C., Shalhav, A. L. & Zagaja, G. P. Impact of Posterior Urethral Plate Repair On Continence Following Robot-Assisted Laparoscopic Radical Prostatectomy. Yonsei Med J. 51, 427–431, doi:10.3349/ymj.2010.51.3.427 (2010).
Rocco, B. et al. Posterior Reconstruction of the Rhabdosphincter Allows a Rapid Recovery of Continence after Transperitoneal Videolaparoscopic Radical Prostatectomy. Eur Urol. 51, 996–1003, doi:10.1016/j.eururo.2006.10.014 (2007).
Krane, L. S., Wambi, C., Bhandari, A. & Stricker, H. J. Posterior Support for Urethrovesical Anastomosis in Robotic Radical Prostatectomy: Single Surgeon Analysis. Can J Urol. 16, 4841–40 (2009).
Joshi, N., de Blok, W., van Muilekom, E. & van der Poel, H. Impact of Posterior Musculofascial Reconstruction on Early Continence After Robot-Assisted Laparoscopic Radical Prostatectomy: Results of a Prospective Parallel Group Trial. Eur Urol. 58, 84–89, doi:10.1016/j.eururo.2010.03.028 (2010).
Jeong, C. W. et al. Effects of New 1-Step Posterior Reconstruction Method on Recovery of Continence after Robot-Assisted Laparoscopic Prostatectomy: Results of a Prospective, Single-Blind, Parallel Group, Randomized, Controlled Trial. The Journal of Urology 193, 935–942, doi:10.1016/j.juro.2014.10.023 (2015).
Sutherland, D. E. et al. Posterior Rhabdosphincter Reconstruction During Robotic Assisted Radical Prostatectomy: Results From a Phase II Randomized Clinical Trial. The Journal of Urology 185, 1262–1267, doi:10.1016/j.juro.2010.11.085 (2011).
Kojima, Y. et al. Bladder Neck Sling Suspension During Robot-Assisted Radical Prostatectomy to Improve Early Return of Urinary Continence: A Comparative Analysis. Urology. 83, 632–640, doi:10.1016/j.urology.2013.09.059 (2014).
Patel, V. R., Coelho, R. F., Palmer, K. J. & Rocco, B. Periurethral Suspension Stitch During Robot-Assisted Laparoscopic Radical Prostatectomy: Description of the Technique and Continence Outcomes. Eur Urol. 56, 472–478, doi:10.1016/j.eururo.2009.06.007 (2009).
Campenni, M. A., Harmon, J. D., Ginsberg, P. C. & Harkaway, R. C. Improved Continence After Radical Retropubic Prostatectomy Using Two Pubo-Urethral Suspension Stitches. Urol Int. 68, 109–112, doi:10.1159/000048429 (2002).
Noguchi, M., Shimada, A., Nakashima, O., Kojiro, M. & Matsuoka, K. Urodynamic Evaluation of a Suspension Technique for Rapid Recovery of Continence After Radical Retropubic Prostatectomy. Int J Urol. 13, 373–378, doi:10.1111/j.1442-2042.2006.01313.x (2006).
Noguchi, M. et al. A Randomized Clinical Trial of Suspension Technique for Improving Early Recovery of Urinary Continence After Radical Retropubic Prostatectomy. Bju Int. 102, 958–963, doi:10.1111/j.1464-410X.2008.07759.x (2008).
Stolzenburg, J. U. et al. Influence of Bladder Neck Suspension Stitches On Early Continence After Radical Prostatectomy: A Prospective Randomized Study of 180 Patients. Asian J Androl. 13, 806–811, doi:10.1038/aja.2011.82 (2011).
Kalisvaart, J. F., Osann, K. E. & Finley, D. S. & Ornstein, D. K. Posterior Reconstruction and Anterior Suspension with Single Anastomotic Suture in Robot-Assisted Laparoscopic Radical Prostatectomy: A Simple Method to Improve Early Return of Continence. Journal of Robotic Surgery. 3, 149–153, doi:10.1007/s11701-009-0151-9 (2009).
Hurtes, X. et al. Anterior Suspension Combined with Posterior Reconstruction During Robot-Assisted Laparoscopic Prostatectomy Improves Early Return of Urinary Continence: A Prospective Randomized Multicentre Trial. Bju Int. 110, 875–883, doi:10.1111/j.1464-410X.2011.10849.x (2012).
Soloway, M. S. & Neulander, E. Bladder-Neck Preservation During Radical Retropubic Prostatectomy. Semin Urol Oncol. 18, 51–56 (2000).
Walsh, P. C. & Marschke, P. L. Intussusception of the Reconstructed Bladder Neck Leads to Earlier Continence After Radical Prostatectomy. Urology. 59, 934–938, doi:10.1016/S0090-4295(02)01596-0 (2002).
Azuma, H. et al. Utility of Transrectal Ultrasonography Guidance and Seven Key Elements of Operative Skill for Early Recovery of Urinary Continence After Laparoscopic Radical Prostatectomy. Int J Oncol. 38, 293–304, doi:10.3892/ijo.2010.865 (2011).
Curto, F. et al. Nerve Sparing Laparoscopic Radical Prostatectomy: Our Technique. Eur Urol. 49, 344–352, doi:10.1016/j.eururo.2005.11.029 (2006).
Liberati, A. et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ. 339, b2700–b2700, doi:10.1136/bmj.b2700 (2009).
DerSimonian, R. & Kacker, R. Random-Effects Model for Meta-Analysis of Clinical Trials: An Update. Contemp Clin Trials. 28, 105–114, doi:10.1016/j.cct.2006.04.004 (2007).
Jadad, A. R. et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials. 17, 1–12, doi:10.1016/0197-2456(95)00134-4 (1996).
JPT, H. & S., G. Eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0(2011). Available at: http://handbook.cochrane.org/ (Date of access: 17th August 2016).
Phillips, B., B., C. & S., D. Oxford Centre for Evidence-based Medicine – Levels of Evidence (2009). Available at: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels- evidence-march-2009/ (Date of access: 17th August 2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant 81470987 and 81170702 to B. Shi), the Tai Shan Scholar Foundation to B. Shi, the Science and Technology Development Project of Shandong Province (Grant 2014GSF118054 to B. Shi; Grant 2014GSF121026 to G. Yin), Natural Science Foundation of Shandong Province (Grant ZR2014HQ062 to Y. Zhu) and Science Foundation of Qilu Hospital of Shandong University (Grant 2015QLMS28 to B. Shi; Grant 2015QLQN21 to Y. Zhu), Medicine and Health Science Technology Development Project of Shandong Province (Grant 2014WS0138 to Y. Zhu).
Author information
Authors and Affiliations
Contributions
Jianfeng Cui, Hu Guo and Gang Yin wrote the main manuscript. Benkang Shi and Gang Yin did the project development. Jianfeng Cui, Yan Li, Shouzhen Chen and Yaofeng Zhu collected and managed data. Shiyu Wang, Yong Wang and and Xigao Liu analyzed the data. Wenbo Wang, Jie Han and Pengxiang Chen prepared all the tables and figures. Shuping Nie edited the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cui, J., Guo, H., Li, Y. et al. Pelvic Floor Reconstruction After Radical Prostatectomy: A Systematic Review and Meta-analysis of Different Surgical Techniques. Sci Rep 7, 2737 (2017). https://doi.org/10.1038/s41598-017-02991-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02991-8
This article is cited by
-
Robot-assisted radical prostatectomy using a novel urethral reconstruction technique vs standard vesicourethral anastomosis. A retrospective cohort study
World Journal of Urology (2022)
-
Advanced Reconstruction of Vesicourethral Support (ARVUS) during robot-assisted radical prostatectomy: first independent evaluation and review of other factors influencing 1 year continence outcomes
World Journal of Urology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.