Abstract
Autism spectrum disorders (ASD) are characterized by a wide genetic and clinical heterogeneity. However, some biochemical impairments, including decreased melatonin (crucial for circadian regulation) and elevated platelet N-acetylserotonin (the precursor of melatonin) have been reported as very frequent features in individuals with ASD. To address the mechanisms of these dysfunctions, we investigated melatonin synthesis in post-mortem pineal glands - the main source of melatonin (9 patients and 22 controls) - and gut samples - the main source of serotonin (11 patients and 13 controls), and in blood platelets from 239 individuals with ASD, their first-degree relatives and 278 controls. Our results elucidate the enzymatic mechanism for melatonin deficit in ASD, involving a reduction of both enzyme activities contributing to melatonin synthesis (AANAT and ASMT), observed in the pineal gland as well as in gut and platelets of patients. Further investigations suggest new, post-translational (reduced levels of 14-3-3 proteins which regulate AANAT and ASMT activities) and post-transcriptional (increased levels of miR-451, targeting 14-3-3ζ) mechanisms to these impairments. This study thus gives insights into the pathophysiological pathways involved in ASD.
Similar content being viewed by others
Introduction
Autism spectrum disorders (ASD) are characterized by deficits in social communication and presence of stereotypy and restrictive patterns of interest. The genetic architecture of ASD is heterogeneous, shaped by a combination of hundreds of frequent and rare variants that can be different from one individual to another1. In this context of wide genetic heterogeneity, the identification of common biological features may reveal shared pathophysiological pathways. Several biological abnormalities have been reported in individuals with ASD, including neurochemical, immunological, endocrine or metabolic traits2,3,4. Among those, elevated blood serotonin is one of the most replicated findings5,6,7,8. A deficit in melatonin, from blood or urine of individuals with ASD, has also been described in several studies8,9,10,11,12,13, and is associated with increased peripheral N-acetylserotonin (NAS), the intermediate metabolite between serotonin and melatonin8.
Melatonin is a hormone mainly synthesized in the pineal gland during the night. It is a biological signal of light/dark cycles and is considered as a major circadian synchronizer14, 15. It is also a modulator of metabolism, immunity, reproduction and digestive functions16 and can directly modulate neuronal networks17. Melatonin appears as a therapeutic target of the frequently reported sleep disorders associated with ASD18,19,20. NAS also displays intrinsic biological properties: it is an agonist of the TrkB receptor and may thus share the neurotrophic properties of brain-derived neurotrophic factor (BDNF), the canonical TrkB ligand21, 22.
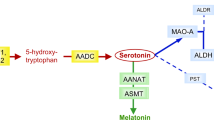
Serotonin conversion into melatonin involves two sequential enzymatic steps: N-acetylation of serotonin into N-acetylserotonin (NAS) by arylalkylamine N-acetyltransferase (AANAT, EC: 2.3.1.87), determining the timing of melatonin secretion, followed by methylation by acetylserotonin O-methyltransferase (ASMT, also called hydroxyindole O-methyltransferase HIOMT, EC: 2.1.1.4), determining the amount of melatonin secretion (Fig. 1a). The regulation of melatonin synthesis displays inter-species variability, including within mammals. In humans, it is considered to involve post-translational mechanisms, including AANAT phosphorylation, and stabilization of both AANAT and ASMT by formation of complexes with 14-3-3ζ proteins23, 24. In addition, 14-3-3ζ protein has been shown to be a target of miR-45125,26,27 which confers resistance to the deleterious effects of oxidative stress, which may play a role in the pathogenesis of ASD, promoting neuronal damage in genetically predisposed individuals4.
Exploration of the serotonin-melatonin pathway in the blood. (a) The serotonin and melatonin synthesis pathway. (b) Platelet AANAT activity. (c) Platelet ASMT activity. (d) Correlation between platelet NAS concentration and ASMT activity (regression after log transformation of ASMT, n = 579). (e) Correlation between plasma melatonin and platelet ASMT activity (regression after log transformation of melatonin and ASMT, n = 641). (f) Contour plot of platelet NAS concentration, AANAT and ASMT activities in patients (red dots) and controls (green dots). (g) Contour plot of plasma melatonin concentration, platelet AANAT and ASMT activities in patients (red dots) and controls (green dots). Blood samples were taken in the morning from 239 individuals with ASD, their first-degree relatives (303 parents and 78 unaffected sibs), and 278 controls. Boxes indicate medians and quartiles. Dashed lines indicate the threshold of the 95th (for NAS) or 5th (for melatonin, AANAT and ASMT) percentile of the control group. Groups were compared using the Wilcoxon two-sample test.
Based on ASMT assessment in blood platelets, we previously suggested that melatonin deficit in patients with ASD results from impaired melatonin synthesis12. Here, we investigated both AANAT and ASMT activities, not only in blood samples (platelets) from a large cohort of patients, their relatives and controls, but also in post-mortem tissues (pineal gland, the major source of melatonin, and gut [ileum samples], the major source of serotonin) from individuals with ASD and controls. We addressed the contribution of rare variants in AANAT and ASMT genes in ASD. Finally, we investigated 14-3-3 proteins in blood platelets and tissues, and miR-451 in plasma of patients with ASD.
Results
Disruption of AANAT and ASMT activities in blood platelets from individuals with ASD
We assessed ASMT enzyme activity in blood platelets from a large cohort of patients with ASD, their first-degree relatives and controls in order to achieve sample sizes of 239 patients, 303 parents, 78 unaffected siblings and 278 controls. Confirming previous results obtained on a smaller cohort12 (Supplementary Figure 1a–c), ASMT activity was significantly decreased in patients’ platelets compared to controls, and to a lesser extent in their first-degree relatives (Fig. 1c). A separate analysis of the two data sets is presented in Supplementary Figure 1d–e.
For the first time we also investigated AANAT activity in blood platelets. AANAT activity was significantly diminished in blood platelets from patients with ASD, but not in their relatives (Fig. 1b). These results assert a global disruption of melatonin synthesis in ASD, involving both AANAT and ASMT enzymes.
Platelet NAS and plasma melatonin were significantly correlated with ASMT activity in all status groups (Fig. 1d–e), with the strongest correlation observed for patients with ASD (NAS-ASMT regression after log transformation of ASMT: n = 193, r2 = 0.57, p < 0.0001; melatonin-ASMT linear regression: n = 201, r2 = 0.57, p < 0.0001). Accordingly, a profile analysis shows that in ASD patients a low plasma melatonin level and elevated platelet NAS content were both significantly associated with low platelet activities of both AANAT and ASMT (Supplementary Figure 2a). The contour plots (i.e. relationships between AANAT activity, ASMT activity and platelet NAS or plasma melatonin, and in ASD patients and controls) indicate ASMT activity as the most related to plasma melatonin and platelet NAS (Fig. 1f–g).
No correlation was observed between whole blood serotonin and either AANAT or ASMT activity (data not shown), indicating that the disruption of melatonin synthesis in platelets of patients with ASD is not sufficient to induce serotonin accumulation. This is consistent with the fact that melatonin synthesis represents only a small minority (about 1%) of serotonin catabolism.
Thus, taking as a threshold the 10th percentile of the control group, a large proportion (74%) of ASD patients displayed a reduced platelet ASMT activity, associated with a decreased plasma melatonin and an increased platelet NAS content. Supplementary Figure 2b recapitulates the frequencies of the different biochemical profiles observed for patients with ASD: the most frequently observed profiles associated low plasma melatonin, low platelet ASMT activity, and high platelet NAS content (40% of ASD patients) with or without increased whole blood serotonin or decreased platelet AANAT activity. Interestingly, only 6% of ASD patients displayed a totally “normal” biochemical profile for the serotonin-melatonin pathway compared to 18% in the relatives and 70% in the controls. Finally, a significant association was observed between ASMT deficit and insomnia, as previously reported for melatonin deficit8. In contrast, no significant clinical correlates of these biochemical impairments were observed for core symptoms of ASD (Supplementary Table 1).
Disruption of the serotonin-NAS-melatonin pathway in autopsy-derived pineal glands from individuals with ASD
Considering that the regulation of the serotonin-NAS-melatonin pathway is likely tissue-specific and blood platelets are only a very minor source of melatonin, we assessed the complete serotonin-melatonin pathway (i.e. serotonin, NAS, melatonin, AANAT, and ASMT - Fig. 1a) in autopsy-derived pineal glands from 9 individuals with ASD and 22 controls. As melatonin synthesis is under strong circadian regulation in the pineal gland, analyses were stratified by time of death. Indeed, marked variations according to time of death were observed for melatonin (Fig. 2c, Kruskal-Wallis test: p = 0.009), NAS (Fig. 2b, p = 0.0005), AANAT (Fig. 2d, p = 0.003) and ASMT (Fig. 2g, p = 0.008), but not serotonin (Fig. 2a, p = 0.53).
Characterization of the serotonin-melatonin pathway in autopsy-derived pineal gland samples from 9 individuals with ASD and 22 controls. (a) Serotonin content. (b) NAS content. (c) Melatonin content. (d) AANAT activity. (e) Correlation between NAS content and AANAT activity and regression line (green) for the control group (regression after log transformation of AANAT). (f) Correlation between melatonin content and AANAT activity, and regression line for the control group (green). (g) ASMT activity. (h) Correlation between NAS content and ASMT activity, and regression line for the control group (green). (i) Correlation between melatonin content and ASMT activity, and regression line for the control group (green). The ASD group was compared to the control group using Wilcoxon two-sample test. Boxes indicate medians and quartiles.
Overall, melatonin (Fig. 2c), ASMT (Fig. 2g) and AANAT (Fig. 2d) were significantly decreased in individuals with ASD as compared to controls. Only two patients with ASD had nocturnal time of death, limiting the statistical power for nighttime analyses. Pooling evening and night tissue data reassert lower values for ASD patients vs. controls for AANAT (p = 0.0009), ASMT (p = 0.01) and melatonin (p = 0.03). Additionally, the melatonin and ASMT decrease remained significant even when considering individuals with diurnal (lights on) time of death. In control samples (but not in patients), the melatonin content of pineal glands displayed moderately strong, but significant correlations with both AANAT (Fig. 2f) and ASMT (Fig. 2i) activities.
NAS was not increased in the pineal glands of individuals with ASD (Fig. 2b), in contrast to our findings in blood platelets (Supplementary Figure 1b). The NAS content was correlated with AANAT and ASMT activities in control samples, but not in patients (Fig. 2e and h).
After correction for post mortem interval, no significant difference was observed for serotonin levels between individuals with ASD and controls (Fig. 2a).
Thus, although pineal glands do not reflect the peripheral increases of NAS and serotonin, they reassert the melatonin deficit observed in periphery, as well as a deficit in both ASMT and AANAT activities, confirming the impairment of melatonin synthesis in ASD.
Disruption of the serotonin-NAS-melatonin pathway in autopsy-derived ilea from individuals with ASD
The gastro-intestinal tract is the major source of serotonin, and another minor source of melatonin. Autopsy-derived ileum samples were obtained from 13 individuals with ASD and from 11 age- and sex-matched controls. Samples were investigated for the whole serotonin-melatonin pathway. Since tissue conservation is critical for autopsy-derived samples, and especially for gastro-intestinal tissues, the conservation state of samples and the presence of enterochromaffin cells (synthesizing serotonin and melatonin) were confirmed by immunohistochemistry using an anti-chromogranin A antibody (Fig. 3a). Enterochromaffin cells were detectable in all samples, including those displaying altered histological structures. In contrast to pineal glands, no effect of time of death was observed and the analyses were thus not stratified.
Characterization of the serotonin-melatonin pathway in autopsy-derived ileum samples from 13 individuals with ASD and 11 controls. (a) Example of chromogranin A immunostaining in one sample from a patient with ASD, assessing the presence of enterochromaffin cells. (b) Serotonin content. (c) NAS content. (d) Melatonin content. (e) AANAT activity. (f) Correlation between NAS content and AANAT activity (regression after log transformation of NAS). (g) Correlation between melatonin content and AANAT activity. (h) ASMT activity. (i) Correlation between NAS content and ASMT activity (regression after log transformation of NAS). (j) Correlation between melatonin content and ASMT activity. (k) Contour plot of NAS content, AANAT and ASMT activities in patient (red dots) and control (green dots) samples. (l) Contour plot of melatonin content, AANAT and ASMT activities in patients (red dots) and control (green dots) samples. The ASD group was compared to the control group using Wilcoxon two-sample test. Boxes indicate medians and quartiles.
We observed no difference between individuals with ASD and controls for the serotonin content (Fig. 3b). In contrast, the melatonin content (Fig. 3d) of ileum samples as well as AANAT (Fig. 3e) and ASMT (Fig. 3h) activities were significantly decreased in patients with ASD compared with controls. Melatonin and AANAT activity were negatively correlated with post mortem interval, but the difference between individuals with ASD and controls remained significant after correction for post mortem interval (Wilcoxon two-sample test, p = 0.01 and 0.005 respectively). Although the melatonin level was significantly correlated with both AANAT (Fig. 3g) and ASMT (Fig. 3j) activities, the contour plots (Fig. 3l) indicated that ASMT activity was the most correlated factor to melatonin content in ileum samples from patients and controls. As in blood platelets (Supplementary Figure 1b), NAS was increased in ileum samples of individuals with ASD (Fig. 3c). A significant correlation was also observed between NAS, AANAT (Fig. 3f,k) and ASMT (Fig. 3i,k) activities.
Thus, the disruption of melatonin synthesis observed in the pineal gland of patients with ASD is also detectable in a peripheral tissue producing melatonin such as the gut, and, as in blood platelets, is associated with increased NAS. These results assert a global disruption of melatonin synthesis in ASD, involving both AANAT and ASMT enzymes, and affecting the pineal gland as well as peripheral tissues.
Contribution of rare genetic variants in AANAT and ASMT in ASD
Considering that the biochemical impairment of melatonin synthesis observed in patients with ASD is shared by first-degree relatives (Fig. 1c, Supplementary Figure 1), we hypothesized that these impairments may be caused by rare mutations and hence sequenced AANAT and ASMT genes. The results were analyzed together with previously published sequencing data12, 28,29,30: taken together, 431 individuals with ASD and 365 controls were directly sequenced for AANAT, as well as 491 individuals with ASD and 490 controls for ASMT, respectively (Table 1). The functional impact of each mutation was determined in silico and in vitro after targeted mutagenesis. Overall, 4.7% of individuals with ASD and 3.9% of controls had a deleterious variant in one of the two genes encoding melatonin synthesis enzymes (p = 0.56). Taking as a threshold the 5th percentile of the control group (0.88 and 2.9 pmol/109 platelets/30 min for ASMT and AANAT, respectively), 2 patients with ASD were carrying ASMT deleterious variants (N17K and C273S) and displayed reduced platelet ASMT activity, while all patients carrying AANAT deleterious variants displayed normal platelet AANAT activity, suggesting that heterozygous AANAT mutations may not have a major impact in vivo, at least for peripheral, low-level enzyme expression (Supplementary Table 2).
We also sequenced all ASMT exons of 7 patients and 17 controls that we assessed for enzyme activity in the pineal gland. For ASMT, we identified 2 previously identified rare non synonymous variants: K81E in a patient and E288D in a control. However, we have previously shown, using functional assays and in vitro mutagenesis, that these variants do not affect ASMT activity31.
Finally, we genotyped a recurrent ASMT microduplication (spanning exon 1 to 7), identified in 12 out of 290 individuals with ASD (4.1%) and in 10 out of 324 controls (3.1%) (OR[CI0.95] = 1.4[0.6–3.2]; Pearson’s chi-square: p = 0.48). Thus, using this PCR approach, we do not confirm the enrichment of microduplication of ASMT in individuals with ASD32. Among patients carrying the microduplication and for whom biochemical and clinical data were available, 5 out of 8 displayed reduced platelet ASMT activity (Supplementary Table 2).
In our previous study8, we reported an association between melatonin deficit and sleep disorders in patients with ASD. Among patients carrying rare variants and for whom sleep information was available, one out of 4 patients with an ASMT deleterious variant, 2 out of 4 with a deleterious AANAT variant, and 5 out of 8 patients with the ASMT microduplication reported insomnia and/or sleep phase delay (Supplementary Table 2).
Reduction of 14-3-3 proteins and increase of miR-451 in samples from patients with ASD
The low frequency of rare variants in AANAT and ASMT genes in patients with ASD is not sufficient to explain the high frequency of melatonin deficit observed in these patients. In a previous study8, we proposed that post-translational regulations of AANAT and ASMT activities, involving the formation of complexes with 14-3-3 proteins23, 24, may be impaired in ASD, and reported low 14-3-3 levels in a small platelet sample from ASD patients. Here, we extended these preliminary findings to larger groups of controls (n = 106 vs. 70), patients (n = 237 vs. 40) and their relatives, confirming a strong reduction of 14-3-3 in platelets of patients with ASD, and revealing intermediate levels in parents (Fig. 4a), as for melatonin, NAS and ASMT (Fig. 1c, Supplementary Figure 1). Furthermore, we measured 14-3-3 in pineal glands from patients with ASD and found that, like AANAT and ASMT activities, it displays significant variations depending on time of death in controls, and is markedly reduced in samples from patients (Fig. 4b). In ileum, 14-3-3 tended to be reduced in patient samples, although this trend did not reach significance, possibly because of a lack of statistical power (Fig. 4c). These findings strengthen our hypothesis of a melatonin deficit resulting from altered 14-3-3-dependent post-translational regulations of AANAT and ASMT activities in ASD. In addition to an immunofluorescent co-localization of the 14-3-3 and the ASMT proteins with p31T-AANAT in human pinealocytes24, we found evidence that 14-3-3 proteins are able to regulate ASMT activity: firstly the pineal ASMT activity of 3 months 14-3-3ζ ko mice on a SV129 background33 was only 60.8 ± 3.8% (n = 8; 4 females, 4 males) as compared to control mice; secondly preincubation with difopein (a blocking agent of 14-3-3-target interactions)34 1 µM for 1 h resulted in a 43.1 ± 4.6% (n = 4) reduction of the activity of a semi-purified bovine ASMT preparation (the one used for the radioenzymatic determination of 5-HT)35.
Since 14-3-3ζ is one of the isoforms most implicated in the regulation of melatonin synthesis23, 24, 36 and since 14-3-3ζ was reported to be repressed by miR-45125,26,27, we measured this micro-RNA in plasma and pineals. Plasmatic and pineal miR-451 were significantly increased in patients with ASD compared to controls (Fig. 4d and e), thus suggesting a post-transcriptional epigenetic mechanism for 14-3-3 impairment.
Discussion
This study investigates for the first time melatonin synthesis in the pineal gland and in the gut from patients with ASD. The pineal gland is the only source of elevated nocturnal melatonin with levels compatible with most roles attributed to this hormone. Our findings reinforce the hypothesis that the nocturnal increase in circulating melatonin is reduced in patients with ASD. The results reveal that this melatonin deficit is caused by enzymatic disruption of both AANAT and ASMT, the two enzymes involved in melatonin synthesis. These impairments were observed in the pineal gland as well as in gut and blood platelets. In these two latter tissues, ASMT appeared as the limiting factor of melatonin synthesis, both in controls and ASD patients. In contrast, the regulation of melatonin level appeared to be more complex in the pineal gland, where the activities of both enzymes determine the melatonin level. This is consistent with the fact that, unlike in gut and platelets, ASMT activity was much higher than AANAT activity in pineal glands. Furthermore, AANAT and ASMT activities are cycling in the pineal gland and alternate as the limiting factor of melatonin synthesis according to the time of the daily cycle37.
Since melatonin secretion is known to be synchronized with light exposure, it may be speculated if the melatonin deficit observed in plasma or in the pineal gland is part of the pathophysiology of ASD, or rather a consequence of environmental bias resulting from ASD condition, e.g. insomnia associated with increased light exposure at night. However, tissue or blood samples were not collected in controlled light conditions. The pineal gland receives environmental cues from the retino-hypothalamic tract, and could be affected by such environmental bias. In contrast, melatonin synthesis in peripheral tissues such as blood platelets and gastro-intestinal tract is not directly regulated by light exposure: thus, the consistent findings of impaired melatonin synthesis in pineal glands, but also in peripheral tissues of patients with ASD, are not suggestive of a secondary or artefactual phenomenon.
One limitation to the tissue study is the small number of patient samples, limiting statistical power, especially in the pineal gland where stratification according to time of death is needed. In particular, only two patients with pineal gland samples had nocturnal time of death. However, in spite of this limitation, melatonin and ASMT decrease remained significant even for patients with diurnal time of death, when ASMT activity and melatonin secretion are at lowest level and the sensitivity to detect impairment in this pathway is expected to be lower. This is consistent with the significant decrease of plasmatic melatonin observed in patients with ASD even when sampled in the morning8.
In gut and platelets, the reduction of ASMT activity observed in patients with ASD appeared to be correlated with an accumulation of NAS, the intermediate metabolite between serotonin and melatonin. This increase of NAS was not observed in the pineal gland, and this may also result from the different stoichiometry of the two enzymes in this tissue compared with “peripheral” sources. Indeed, in contrast with data from platelets and ileum, pineal NAS was not correlated with ASMT activity, but with AANAT activity, although with a complex pattern as observed for melatonin. In the pineal glands of ASD patients, NAS levels may be determined by its degradation (by ASMT) and its synthesis (by AANAT), both being impaired. In platelets, unlike melatonin which is a diffusible molecule, NAS is co-stored with 5-HT in dense granules and its release is controlled38. By contrast, using high concentrations (10 mM) of indoleamines, it has been reported that melatonin and NAS are highly permeant molecules that will leak out of a cellular model for pinealocytes as rapidly as they are synthesized (ratio of estimated extracellular concentrations for melatonin, NAS, and 5-HT: 1:0.18:0.006)39. Whether or not NAS is accumulated in the brain of patients with ASD, where it may exert its proper biological functions, and especially its neurotrophic effect21, 22, is still to be determined.
The exact mechanisms of the disruption of AANAT and ASMT activities in ASD patients remain to be elucidated. Considering the familial aggregation of these biochemical traits, we considered a genetic hypothesis and screened AANAT and ASMT genes for rare variants. However, although rare variants may explain melatonin deficit in a subset of families, their low frequency was far from sufficient to explain the high rates of biochemical impairments reported in patients, and we found no significant enrichment of these variants in the population of patients with ASD compared with controls. Some functional polymorphisms in the ASMT promoter (rs4446909 and rs5989681) may have a role as they have been associated with the risk of ASD12. This association finding was not fully replicated in independent studies, although a similar trend was observed29, 40, 41. Interestingly, the same SNPs have also been associated with bipolar disorder42, 43, major depressive disorder44, and depressive symptoms in individuals with sleep phase delay45.
As an alternative to a defect in the genes coding for AANAT and ASMT, we propose that the abnormal reduction of 14-3-3 proteins26, first identified as activators of tryptophan and tyrosine hydroxylases46, could lead to a defect of AANAT23, 36 and ASMT activities24, present study, and ultimately to a melatonin deficit in ASD. Indeed, the 14-3-3 protein level was decreased in all three materials investigated in patients compared to controls. This post-translational hypothesis is consistent with the impairments of both AANAT and ASMT and, as suggested by exome sequencing in multiplex autism families47, polymorphisms in YWHA genes involved in 14-3-3 protein synthesis, already reported for ADHD, schizophrenia, and bipolar disorder48, are presently investigated in our lab. Finally, the platelet and pineal 14-3-3 decrease was found to be associated with an increase of plasma and pineal miR-451, reasserting in ASD the regulatory role of miR-451 towards 14-3-325,26,27.
The roles of 14-3-3, miR-451 and melatonin deficits in the etiologic architecture of ASD remain to be elucidated. ASD are complex, heterogeneous and multifactorial diseases1, 49, 50. The high frequency of melatonin deficit in patients8 (Supplementary Figure 2b) is particularly striking within this paradigm, and might suggest that 14-3-3/melatonin impairments are part of the core pathological processes in ASD. In a multifactorial paradigm, a 14-3-3/melatonin deficit may be a susceptibility factor which, in conjunction with genetic variants in ASD-associated genes, would result in a pathological phenotype. The observation of melatonin impairments in parents of patients with ASD can fit with this hypothesis. Beyond the hundreds of reported susceptibility genes and loci in ASD, functional convergences have been identified to common pathways, including synaptic function, neuronal development and signaling, or chromatin remodeling. Non-genetically determined impairments, such as immune dysfunction, have also been implicated49. Alternatively, a 14-3-3/melatonin deficit may be a common phenomenon secondary to the disruption of one or several of these processes. In this case, considering the pleiotropic functions of the hormone melatonin and of the regulatory 14-3-3 proteins, it is likely that 14-3-3 and melatonin deficits would in turn exacerbate neuronal dysfunction(s). These findings provide support for future exploration into the potential role of the melatonin synthesis pathway in the treatment of ASD.
In conclusion, this study provides support for a frequent melatonin synthesis defect in ASD and reports for the first time that this abnormal melatonin reduction is caused by deficits of both AANAT and ASMT activities in pineal glands, gut, and blood platelets of patients. These results also open new perspectives on the post-translational and post-transcriptional mechanisms involved and provide insights into the common pathways of autism pathophysiology.
Patients and Methods
Methods were performed in accordance with the relevant guidelines and regulations
Autopsy-derived tissue samples
Ileum and pineal gland samples from individuals with ASD and controls were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders of the University of Maryland (Baltimore, MD, USA), the Autism Tissue Program (Autism Speaks, Princeton, NJ, USA) and from the Anatomy Department of the Goethe Universität (Frankfurt, Germany). Written informed consents were obtained from the donors’ next of kin. Samples were frozen after autopsy and kept at −80 °C. Groups’ characteristics are described in Supplementary Table 3. Biochemical analyses were performed after tissue disruption and homogenization. The impact of post mortem interval (PMI) was assessed by linear regression (parameter measured = f[PMI]) in order to take into account the post mortem stability of the parameter investigated. For parameters displaying correlation with PMI, a correction was applied as follows: parameter corrected = parameter measured − (correlation coefficient × PMI). All figures present the raw data. Analyses performed with corrected data are described in the main text. Despite the fact that AANAT is known to display a very short half-life (a few minutes) in rodents15, there are major differences in its regulation among species. AANAT and ASMT activities have previously been shown to be stable when measured in human pineal organs held under the usual post-mortem conditions51, and no significant correlation was observed here between AANAT and ASMT activities and PMI.
Subjects, clinical evaluations, blood samples
The clinical evaluations and blood sampling of patients with ASD (diagnosed according to DSM-IV), their relatives, and control subjects investigated for genetics and blood biochemistry have been detailed previously8. The local Institutional Review Boards (Comité de Protection des Personnes Ile de France IX) approved this study. Written informed consents were obtained after oral and written information from all participants of the study, and from the children’s parents when subjects were under 18.
Biochemical measurements
Melatonin in plasma and tissues was measured using a radioimmunoassay (RK-MEL, Bühlmann, Switzerland) according to the manufacturer’s instructions. Serotonin was measured by high-performance liquid chromatography52. NAS, as well as enzyme activities of AANAT, ASMT were determined by radioenzymology. Technical details of enzymatic assays and circulating miR-451 determination are included as supplementary information.
The amount of 14-3-3 proteins was determined using the commercial 14-3-3 Pro ELISA kit from MyBioSource (San Diego, CA, USA). The immunogen used to produce the antibodies is recombinant full length human 14-3-3 γ. The kit has about 40% cross reactivity with 14-3-3 ζ, ε, σ, τ (MyBioSouce, personal communication).
Sequencing of AANAT and ASMT and genotyping of ASMT microduplication
AANAT (the 3 coding exons) and ASMT (promoter B and the 9 coding exons) were sequenced according to the previously published protocols12, 28. Technical details are included in Supplementary Information. Mapping and genotyping of ASMT microduplication are detailed in Supplementary Table 5.
Statistical analyses
Statistical analyses were performed using JMP Pro 10 (SAS, Toronto, ON, Canada) and Stata 11.0 (StataCorp LP, College Station, TX, USA). Since most biochemical parameters were not normally distributed, categorical and non-parametric statistical tests were preferred. Error type I was chosen at 0.05. The statistical tests used for this study were Wilcoxon two-sample test, Kruskall-Wallis test, Pearson’s chi-square test, Fisher’s exact test, and linear regression.
References
Bourgeron, T. From the genetic architecture to synaptic plasticity in autism spectrum disorders. Nat. Rev. Neurosci. 16, 551–563, doi:10.1038/nrn3992 (2015).
Lam, K. S., Aman, M. G. & Arnold, L. E. Neurochemical correlates of autistic disorder: a review of the literature. Res. Dev. Disabil. 27, 254–289, doi:10.1016/j.ridd.2005.03.003 (2006).
Rossignol, D. A. & Frye, R. E. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol. Psychiatry 17, 290–314, doi:10.1038/mp.2010.136 (2012).
Frustaci, A. et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic. Biol. Med. 52, 2128–2141, doi:10.1016/j.freeradbiomed.2012.03.011 (2012).
Schain, R. J. & Freedman, D. X. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J. Pediatr. 58, 315–320, doi:10.1016/S0022-3476(61)80261-8 (1961).
Anderson, G. M., Horne, W. C., Chatterjee, D. & Cohen, D. J. The hyperserotonemia of autism. Ann. N. Y. Acad. Sci. 600, 331–332, doi:10.1111/nyas.1990.600.issue-1 (1990).
Leboyer, M. et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biol. Psychiatry 45, 158–163, doi:10.1016/S0006-3223(97)00532-5 (1999).
Pagan, C. et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 4, e479, doi:10.1038/tp.2014.120 (2014).
Nir, I. et al. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J. Autism Dev. Disord. 25, 641–654, doi:10.1007/BF02178193 (1995).
Kulman, G. et al. Evidence of pineal endocrine hypofunction in autistic children. Neuroendocrinology Lett. 21, 31–34 (2000).
Tordjman, S., Anderson, G. M., Pichard, N., Charbuy, H. & Touitou, Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry 57, 134–138, doi:10.1016/j.biopsych.2004.11.003 (2005).
Melke, J. et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol. Psychiatry 13, 90–98, doi:10.1038/sj.mp.4002016 (2008).
Tordjman, S. et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 37, 1990–1997, doi:10.1016/j.psyneuen.2012.04.013 (2012).
Erren, T. C. & Reiter, R. J. Melatonin: a universal time messenger. Neuroendocrinology Lett. 36, 187–192 (2015).
Johnston, J. D. & Skene, D. J. Regulation of mammalian neuroendocrine physiology and rhythms by melatonin. J. Endocrinol. 226, T187–T198, doi:10.1530/JOE-15-0119 (2015).
Claustrat, B., Brun, J. & Chazot, G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 9, 11–24, doi:10.1016/j.smrv.2004.08.001 (2005).
Bourgeron, T. The possible interplay of synaptic and clock genes in autism spectrum disorders. Cold Spring Harb. Symp. Quant. Biol. 72, 645–654, doi:10.1101/sqb.2007.72.020 (2007).
Andersen, I. M., Kaczmarska, J., Mc Grew, S. G. & Malow, B. A. Melatonin for insomnia in children with autism spectrum disorders. J. Child Neurol. 23, 482–485, doi:10.1177/0883073807309783 (2008).
Wright, B. et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: a randomised controlled crossover trial. J. Autism Dev. Disord. 41, 175–184, doi:10.1007/s10803-010-1036-5 (2011).
Malow, B. et al. Melatonin for sleep in children with autism: a controlled trial examining dose, tolerability, and outcomes. J. Autism Dev. Disord. 42, 1729–1737; author reply 1738 (2012).
Jang, S. W. et al. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 107, 3876–3881, doi:10.1073/pnas.0912531107 (2010).
Choudhury, A., Singh, S., Palit, G., Shukla, S. & Ganguly, S. Administration of N-acetylserotonin and melatonin alleviate chronic ketamine-induced behavioural phenotype accompanying BDNF-independent and dependent converging cytoprotective mechanisms in the hippocampus. Behav. Brain Res. 297, 204–212, doi:10.1016/j.bbr.2015.10.027 (2016).
Obsil, T., Ghirlando, R., Klein, D. C., Ganguly, S. & Dyda, F. Crystal structure of the 14-3-3zeta:serotonin N-acetyltransferase complex. a role for scaffolding in enzyme regulation. Cell 105, 257–267, doi:10.1016/S0092-8674(01)00316-6 (2001).
Maronde, E. et al. Dynamics in enzymatic protein complexes offer a novel principle for the regulation of melatonin synthesis in the human pineal gland. J. Pineal Res. 51, 145–155, doi:10.1111/j.1600-079X.2011.00880.x (2011).
Yu, D. et al. MiR-451 protects against erythroid oxidant stress by repressing 14-3-3zeta. Genes Dev. 24, 1620–1633, doi:10.1101/gad.1942110 (2010).
Bergamaschi, A. & Katzenellenbogen, B. S. Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene 31, 39–47, doi:10.1038/onc.2011.223 (2012).
Wu, L. et al. Altered expression of 14-3-3ζ protein in spinal cords of rat fetuses with spina bifida aperta. PloS One 8, e70457, doi:10.1371/journal.pone.0070457 (2013).
Chaste, P. et al. Genetic variations of the melatonin pathway in patients with attention-deficit and hyperactivity disorders. J. Pineal Res 51, 394–399, doi:10.1111/jpi.2011.51.issue-4 (2011).
Jonsson, L. et al. Mutation screening of melatonin-related genes in patients with autism spectrum disorders. BMC Med. Genomics 3, 10, doi:10.1186/1755-8794-3-10 (2010).
Pagan, C. et al. Mutation screening of ASMT, the last enzyme of the melatonin pathway, in a large sample of patients with intellectual disability. BMC Med. Genet. 12, 17, doi:10.1186/1471-2350-12-17 (2011).
Goubran-Botros, H. et al. Crystal structure and functional mapping of human ASMT, the last enzyme of the melatonin synthesis pathway. J. Pineal Res. 54, 46–57, doi:10.1111/j.1600-079X.2012.01020.x (2013).
Cai, G. et al. Multiplex ligation-dependent probe amplification for genetic screening in autism spectrum disorders: efficient identification of known microduplications and identification of a novel microduplication in ASMT. BMC Med. Genomics 1, 50, doi:10.1186/1755-8794-1-50 (2008).
Cheah, P. S. et al. Neurodevelopmental and neuropsychiatric behaviour defects arise from 14-3-3ζ deficiency. Mol. Psychiatry 17, 451–466, doi:10.1038/mp.2011.158 (2012).
Aghazadeh, Y. & Papadopoulos, V. The role of the 14-3-3 protein family in health, disease, and drug development. Drug Discovery Today 21, 278–287, doi:10.1016/j.drudis.2015.09.012 (2016).
Saavedra, J. M., Brownstein, M. & Axelrod, J. A specific and sensitive enzymatic-isotopic microassay for serotonin in tissues. J. Pharmacol. Exper. Ther 186, 508–515 (1973).
Ganguly, S. et al. Melatonin synthesis: 14-3-3-dependent activation and inhibition of arylalkylamine N-acetyltransferase mediated by phosphoserine-205. Proc. Natl. Acad. Sci. USA 102, 1222–1227, doi:10.1073/pnas.0406871102 (2005).
Liu, T. & Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 39, 91–96, doi:10.1111/jpi.2005.39.issue-1 (2005).
Launay, J. M., Barré, N., Costa, J. L. & Da Prada, M. The melatonin pathway in blood platelets. Melatonin and the Pineal Gland. From Basic Science to Clinical Application. (eds Touitou, Y., Arendt, J. & Pévet, P.) 35–40 (Libbey, 1993).
Yu, H., Dickson, E. J., Jung, S. R., Koh, D. S. & Hille, B. High membrane permeability for melatonin. J. Gen. Physiol. 147, 63–76, doi:10.1085/jgp.201511526 (2015).
Toma, C. et al. Is ASMT a susceptibility gene for autism spectrum disorders? A replication study in European populations. Mol. Psychiatry 12, 977–979, doi:10.1038/sj.mp.4002069 (2007).
Veatch, O. J. et al. Genetic variation in melatonin pathway enzymes in children with autism spectrum disorder and comorbid sleep onset delay. J. Autism Dev. Disord. 45, 100–110, doi:10.1007/s10803-014-2197-4 (2015).
Etain, B. et al. Genetic and functional abnormalities of the melatonin biosynthesis pathway in patients with bipolar disorder. Hum. Mol. Genet. 21, 4030–4037, doi:10.1093/hmg/dds227 (2012).
Geoffroy, P. A. et al. Genetic association study of circadian genes with seasonal pattern in bipolar disorders. Sci. Rep 5, 10232, doi:10.1038/srep10232 (2015).
Galecki, P. et al. Single-nucleotide polymorphisms and mRNA expression for melatonin synthesis rate-limiting enzyme in recurrent depressive disorder. J. Pineal Res. 48, 311–317, doi:10.1111/jpi.2010.48.issue-4 (2010).
Kripke, D. F. et al. Polymorphisms in melatonin synthesis pathways: possible influences on depression. J. Circadian Rhythms 9, 8, doi:10.1186/1740-3391-9-8 (2011).
Ichimura, T., Isobe, T., Okuyama, T., Yamauchi, T. & Fujisawa, H. Brain 14-3-3 protein is an activator protein that activates tryptophan 5-monooxygenase and tyrosine 3-monooxygenase in the presence of Ca2+,calmodulin-dependent protein kinase II. FEBS Lett. 219, 79–82, doi:10.1016/0014-5793(87)81194-8 (1987).
Toma, C. et al. Exome sequencing in multiplex autism families suggests a major role for heterozygous truncating mutations. Mol. Psychiatry 19, 784–790, doi:10.1038/mp.2013.106 (2014).
Jacobsen, K. K., Kleppe, R., Johansson, S., Zayats, T. & Haavik, J. Epistatic and gene wide effects in YWHA and aromatic amino hydroxylase genes across ADHD and other common neuropsychiatric disorders: association with. YWHAE. Am. J. Med. Genet. part B 168B, 423–432, doi:10.1002/ajmg.b.32339 (2015).
Voineagu, I. et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474, 380–384, doi:10.1038/nature10110 (2011).
Pinto, D. et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am. J. Human. Genet. 94, 677–94, doi:10.1016/j.ajhg.2014.03.018 (2014).
Ackermann, K. et al. Characterization of human melatonin synthesis using autoptic pineal tissue. Endocrinology 147, 3235–3242, doi:10.1210/en.2006-0043 (2006).
Kema, I. P. et al. High performance liquid chromatographic profiling of tryptophan and related indoles in body fluids and tissues of carcinoid patients. Clin. Chim. Acta 221, 143–158, doi:10.1016/0009-8981(93)90029-4 (1993).
Acknowledgements
We thank the patients and their families, and the controls who accepted to participate in this study. Some tissue used in this research was obtained from Autism BrainNet that is sponsored by the Simons Foundation, and from the Maryland Brain and Tissue Bank. The authors also acknowledge the Autism Tissue Program that was the predecessor to Autism BrainNet. The Clinical Investigation Centers of Robert-Debré and Henri Mondor Hospitals obtained and processed blood samples, the Hematology departments from both hospitals (Dr MF Hurtaud and Pr M Imbert) performed platelet counts; the Anatomopathology department of Lariboisière Hospital (Pr F Gray) performed immunohistochemistry analyses. This work was supported by academic institutions (Institut Pasteur, Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Assistance Publique – Hôpitaux de Paris, University Paris Diderot); academic grants: ANR (SynDivAutism) and Neuron-ERANET (EUHF-AUTISM); charity foundations: Bettencourt-Schueller foundation, Orange foundation, FondaMental foundation, Conny-Maeva foundation, Cognacq-Jay foundation.
Author information
Authors and Affiliations
Contributions
C.P., R.D., M.L., J.M.L. and T.B. designed the study. C.P., H.G.B., R.B., M.B., N.L., K.M., F.A., J.C., P.C., S.J., F.F., G.H., E.M. contributed to generate the data. C.P., J.M.L., T.B. drafted the manuscript. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pagan, C., Goubran-Botros, H., Delorme, R. et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci Rep 7, 2096 (2017). https://doi.org/10.1038/s41598-017-02152-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02152-x
This article is cited by
-
Impact of IDO activation and alterations in the kynurenine pathway on hyperserotonemia, NAD+ production, and AhR activation in autism spectrum disorder
Translational Psychiatry (2023)
-
Sleep and Autism Spectrum Disorder: A Comprehensive Review of Diagnosis, Markers, Interventions, and Treatments
Sleep and Vigilance (2023)
-
Biological correlates of altered circadian rhythms, autonomic functions and sleep problems in autism spectrum disorder
Annals of General Psychiatry (2022)
-
A perspective on molecular signalling dysfunction, its clinical relevance and therapeutics in autism spectrum disorder
Experimental Brain Research (2022)
-
Deficiency of the ywhaz gene, involved in neurodevelopmental disorders, alters brain activity and behaviour in zebrafish
Molecular Psychiatry (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.