Abstract
Human brain samples were collected from 46 autopsy cases, including 23 fatal heat stroke cases and 23 age-matched controls. Nine candidate reference genes (PES1, POLR2A, IPO8, HMBS, SDHA, GAPDH, UBC, B2M, ACTB) were evaluated in the cerebral cortex of 10 forensic autopsy cases (5 heat stroke and 5 controls), using the geNorm module in qBaseplus software. SDHA, POLR2A, IPO8 and HMBS were identified as the most stable reference genes. Using these validated reference genes, mRNA expressions of Matrix metalloproteinases (MMPs, MMP2 and MMP9), Claudin5 (CLDN5), Occludin (OCLN), Zona occludens protein-1 (ZO1) and Aquaporins (AQPs, AQP1 and AQP4) in the cerebral cortex were examined. Relative mRNA quantification using Taqman real-time PCR assay demonstrated increased calibrated normalized relative quantity (CNRQ) values of MMP9, CLDN5, OCLN, ZO1 and AQP4 in heat stroke cases. Heat stroke cases showed an increase in brain water content, which was found to be positively correlated with MMP9, OCLN, ZO1 and CLDN5 mRNA. When using one conventional reference gene (GAPDH or ACTB) for normalization, no difference was detected between heat stroke and controls. In immunostaining, only AQP4 showed more intense staining in most heat stroke cases. The present study, for the first time, reports increased cerebral MMP9, CLDN5, OCLN, ZO1 and AQP4 in heat stroke and suggest a crucial role of reference gene selection when using postmortem human tissues.
Similar content being viewed by others
Introduction
Heat stroke is defined as a form of hyperthermia associated with a systemic inflammatory response leading to a syndrome of multi organ dysfunction in which encephalopathy predominates1. The mortality is as high as 10–15% in patients with heat stroke. Nearly 30% of patients with heat stroke are accompanied by central nervous system (CNS) dysfunction that results in delirium, convulsions, or coma2. Brain edema is an important factor associated with brain damage causing long-term disability and death in patients with heat-related illness. In fact, forensic autopsy data showed profound brain edema in heat stroke cases3. However, this phenomenon has not been fully emphasized in clinical treatment. The potential mechanism of brain edema formation following heat stroke has not been fully clarified.
Matrix metalloproteinases (MMPs) belong to a family of calcium-dependent zinc-containing endopeptidases, which are involved in the tissue remodeling and degradation of the extracellular matrix (ECM)4. Considerable research has been conducted on the role of two secreted MMPs, MMP2, and MMP9. Both of them have positive and negative roles in the healthy and diseased CNS5. MMP9 is responsible for blood-brain barrier (BBB) opening in several pathological conditions and the marked increase of MMP9 causes severe BBB disruption. MMP9-mediated BBB disruption may be a crucial step in the pathogenesis of some neuroinflammatory diseases6,7,8. Claudin5 (CLDN5), Occludin (OCLN) and Zona occludens protein-1 (ZO1) are key tight junction (TJ) proteins that play an important role in modulation of BBB permeability9. Decreased expression of CLDN5, OCLN and ZO1 has been reported to be closely associated with BBB damage10. Aquaporins (AQPs) are water channels that facilitate water transport. AQP1 and AQP4 are presumed as major contributors to participate in brain water homeostasis11. AQPs, in particular AQP4, appear to play a crucial role in cerebral volume regulation following trauma, inflammation, ischemia, tumors as well as metabolic disturbances12, 13.
Experimental studies reported that hyperthermia caused by heat exposure is closely associated with the breakdown of the BBB followed by brain edema14. The disruption of the BBB is characterized by the degradation of the junctional complex proteins and increase in multiple matrix metalloproteinases. However, these studies have generally been restricted to rodent models that have inevitably inherent deficiencies, including a lissencephalic brain and small head size relative to body size. Furthermore, heat stroke in human is rarely as pure as in experimental models, it is necessary to investigate the human materials following fatal heat stroke.
The present study analyzed the gene expressions of MMP2, MMP9, CLDN5, OCLN, ZO1, AQP1 and AQP4, in the brains of forensic autopsy cases, using reverse transcription quantitative PCR (RT-qPCR), combined with immunohistochemical detections, to investigate the molecular pathology of brain edema in fatal heat stroke cases with special regard to the importance of reference gene selection.
Results
Brain water content
There was a significant increase in brain water content in the heat stroke group as compared to the control group (Table 1, heat stroke: 82.3% ± 2.9%; control: 79.3% ± 2.6%, p < 0.05, Student’s t test).
RNA concentration, purity and integrity
RNA concentrations ranged from 25.9 to 348.7 ng/μL (mean 194.1 ng/μL). There were no age or postmortem interval dependences on Pearson correlation analysis (p > 0.05). RNA concentrations showed no significant differences between the heat stroke group and control group.
RNA purity, determined using 260/280 absorbance (A260/A280) ratios, ranged from 1.75 to 2.19 (mean 1.98). There were no age or postmortem interval dependences on Pearson correlation analysis (p > 0.05). RNA purity showed no significant differences between the heat stroke group and the control group.
RIN values showed substantial variations in each group. There were no age or postmortem interval dependences on Pearson correlation (p > 0.05). RIN values were evidently lower in the heat stroke group as compared to the control group (Table 1, heat stroke: 3.6 ± 1.0; control: 4.9 ± 1.1, p < 0.05, Student’s t test).
Amplification efficiency
The amplification efficiencies of targets and reference genes ranged from 86.2% (ACTB) to 104.6% (UBC), showing small inter-individual variations (standard deviation, SD < 5%). Details are shown in Table 2.
Reference genes validation
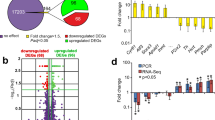
The geNorm module in qBaseplus software ranked the 9 reference genes. The most stable one was SDHA followed by POLR2A, IPO8 and HMBS. The least stable one was ACTB (Fig. 1). Pairwise variation (V) was calculated based on normalization factor values (NFn and NFn + 1) after the inclusion of the least stable reference gene and indicated if the extra reference gene added to the stability of the normalization factor. The V-value was the lowest when the fifth most stable gene (PES1) was added (Fig. 2). However, when the fourth most stable gene (HMBS) was added, V3/4 showed a V-value of 0.153, near the threshold of 0.15. Therefore, to save on cost and time, four reference genes SDHA, POLR2A, IPO8 and HMBS, were selected for normalization.
Data analysis
Normalization against validated reference genes
Raw Ct values and amplification efficiencies of targets and 4 validated reference genes, SDHA, POLR2A, IPO8 and HMBS, were imported into the qBaseplus software. CNRQ values were exported and statistically investigated.
There were no gender-related differences, or age or postmortem interval dependence in CNRQ values of target genes on Pearson correlation (p > 0.05).
CNRQ values of AQP4, CLDN5, OCLN, ZO1 and MMP9 were significantly higher in the heat stroke group as compared to the control group (Fig. 3).
CNRQ values of CLDN5 and MMP9 were found to be positively correlated with brain water contents (r2 = 0.1225 and 0.1486, p < 0.05).
Normalization against ACTB or GAPDH
When ACTB or GAPDH alone was used for normalization, there was no significant difference in the expression of any target gene (Figs 4 and 5).
Immunostaining
Immunostaining showed substantial interindividual variations in each group. AQP1 (Fig. 6a and b) and AQP4 (Fig. 6c and d, Fig. 7) were mainly detected in glial cells which were morphologically identified as astrocytes, and only AQP4 showed more intense staining in most heat stroke cases. CLDN5 was strongly positive in capillary endothelia, and no significant differences in distribution or intensity were detected between heat stroke and control group (Fig. 6e and f, Fig. 7). MMP2 was detected clearly in the neurons, showing no significant differences in distribution or intensity between heat stroke and control group (Fig. 6g and h, Fig. 7). MMP9 was located in glial cells, neurons and capillary endothelia, and no significant differences in distribution or intensity were detected between heat stroke and control group (Fig. 6i and j, Fig. 7). OCLN was positive in capillary endothelia, sporadically in neurons and glial cells, and no significant differences in distribution or intensity were detected between heat stroke and control group (Fig. 6k and l, Fig. 7). ZO1 was located in capillary endothelia, and no significant differences in distribution or intensity were detected between heat stroke and control group (Fig. 6m and n, Fig. 7).
Immunostaining of AQP1 (a and b), AQP4 (c and d), CLDN5 (e and f), MMP2 (g and h), MMP9 (i and j), OCLN (k and l) and ZO1 (m and n) in the brain. Peracute death due to blunt injury (a,c,e,g,i,k and m), a 52-year-old male, 27 h postmortem. Death due to heat stroke (b,d,f,h,j,l and n), a 64-year-old male, 30 h postmortem.
Discussion
RT-qPCR is increasingly applied to determine changes in gene expressions due to the high sensitivity and accuracy of the technique. The most common procedures in RT-qPCR are relative measurements of genes of interest after normalization with the endogenous reference gene(s). Accurate and reliable relative RT-qPCR requires ideal reference gene(s). However, expressions of several conventional reference genes were shown to vary due to nutritional or hormonal factors, biological processes, and/or tissue or cell types; a single endogenous reference gene cannot meet the criteria of an ideal reference gene15. In the field of molecular neurobiology, RT-qPCR, using postmortem autopsy tissues, has become a hotspot16,17,18. It can provide novel biomarkers and disease-modifying therapeutic targets for some CNS diseases. Previous studies, using different normalization strategies, demonstrated contradicting results, suggesting the importance of normalization19, 20.
In the present study, with the help of geNorm module in qBaseplus software, nine reference genes were evaluated. The V-value was the lowest when the fifth most stable gene (PES1) was added. Further addition of genes increased V-values, indicating a negative influence on the normalization process. Ideally, a threshold V-value of 0.15 is recommended as a cut-off value by geNorm to determine the optimal number of reference genes21. However, when the fourth most stable gene (HMBS) was added, V3/4 showed a V-value of 0.153, near the threshold of 0.15. Therefore, to save on cost and time, four reference genes SDHA, POLR2A, IPO8 and HMBS, were selected for normalization. In the present study, CLW/H ratios were higher in the heat stroke group as compared to the control group, indicating brain edema in heat stroke cases. However, using mRNA measurements of intracerebral MMPs, CLDN5, OCLN, ZO1, and AQPs as markers of brain edema, inconsistent results were detected by different normalization methods. When those four validated reference genes, SDHA, POLR2A, IPO8 and HMBS, were used for normalization, increased cerebral expressions of AQP4, CLDN5, OCLN, ZO1 and MMP9 were detected in heat stroke group. However, these findings cannot be detected when GAPDH or B2M alone was used for normalization. Expression stability values of these five reference genes calculated by geNorm showed ACTB as the least stable one, followed by GAPDH. Therefore, gene expression levels that normalized against four validated reference genes were believed to be accurate and reliable; ACTB and GAPDH, two conventional reference genes, were not suitable for normalization of human postmortem brain tissues.
Another considerable factor influencing the accuracy of gene expression analysis using RT-qPCR is the integrity of RNA22. However, unlike animal experimentation, RNA degradation is inevitable and unpredictable for human tissues collected at autopsy. In the present study of the human brain tissues, RIN values showed no postmortem interval-dependent changes but were significantly lower in the heat stroke group as compared to the control group, indicating that RNA quality was more seriously affected in cases of hyperthermia.
The up-regulations of MMP2 and MMP9 in brain are associated with an increase of BBB permeability by degrading the endothelial basal lamina of the BBB which results in vasogenic edema23. Despite the well documented effects of systemic inflammatory response, the impact of hyperthermia on the BBB has been overlooked and the probable mechanism has not been fully addressed. In the present study, brain tissues in heat stroke group showed evidently higher CNRQ values of MMP9, but not MMP2. These findings suggest independent contributions of MMP2 and MMP9 in the brain tissues of heat stroke group, which require further investigation. MMP9 was regarded as a key player in the alteration of BBB permeability. Several studies in animal models have shown that increased MMP9 is closely related to the breakdown of BBB, by digesting ECM24, 25. Both inhibiting MMP9 and deleting MMP9 gene can attenuate the BBB disruption26, 27.
In addition, intercellular junctions between endothelial cells are essential for vascular integrity and function. Breaching of endothelial barriers is a key event in the development of brain edema. The loss of CLDN5, OCLN and ZO1 in TJs can open the BBB, lead to brain edema and neuronal cell death. Decreased expression of CLDN5, OCLN and ZO1 is closely associated with BBB damage28. However, in the present study, increased CLDN5, OCLN and ZO1 mRNA expression was detected in the heat stroke group. These findings might be considered a compensatory mechanism to mend junctional complexes and restore barrier function.
AQP4, the principal AQP in mammalian brain, highly correlates with a variety of pathophysiological processes of brain edema. Increased expression of AQP4 in the brain indicated that AQPs participated in the formation of brain edema29. In the vasogenic edema resolution phase, an increase of AQP4 was observed in several studies30, 31. Therefore, in heat stroke cases, AQP4 might play a beneficial role in eliminating accumulating water from the extracellular space of the CNS, suggesting an activation of the self-protective system.
In the present study, the immunostaining did not detect any evident differences in distribution or intensity among all the causes of death except for AQP4. These findings may be caused by a lower sensitivity of immunostaining in detecting changes in gene products than that with quantitative analyses of gene expressions using RT-qPCR. The major limitation of the present study is that the protein levels of all targets have not been examined because of the limitation of the postmortem materials, which need further investigation.
In conclusion, the present study shows that brain edema is evident in heat stroke, and human brain retains a self-protective response capacity in victims who died due to heat stroke. Systematic analysis of gene expressions using RT-qPCR is a useful procedure and validation of reference genes is crucial.
Materials and Methods
Sample collection
A total of 46 human forensic autopsy cases were selected from autopsy documents. The demographics of study subjects are described in Table 1. In each case, the cause of death was carefully diagnosed on the basis of autopsy examination, including macromorphological, histological, toxicological and biochemical analyses. Cases were divided into two groups as follows: 23 fatal heat stroke cases and 23 age, gender and postmortem interval (PMI)-matched control subjects, including three hanging cases, three strangulation cases, five blunt injury cases, six fire fatality cases, and six acute cardiac death cases. A thorough neuropathological analysis was performed as part of our routine investigation, and cases with any preexisting neurological pathologies were excluded in the present study.
In the present study, clearly accountable cases without any other complications that may have contributed to the death, supported by well-established circumstantial evidence, were collected. Postmortem interval was defined as the estimated time from death to autopsy which was estimated on the basis of autopsy findings and circumstantial evidence recorded in autopsy documents. The definition and criteria of deaths due to heat stroke are described in our previous report32; drug abusers and chronic alcoholics were excluded from the heat stroke group. This work was approved by our institutional Ethics Committee of Southern Medical University. All sampling methods were followed anatomical practices and carried out in accordance with regulations of Methods of extraction, fixation, packing and inspection of forensic pathology of The PRC Public Safety Industry Standard (GA/T 148–1996) and Forensic pathology materials extraction, fixed operating instructions of Southern Medical University (NYSJ-JS-BL04). As one of our routine work, the informed consent paper was obtained from the immediate family members of deceased before starting autopsy.
Brain water content
Brain water content was measured by a halogen moisture analyzer (model HB43, METTLER TOLEDO, Switzerland) automatically according to the manufacturer’s instructions20. Briefly, brain tissue samples were taken from consistent sites in the parietal lobe of left cerebral hemispheres at autopsy. About one gram of brain tissue was weighted first to obtain a wet weight (WW), then placed in the analyzer at 150 °C for about 30 min and weighted again to obtain a dry weight (DW). The water content was calculated using the equation: (WW − DW)/WW × 100%.
Toxicological analyses
The procedures of drug testing and analysis, including chemicals and reagents, sample preparation and conditions of the instrument were performed by gas chromatography/mass spectrometry33.
Extraction of total RNA and cDNA synthesis
Tissue specimens were taken from consistent sites in the central anterior of left cerebral hemispheres (precentral gyrus) at autopsy, then immediately submerged in 1 ml of RNA stabilization solution (RNAlaterTM, Ambion, Austin). Total RNA was isolated from 100 mg of sample using RNAiso Plus (Takara Bio, Inc., Shiga, Japan) according to the manufacturer’s instructions. After extraction, the RNA concentration was estimated by spectrophotometric analysis using a NanoDrop 1000 (Thermo Scientific, Wilmington, USA). cDNA copies of total RNA were obtained using a High Capacity RNA-to-cDNA kit (Applied Biosystems Japan, Ltd.), then were adjusted to a concentration equivalent to 5 ng/μl of total RNA using nuclease-free water.
Evaluation of the quality and integrity of RNA samples
RNA purity was determined using 260/280 absorbance (A260/A280) ratios. The RNA integrity number (RIN) was determined using a RNA 6000 Nano Labchip kit in an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, USA) following the manufacturer’s protocol.
Reference genes selection
Nine candidate reference genes were evaluated in the brain tissue of 10 forensic autopsy cases (Heat stroke, n = 5 and control, n = 5): pescadillo homolog 1 (PES1), polymerase (RNA) II (DNA directed) polypeptide A (POLR2A), importin 8 (IPO8), hydroxymethylbilane synthase (HMBS), succinate dehydrogenase complex (SDHA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), ubiquitin C (UBC), beta-2-microglobulin (B2M), beta-actin (ACTB). PES1, POLR2A, IPO8, HMBS, SDHA were chosen from the relevant literature and have been validated in postmortem brain tissues34, 35. GAPDH, B2M and ACTB were conventional reference genes. Details are shown in Table 2.
Quantitative real-time reverse transcriptase polymerase chain reaction (RT-qPCR)
The PCR primers and probes (TaqMan Gene Expression Assay) were purchased from Applied Biosystems, Inc. (Carlsbad, CA, USA). Details are shown in Table 2. RT-qPCR reactions were run in 96-well reaction plates with an Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). RT-qPCR was performed with 10 μl cDNA (corresponding to the cDNA reverse transcribed from approximately 50ng RNA) in 20 μl reaction mix containing 10 μl TaqMan Gene Expression Master Mix (2×) and the above-mentioned TaqMan Gene Expression Assays (lyophilized powder). Thermal cycling conditions included 1 cycle at 50 °C for 2 min, 1 cycle at 95 °C for 10 min, followed by 40 cycles of amplification at 95 °C for 15 sec, and 60 °C for 1 min. The threshold cycle (Ct) was calculated by the instrument software automatically (threshold value at 0.2). Raw fluorescent data (normalized reporter values, Rn values) were also exported.
Amplification efficiency calculation
Amplification efficiencies were calculated from raw fluorescent data (Rn values), using a completely objective and noise-resistant algorithm, the Real-time PCR Miner program36 (http://ewindup.info/miner/).
Data analysis
Normalization against validated reference genes
Raw Ct values and calculated amplification efficiencies of these 9 reference genes were imported into the qBaseplus software37. In qBaseplus software, geNorm module was used to identify the most stable reference genes and determine the minimum number of reference genes21.
After determining the minimum number of reference genes, raw Ct values and amplification efficiencies of targets and validated reference genes were imported into the qBaseplus software again. Using a calibrator case (peracute death due to blunt injury, 52-year-old male; 27 h postmortem), calibrated normalized relative quantity (CNRQ) values were exported from the qBaseplus software, and statistically investigated.
Normalization against conventional reference gene (ACTB or GAPDH)
The expression levels of mRNA transcripts are described as the ratios of the targets normalized to the endogenous reference (GAPDH or B2M), using the 2−ΔΔCt method, as the ratios for fold change relative to the above mentioned calibrator. The 2−ΔΔCt method assumes that the amplification efficiency of the reaction is ideal (100%) and constant for each sample.
Immunostaining
The brains were fixed in buffered 4% formaldehyde for 2 weeks and the cerebral hemispheres were cut coronally into 1-cm-thick sections, and the sections of cerebellum and brain stem were also prepared. Paraffin-embedded brain tissue specimens were taken from the standardized anatomical regions. Serial sections (5 μm thick) were cut and stained with hematoxylin-eosin (HE) as part of routine laboratory investigation. In the present study, parietal lobes of left cerebral hemispheres were used for immunostaining.
Mouse monoclonal anti-AQP1 antibody (Abcam, Cambridge, code ab9566, diluted 500-fold), rabbit polyclonal anti-AQP4 antibody (Santa Cruz Biotechnology, Santa Cruz, code sc-20812, diluted 500-fold), rabbit polyclonal anti-CLDN5 antibody (Abcam, Cambridge, code ab53765, diluted 500-fold), rabbit polyclonal anti-MMP2 antibody (Abcam, Cambridge, code ab79781, diluted 100-fold), rabbit polyclonal anti-OCLN antibody (Abcam, Cambridge, code ab168986, diluted 200-fold), rabbit polyclonal anti-ZO1 antibody (Santa Cruz Biotechnology, Santa Cruz, code sc-33725, diluted 500-fold) and rabbit polyclonal anti-MMP9 antibody (Abcam, Cambridge, code ab38898, diluted 800-fold) were used. Following overnight incubation with the primary antibodies described above at room temperature, immunoreactions were visualized by the polymer method (ChemMate Envision, Dako, Tokyo, code k5027) and color was developed with 3,3′-diaminobenzidine tetrahydrochloride (DAB liquid system, Dako, Tokyo, code k3466), according to the manufacturer’s instructions (counterstaining with hematoxylin).
Image J. 1.46 R (NIH, USA) was used to calculate the intensity and extent of staining for the detected molecules. The per-area density of staining was calculated in this manner to reflect the percentage of the positive staining, resulting in a semi-quantitative analysis. A total of 5 microscopic fields were randomly selected, and their images were cropped. The integral optical density (IOD) levels of the stained cells in the tissue samples were then calculated by image analysis. Results were expressed as the mean ± standard deviation (SD) per tissue examined. Using a calibrator case (peracute death due to blunt injury, 52-year-old male; 27 h postmortem), ratios for fold change relative to the calibrator were used for statistical analysis.
Statistic
All the RT-qPCR experiments were performed in triplicate, and results are reported as the mean ± SD. Correlation analyses between pairs of parameters were performed using linear regression (Pearson correlation analysis). The Student’s t test (two-tailed) was used to compare groups. Statistical analyses were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, USA). Values of p < 0.05 were considered as statistically significant.
References
Bouchama, A. & Knochel, J. P. Heat Stroke. N Engl J Med. 346, 1978–1988, doi:10.1056/NEJMra011089 (2002).
Liu, Z. F. et al. Pathological Changes in the Lung and Brain of Mice During Heat Stress and Cooling Treatment. World J Emerg Med. 2, 50–53, doi:10.5847/wjem.j.1920-8642.2011.01.009 (2011).
Palmiere, C. & Mangin, P. Hyperthermia and Postmortem Biochemical Investigations. Int J. Legal Med. 127, 93–102, doi:10.1007/s00414-012-0722-6 (2013).
Woessner, F. J. The Family of Matrix Metalloproteinases. Ann Ny Acad Sci 732, 11–21, doi:10.1111/nyas.1994.732.issue-1 (1994).
Agrawal, S., Lau, L. & Yong, V. MMPs in the Central Nervous System: Where the Good Guys Go Bad. Semin Cell Dev Biol. 19, 42–51, doi:10.1016/j.semcdb.2007.06.003 (2008).
Nagel, S. et al. Minocycline and Hypothermia for Reperfusion Injury After Focal Cerebral Ischemia in the Rat—Effects On BBB Breakdown and MMP Expression in the Acute and Subacute Phase. Brain Res. 1188, 198–206, doi:10.1016/j.brainres.2007.10.052 (2008).
Li, D. D., Song, J. N., Huang, H., Guo, X. Y. & An, J. Y. The Roles of MMP-9/TIMP-1 in Cerebral Edema Following Experimental Acute Cerebral Infarction in Rats. Neurosci Lett. 550, 168–172, doi:10.1016/j.neulet.2013.06.034 (2013).
Chang, C. Y. et al. Disruption of in Vitro Endothelial Barrier Integrity by Japanese Encephalitis virus-Infected Astrocytes. Glia. (2015).
Furuse, M. & Tsukita, S. Claudins in Occluding Junctions of Humans and Flies. Trends Cell Biol. 16, 181–188, doi:10.1016/j.tcb.2006.02.006 (2006).
Jiao, H., Wang, Z., Liu, Y., Wang, P. & Xue, Y. Specific Role of Tight Junction Proteins Claudin-5, Occludin, and ZO-1 of the Blood–Brain Barrier in a Focal Cerebral Ischemic Insult. J. Mol Neurosci. 44, 130–139, doi:10.1007/s12031-011-9496-4 (2011).
Zador, Z., Bloch, O., Yao, X. & Manley, G. T. Aquaporins: Role in Cerebral Edema and Brain Water Balance. Prog Brain Res. 161, 185–194, doi:10.1016/S0079-6123(06)61012-1 (2007).
Manley, G. T. et al. Aquaporin-4 Deletion in Mice Reduces Brain Edema After Acute Water Intoxication and Ischemic Stroke. Nat Med. 6, 159–163, doi:10.1038/72256 (2000).
Badaut, J., Fukuda, A. M., Jullienne, A. & Petry, K. G. Aquaporin and Brain Diseases ☆ ☆☆. Biochimica et Biophysica Acta (BBA) - General Subjects. 1840, 1554–1565, doi:10.1016/j.bbagen.2013.10.032 (2014).
Sharma, H. S. Hyperthermia Induced Brain Oedema: Current Status and Future Perspectives. Indian J. Med Res. 123, 629–652 (2006).
de Jonge, H. J. et al. Evidence Based Selection of Housekeeping Genes. Plos One. 2, e898, doi:10.1371/journal.pone.0000898 (2007).
Narayanan, K. L., Chopra, V., Rosas, H. D., Malarick, K. & Hersch, S. Rho Kinase Pathway Alterations in the Brain and Leukocytes in Huntington’s Disease. Mol Neurobiol. (2016).
Patel, N., Crider, A., Pandya, C. D., Ahmed, A. O. & Pillai, A. Altered mRNA Levels of Glucocorticoid Receptor, Mineralocorticoid Receptor, and Co-Chaperones (FKBP5 and PTGES3) in the Middle Frontal Gyrus of Autism Spectrum Disorder Subjects. Mol Neurobiol. 53, 2090–2099, doi:10.1007/s12035-015-9178-2 (2016).
Lee, S. T. et al. Altered Expression of miR-202 in Cerebellum of Multiple-System Atrophy. Mol Neurobiol. 51, 180–186, doi:10.1007/s12035-014-8788-4 (2015).
Huth, A., Vennemann, B., Fracasso, T., Lutz-Bonengel, S. & Vennemann, M. Apparent Versus True Gene Expression Changes of Three Hypoxia-Related Genes in Autopsy Derived Tissue and the Importance of Normalisation. Int J. Legal Med. 127, 335–344, doi:10.1007/s00414-012-0787-2 (2013).
Wang, Q. et al. Molecular Pathology of Brain Edema After Severe Burns in Forensic Autopsy Cases with Special Regard to the Importance of Reference Gene Selection. Int J. Legal Med. 127, 881–889, doi:10.1007/s00414-013-0868-x (2013).
Vandesompele, J. et al. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 3, H34, doi:10.1186/gb-2002-3-7-research0034 (2002).
Fleige, S. & Pfaffl, M. W. RNA Integrity and the Effect On the Real-Time qRT-PCR Performance. Mol Aspects Med. 27, 126–139, doi:10.1016/j.mam.2005.12.003 (2006).
Zhang, H., Adwanikar, H., Werb, Z. & Noble-Haeusslein, L. J. Matrix Metalloproteinases and Neurotrauma: Evolving Roles in Injury and Reparative Processes. Neuroscientist. 16, 156–170, doi:10.1177/1073858409355830 (2010).
ElAli, A., Doeppner, T. R., Zechariah, A. & Hermann, D. M. Increased Blood-Brain Barrier Permeability and Brain Edema After Focal Cerebral Ischemia Induced by Hyperlipidemia: Role of Lipid Peroxidation and Calpain-1/2, Matrix Metalloproteinase-2/9, and RhoA Overactivation. Stroke. 42, 3238–3244, doi:10.1161/STROKEAHA.111.615559 (2011).
Ludewig, P. et al. Carcinoembryonic Antigen-Related Cell Adhesion Molecule 1 Inhibits MMP-9-mediated Blood-Brain-Barrier Breakdown in a Mouse Model for Ischemic Stroke. Circ Res. 113, 1013–1022, doi:10.1161/CIRCRESAHA.113.301207 (2013).
Rosenberg, G. A., Estrada, E. Y. & Dencoff, J. E. Matrix Metalloproteinases and TIMPs are Associated with Blood-Brain Barrier Opening After Reperfusion in Rat Brain. Stroke. 29, 2189–2195, doi:10.1161/01.STR.29.10.2189 (1998).
Svedin, P., Hagberg, H., Savman, K., Zhu, C. & Mallard, C. Matrix Metalloproteinase-9 Gene Knock-Out Protects the Immature Brain After Cerebral Hypoxia-Ischemia. J. Neurosci. 27, 1511–1518, doi:10.1523/JNEUROSCI.4391-06.2007 (2007).
Yang, Y., Estrada, E. Y., Thompson, J. F., Liu, W. & Rosenberg, G. A. Matrix Metalloproteinase-Mediated Disruption of Tight Junction Proteins in Cerebral Vessels is Reversed by Synthetic Matrix Metalloproteinase Inhibitor in Focal Ischemia in Rat. J Cereb Blood Flow Metab. 27, 697–709, doi:10.1038/sj.jcbfm.9600375 (2007).
Tait, M. J., Saadoun, S., Bell, B. A. & Papadopoulos, M. C. Water Movements in the Brain: Role of Aquaporins. Trends Neurosci. 31, 37–43, doi:10.1016/j.tins.2007.11.003 (2008).
Fukuda, A. M. et al. Delayed Increase of Astrocytic Aquaporin 4 After Juvenile Traumatic Brain Injury: Possible Role in Edema Resolution? Neuroscience. 222, 366–378, doi:10.1016/j.neuroscience.2012.06.033 (2012).
Tourdias, T. et al. Differential Aquaporin 4 Expression During Edema Build-Up and Resolution Phases of Brain Inflammation. J Neuroinflammation 8, 143, doi:10.1186/1742-2094-8-143 (2011).
Wang, Q. et al. Evaluation of Human Brain Damage in Fatalities Due to Extreme Environmental Temperature by Quantification of Basic Fibroblast Growth Factor (bFGF), Glial Fibrillary Acidic Protein (GFAP), S100β and Single-Stranded DNA (ssDNA) Immunoreactivities. Forensic Sci Int. 219, 259–264, doi:10.1016/j.forsciint.2012.01.015 (2012).
Tominaga, M. et al. Postmortem Analyses of Drugs in Pericardial Fluid and Bone Marrow Aspirate. J. Anal Toxicol. 37, 423–429, doi:10.1093/jat/bkt047 (2013).
Koppelkamm, A. et al. Validation of Adequate Endogenous Reference Genes for the Normalisation of qPCR Gene Expression Data in Human Post Mortem Tissue. Int J. Legal Med. 124, 371–380, doi:10.1007/s00414-010-0433-9 (2010).
Wang, Q. et al. Stability of Endogenous Reference Genes in Postmortem Human Brains for Normalization of Quantitative Real-Time PCR Data: Comprehensive Evaluation Using geNorm, NormFinder, and BestKeeper. Int J. Legal Med. 126, 943–952, doi:10.1007/s00414-012-0774-7 (2012).
Zhao, S. & Fernald, R. D. Comprehensive Algorithm for Quantitative Real-Time Polymerase Chain Reaction. J. Comput Biol. (2005).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. QBase Relative Quantification Framework and Software for Management and Automated Analysis of Real-Time Quantitative PCR Data. Genome Biol. 8, R19, doi:10.1186/gb-2007-8-2-r19 (2007).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos 81401556 and 81601641), the Guangdong Natural Science Foundation (Nos 2014A030310504 and 2014A030310293), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (No. 2015-311), and the Special Foundation of President of School of Public Health of Southern Medical University (Grant No. GW201523).
Author information
Authors and Affiliations
Contributions
Y.D., J.-T.X. and H.-N.J. collected the samples, carried out RT-qPCR experiments. D.Z. and R.Z. performed the brain water content and toxicological analyses. S.-H.D., J.-T.X. and Y.X. carried out the immunostaining, statistical analysis and helped to draft the manuscript. Q.W. and X.-L.X. designed the study and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, Y., Xu, JT., Jin, HN. et al. Increased cerebral expressions of MMPs, CLDN5, OCLN, ZO1 and AQPs are associated with brain edema following fatal heat stroke. Sci Rep 7, 1691 (2017). https://doi.org/10.1038/s41598-017-01923-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01923-w
This article is cited by
-
Short-term postmortem interval estimation by detection of apoptosis-related protein in skin
Forensic Science, Medicine and Pathology (2024)
-
Forensic neuropathology in the past decade: a scoping literature review
Forensic Science, Medicine and Pathology (2023)
-
Practical application of a Bayesian network approach to poultry epigenetics and stress
BMC Bioinformatics (2022)
-
Tight junction structure, function, and assessment in the critically ill: a systematic review
Intensive Care Medicine Experimental (2018)
-
CXCL1 and CXCR2 as potential markers for vital reactions in skin contusions
Forensic Science, Medicine and Pathology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.