Abstract
An accurate method to predict the mortality in the intensive care unit (ICU) patients has been required, especially in children. The aim of this study is to evaluate the value of serum anion gap (AG) for predicting mortality in pediatric ICU (PICU). We reviewed a data of 461 pediatric patients were collected on PICU admission. Corrected anion gap (cAG), the AG compensated for abnormal albumin levels, was significantly lower in survivors compared with nonsurvivors (p < 0.001). Multivariable logistic regression analysis identified the following variables as independent predictors of mortality; cAG (OR 1.110, 95% CI 1.06–1.17; p < 0.001), PIM3 [OR 7.583, 95% CI 1.81–31.78; p = 0.006], and PRISM III [OR 1.076, 95% CI 1.02–1.14; p = 0.008]. Comparing AUCs for mortality prediction, there were no statistically significant differences between cAG and other mortality prediction models; cAG 0.728, PIM2 0.779, PIM3 0.822, and PRISM III 0.808. The corporation of cAG to pre-existing mortality prediction models was significantly more accurate at predicting mortality than using any of these models alone. We concluded that cAG at ICU admission may be used to predict mortality in children, regardless of underlying etiology. And the incorporation of cAG to pre-existing mortality prediction models might improve predictability.
Similar content being viewed by others
Introduction
To improve the quality of intensive care unit (ICU) care, mortality prediction is important1. However, estimation of mortality is difficult in critically ill patients whose condition may deteriorate. There are some invasive methods to assess the status of patients, such as pulmonary artery wedge pressure measurement, but these take time to institute and have side effects, such as infection. Recently developed assessments based on physiologic variables have limitations related to the high proportion of missing data, because the variables required are not collected for all patients admitted to ICU or not required for direct patient management1. Therefore it is necessary to identify noninvasive, easy tools for mortality prediction in ICUs, especially for pediatric ICU (PICU) patients.
Anion gap (AG) is traditionally one of the most commonly used biomarkers. It is the simplest means of evaluating the acid-base status of patients, and is calculated from the difference between the measured concentration of serum cations and anions. It helps to identify the presence and causes of metabolic acidosis2, 3. In addition, calculation of an initial serum AG in adult patients admitted to ICU has been suggested as a sensitive and specific tool to predict prognosis or mortality4,5,6. It has been shown that patients with a high AG have increased admission rates to hospital, increased rates of admission to ICU, and increased severity of illness, independent of concomitant electrolyte abnormalities4. An elevated AG is also associated with increased in-hospital mortality compared with patients having a normal AG5, 6. However, there is lack of data related to pediatric patients. In pursuit of better mortality prediction and proper management of patients in PICU, research on AG as a mortality predictor is meaningful.
In this study, we researched several factors associated with mortality in children admitted to PICU. Specifically, we investigated whether serum AG measured at the admission to PICU could be strong predictor of mortality, and what its predictive value is compared with other mortality prediction models.
Results
Patients’ characteristics
From December 2009 to February 2015, a total of 461 pediatric patients were included in this study. Predisposing medical diseases were neurologic problems (222 patients, 48.2%), such as intractable epilepsy or status epilepticus; respiratory problems (82 patients, 17.8%), such as respiratory infection or airway obstruction; and hematologic-oncologic problems (69 patients, 15%), such as neutropenic fever or tumor lysis syndrome. The patients’ baseline characteristics are presented in Table 1. The median age of the patients was 3.1 years and 58.1% (268/461) were male. Overall in-hospital mortality was 20.2% (93/461) with a median lengh of stay (LOS) in PICU of 10 days. A total of 349 children (75.7%) required mechanical ventilation within 24 hours of PICU admission. Overall, 281 (61%) patients had a metabolic acidosis. The most common reason for PICU admission was respiratory failure (51.6%).
Clinical Characteristics of survivors and non-survivors
Patients were divided into survivors and non-survivors on the basis of in-hospital mortality (Table 2). Survivors were younger than non-survivors were (median age, 2.4 vs. 5.8 years). Median LOS in PICU was statistically different between survivors and non-survivors, 9 days and 14 days, respectively. Non-survivors tended to require mechanical ventilation within 24 hours of PICU admission. The two groups were distinct in their reasons for PICU admission. The percentage of children with respiratory failure, neurologic problems, and requiring intensive monitoring was higher in survivors than in non-survivors. Meanwhile, a higher proportion of non-survivor had sepsis, post-resuscitation status, and renal failure. Scoring measurements for mortality prediction used in PICU (PIM2, PIM3, and PRISM III) were statistically different between survivors and non-survivors (p < 0.0001).
More than 60% of patients in both groups had metabolic acidosis, but this had no statistical meaning. According to the summary data for measured and calculated variables, non-survivors had higher levels of sodium and lactate and lower levels of albumin, pH, SBE, and HCO3, compared with survivors. Both AG and cAG at admission were significantly higher in non-survivors than in survivors (p < 0.0001). There were significant differences in the median values of delta neutrophil index (DNI), coagulation indices (platelets and partial thrombin time), and C-reactive protein (CRP) between survivors and non-survivors (p < 0.0001).
Corrected anion gap as a predictor of mortality in PICU patients
In the univariable analyses, 15 variables were correlated with in-hospital mortality (p < 0.01). Of these, 9 variables (age, sex, pH, DNI, platelets, cAG and pre-existing mortality prediction models) were introduced into the multivariable logistic regression analysis. cAG was identified as an independent factor associated with in-hospital mortality [odds ratio (OR) 1.110, 95% confidence interval (CI) 1.06–1.17, p < 0.001]. PIM3 and PRISM III were also found to be independently associated with mortality (Table 3).
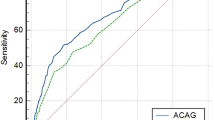
We also considered relationship between cAG at admission and pre-existing mortality prediction models used in PICU. Each model was significantly correlated with cAG, but the correlation coefficients were relatively low. However, receiver operating characteristic (ROC) curves of cAG, PIM 2, PIM3, and PRISM III showed that there were no differences in mortality prediction. The area under the ROC curve (AUC) was 0.728 for cAG, 0.779 for PIM2, 0.822 for PIM3, and 0.808 for PRISM III score (Table 4, Fig. 1).
Receiver operating characteristic (ROC) curves for mortality between corrected anion gap and pre-existing mortality prediction models in PICU. There were no differences between corrected anion gap and other prediction models for mortality. PIM3 showed the best power to predict in-hospital mortality (area under the ROC curve, 0.822 [95% CI, 0.767–0.877]).
The incorporation of cAG to pre-existing mortality prediction models improved their ability to predict mortality (Table 5). The incorporation of cAG to PIM2 gave an NRI of 17.5% (p = 0.001) and IDI of 7.9% (p < 0.001). The PIM3 with cAG provided an NRI of 16.7% (p = 0.002) and IDI of 7.3% (p < 0.001). And the incorporation of cAG to PRISM III yielded an NRI of 9.4% (p = 0.043) and IDI of 5.2% (p < 0.001). All cAG-incorporated models showed good discrimination but, the models of PIM3 with cAG showed was revealed poor calibration in Hosmer–Lemeshow goodness-of-fit test (p = 0.0004) (Supplementary Table S1).
And comparing AUCs between pre-existing models and cAG-incorporated models, all cAG-incorporated models showed improved performance in mortality prediction. But the model of PIM3 with cAG showed no statistical significance as well in AUC comparison, even though AUC of PIM3 with cAG (0.855) was greater than PIM3 (0.822) (Supplementary Table S2). In this study, the best cutoff value of cAG to predict mortality was 18.0 mEq/L (sensitivity 46.2%, specificity 87.0%). Figure 2 shows survival curves based on data from all 461 patients stratified by cutoff value of cAG (cAG < 18.0 vs. cAG ≥ 18.0 mEq/L, p < 0.001 by log-rank test).
Discussion
In this study, serum cAG calculated at the time of PICU admission was higher in non-survivors than in survivors. Increased cAG was associated with in-hospital mortality, and was an independent predictor of mortality in PICU patients. And cAG was correlated with pre-existing mortality prediction models for children. cAG could be a mortality predictor in critically ill children, regardless of the presence of metabolic acidosis or their underlying etiology.
Acid-base derangements are common in critically ill patients. Although the pathogenesis is not fully understood, it is well-known that ongoing acid-base disequilibrium could reflect the severity of disease and is associated with a poor prognosis of the patient7. Many studies have observed a strong association between acidosis and increased organ dysfunction and mortality. However, it remains difficult to ensure accurate measurement of in ICU patients because of their complex and mixed clinical situations of each8, 9.
In recent years, quantitative approaches to acid-base disturbances have been increasingly applied to clinical practice. These aim to give information about unmeasured anions or strong ion differences for quantitative evaluation of acid-base derangements in the ICU10,11,12. Previous studies compared traditional biomarkers, such as pH, base excess, or lactate, as means of assessing acid-base disorders and predicting prognosis in critically ill patients13,14,15,16. However, their reliability has not been established yet. Therefore, reassessing the clinical application of AG, the easiest and most readily available way to calculate acid-base disequilibrium, is useful and meaningful.
AG is a traditional tool used to assess acid-base status and aid the differential diagnosis of metabolic acidosis. Because hypoalbuminemia could affect its interpretation, AG should be corrected for serum albumin level. An elevated cAG usually reflects the presence of metabolic acidosis caused by the overproduction or decreased excretion of organic acids. In addition, elevated cAG has been reported as a predictor of mortality in critically ill patients5, 6, 10, 11. In patients with Streptococcus pneumoniae bacteremia5 and acute myocardial infarction6, the presence of an increased cAG acidosis was associated with increased mortality.
Mortality is considered the most reliable endpoint of clinical management in ICU setting. Therefore, estimating mortality risk is an important of ICU care. Several models are available to predict the risk of death of children in PICU. By keeping these models updated, the clinical meaning and validity of each prediction model have been proved. However, with ongoing revision and updating, these models have become more complicated. They require at least 10 variables to be collected from patients, have different coding rules, and each model relies on complex calculations, making their application difficult1, 17, 18. Therefore, there is an increasing need to establish an easy clinical method for use in PICU settings. Several biomarkers such as DNI19, CRP20, procalcitonin21, thrombocytopenia22 and eosinopenia23 have been suggested for use in PICU.
We conducted this study to test whether cAG at admission can predict mortality or morbidity in PICU patients. Comparing survivors and non-survivors, we determined that an increased cAG at admission to PICU was strongly associated with in-hospital mortality, regardless of the underlying etiology. In terms of the multivariable logistic regression analysis for mortality prediction, cAG at admission was identified as the strongest independent factor associated with in-hospital death. Furthermore, incorporation of cAG at admission to pre-existing mortality prediction models improved their ability of to predict mortality in this study. Considering the complex characteristics of physiology and disease etiologies in children, measurement of cAG at admission to PICU could aid understanding of current status and the clinical outcome of pediatric patients. This study shows that cAG, which is a simple method to calculate, does not require arterial puncture and is readily available, could be a marker of mortality prediction in PICU. The limitation of this study is that data were collected retrospectively. We assessed only the initial cAG at admission to PICU, and obtained the results associated with mortality. And considering incorporation of cAG to pre-existing mortality prediction models, the model of PIM3 with cAG showed good discrimination but poor calibration. There was still a clinical significant improvement in mortality prediction, even if not statistical significant when comparing AUCs between PIM3 and cAG-incorporated PIM3. We think that because predictability of standalone PIM3 is already very powerful and the sample size was relatively small. But owing to the results of NRI, IDI, and an increase in AUC from 0.822 of PIM3 to 0.855 of PIM3 with cAG, cAG is still a useful tool to add on to other mortality prediction models in general. Further studies might be needed as a prospective design in a large multicenter setting to validate the ability of cAG to predict clinical outcome. And serial assessment of cAG could provide much more information about prognosis in PICU.
In conclusion, an elevated cAG at admission was associated with higher mortality in PICU. We suggest that cAG at admission may be used to predict mortality in PICU, regardless of underlying etiology. And the incorporation of cAG to pre-existing mortality prediction models could improve their performance.
Methods
Study population
This was an observational study using data collected retrospectively from the medical records of children admitted to the PICU at Severance Hospital, Seoul, Korea between December 2009 and February 2015. Patients were over 1 month and under 18 years of age and were in the care of the Department of Pediatrics. Patients were admitted to PICU from the emergency department or the general ward. Patients who were discharged or died within 24 hours of PICU admission were excluded. Neonates, patients with cardiac diseases, and patients requiring post-operative care were admitted to and treated in separate specialized units, and were thus excluded from this study.
Data collection
All data were collected and analyzed retrospectively. Blood samples were obtained from indwelling arterial catheters or by venipuncture on admission to PICU. The samples were immediately transported to the central laboratory based in the hospital. Initial arterial blood gases, complete blood cell counts, coagulation indices, serum electrolytes, and levels of albumin were analyzed. The AG was calculated as:
Compensation for abnormal albumin levels was achieving the following equation:
Normal cAG values were defined as 3–12 mEq/L, using the ion-specific electrodes method (Hitachi 747 Manual; Roche Diagnostics, Sydney, NSW, Australia)8. Metabolic acidosis was defined as a standard base excess (SBE) < −2 mEq/L, using following formula7:
Age, sex, in-hospital mortality, length of stay in PICU, underlying etiology, reasons for admission, and requirement for mechanical ventilation within 24 hours of PICU admission were recorded. Concomitant metabolic problems that could affect acid-base status and cAG, such as liver or renal failure, were also recorded. For all patients, Pediatric Index of Mortality (PIM) 218 and PIM 31 were recorded at admission, and the Pediatric Risk of Mortality III (PRISM III)17 was recorded at 24 hours after admission to PICU. PIM and PRISM III scores were calculated on the basis of required variables of each. And then PIM2 and PIM3 were expressed as ‘logit_probability’ based on the logistic regression equation for the derived scores17. But PRISM III was expressed as calculated scores24.
Statistical analysis
Baseline characteristics of patients were compared using Mann-Whitney U test or Fisher’s exact test, as appropriate. Data were reported as numbers (percentages) or medians (inter-quartile range). Groups were compared by the chi-square test or Fisher’s exact test for categorical variables, and the Mann–Whitney U test, as appropriate. Among variables with P < 0.01 in the univariable logistic regression analyses, less than 9 variables without missing values were introduced into multivariable logistic regression analysis. Multivariable logistic regression model was used to identify independent predictors of mortality and to examine the relation between cAG and mortality. The correlation between cAG and pre-existing mortality prediction models was assessed using Spearman’s method. Survival curves were determined using Kaplan-Meyer method, and the differences in survival according to cutoff values of cAG were analyzed by means of the log-rank test. The optimal cutoff value of cAG to predict mortality was chosen by the Youden index and the AUC. Statistical analyses were performed with SPSS 20.0 (SPSS Inc., Chicago, IL).
The predictive values of cAG and PIM2, PIM3, PRISM III for predicting in-hospital mortality were compared by calculating the AUC. Between pre-existing models and cAG-incorporated models (PIM2 with cAG, PIM3 with cAG, and PRISMIII with cAG), Hosmer-Lemeshow goodness-of-fit test for calibration to assess applicability and ROC curve for discrimination and comparisons of AUCs were used by SAS 9.4 (SAS Inc., Cary, NC).
And the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) statistics are introduced to assess the incremental predictive impact of new models that integrate a candidate marker (cAG) to pre-existing models (PIM2, PIM3 or PRISM III)25. Subjects are considered separately who develop and who do not develop events; patients who are dead or survived in this case. For NRI, each subject is assigned to a risk category based on the death probability calculated by the pre-existing mortality prediction models. And then each subject is reassigned to a risk category using new mortality prediction models, constructed by incorporation of cAG to pre-existing mortality prediction models. After calculating the net proportion of subjects with death reassigned to a higher risk category (NRIevemt) and of subjects without events reassigned to a lower risk category (NRInonevent), the NRI is the sum of NRIevent and NRInonevent. And the IDI considers separately the actual change in calculated risk for each individual for those with and those without events. The IDIevent is the difference between the mean probability of a new mortality prediction model for those with the event and the mean probability of the existing models for those with the event. The IDI for those without events (IDInonevent) is the difference in mean probability for those who do not have the event between the reference and new models. The IDI is expressed as the total of IDIevents and IDInonevents. If the value of NRI and IDI are over 0, it means that the performance of new models to predict mortality is improved than existing models in sensitivity and specificity26. NRI and IDI were performed using R software (R version 3.0.1). P < 0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Severance Hospital [Seoul, Korea, IRB No. 4-2012-0369]. All protocols and methods in this study were carried out in accordance with relevant guidelines and regulations. And this study was exempted from the informed consent due to retrospective observational study followed by the institutional review board of Severance Hospital.
References
Straney, L. et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med 14, 673–681, doi:10.1097/PCC.0b013e31829760cf (2013).
Kraut, J. A. & Madias, N. E. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol 2, 162–174, doi:10.2215/CJN.03020906 (2007).
Salem, M. M. & SK, M. Gaps in the anion gap. Arch Intern Med 152, 1625–1629, doi:10.1001/archinte.1992.00400200063011 (1992).
BE, B. Clinical significance of the elevated anion gap. Am J Med 79, 289–296, doi:10.1016/0002-9343(85)90306-7 (1985).
Imran, M. N. et al. Early predictors of mortality in pneumococcal bacteraemia. Ann Acad Med Singap 34, 426–431 (2005).
Sahu, A., Cooper, H. A. & JA, P. The initial anion gap is a predictor of mortality in acute myocardial infarction. Coron Artery Dis 17, 409–412, doi:10.1097/00019501-200608000-00002 (2006).
Adrogué, H. J. & NE, M. Management of life-threatening acid-base disorders. First of two parts. N Engl J Med 338, 26–34, doi:10.1056/NEJM199801013380106 (1998).
Gunnerson, K. J. Clinical review: the meaning of acid-base abnormalities in the intensive care unit part I - epidemiology. Crit Care 9, 508–516, doi:10.1186/cc3796 (2005).
Kaplan, L. J. & Frangos, S. Clinical review: Acid-base abnormalities in the intensive care unit–part. Crit Care 9, 198–203, doi:10.1186/cc2912 (2005).
Kaplan, L. J. & Kellum, J. A. Initial pH, base deficit, lactate, anion gap, strong ion difference, and strong ion gap predict outcome from major vascular injury*. Critical Care Medicine 32, 1120–1124, doi:10.1097/01.CCM.0000125517.28517.74 (2004).
Rocktaeschel, J., Morimatsu, H., Uchino, S. & Bellomo, R. Unmeasured anions in critically ill patients: can they predict mortality? Crit Care Med 31, 2131–2136, doi:10.1097/01.CCM.0000079819.27515.8E (2003).
Morris, C. G. & Low, J. Metabolic acidosis in the critically ill: part 1. Classification and pathophysiology. Anaesthesia 63, 294–301, doi:10.1111/j.1365-2044.2007.05370.x (2008).
Martin, M. et al. Diagnosis of Acid-Base Derangements and Mortality Prediction in the Trauma Intensive Care Unit: The Physiochemical Approach. The Journal of Trauma: Injury, Infection, and Critical Care 58, 238–243, doi:10.1097/01.TA.0000152535.97968.4E (2005).
Fencl, V., Jabor, A., Kazda, A. & J, F. Diagnosis of metabolic acid–base disturbances in critically ill patients. Am J Respir Crit Care Med 162, 2246–2251, doi:10.1164/ajrccm.162.6.9904099 (2000).
Smith, I. et al. Base excess and lactate as prognostic indicators for patients admitted for intensive care. Intensive Care Med 27, 74–83, doi:10.1007/s001340051352 (2001).
Levraout, J. et al. Reliability of anion gap as an indicator of blood lactate in critically ill patients. Intensive Care Med 23, 417–422, doi:10.1007/s001340050350 (1997).
Taori, R. N., Lahiri, K. R. & Tullu, M. S. Performance of PRISM (Pediatric Risk of Mortality) score and PIM (Pediatric Index of Mortality) score in a tertiary care pediatric ICU. Indian J Pediatr 77, 267–271, doi:10.1007/s12098-010-0031-3 (2010).
Slater, A., Shann, F. & Pearson, G., Paediatric Index of Mortality Study, G.. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 29, 278–285, doi:10.1007/s00134-002-1601-2 (2003).
Seok, Y. et al. Delta neutrophil index: a promising diagnostic and prognostic marker for sepsis. Shock 37, 242–246, doi:10.1097/SHK.0b013e3182454acf (2012).
Ho, K. M., Lee, K. Y., Dobb, G. J. & Webb, S. A. C-reactive protein concentration as a predictor of in-hospital mortality after ICU discharge: a prospective cohort study. Intensive Care Med 34, 481–487, doi:10.1007/s00134-007-0928-0 (2008).
Casado-Flores, J. et al. Serum procalcitonin in children with suspected sepsis: a comparison with C-reactive protein and neutrophil count. Pediatr Crit Care Med 4, 190–195, doi:10.1097/01.PCC.0000059420.15811.2D (2003).
Agrawal, S., Sachdev, A., Gupta, D. & Chugh, K. Platelet counts and outcome in the pediatric intensive care unit. Indian Journal of Critical Care Medicine: Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine 12, 102–108, doi:10.4103/0972-5229.43678 (2008).
Abidi, K. et al. Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med 37, 1136–1142, doi:10.1007/s00134-011-2170-z (2011).
Costa, G. A., Delgado, A. F., Ferraro, A. & Okay, T. S. Application of the pediatric risk of mortality (PRISM) score and determination of mortality risk factors in a tertiary pediatric intensive care unit. Clinics 65, 1087–1092, doi:10.1590/S1807-59322010001100005 (2010).
Pencina, M. J., D’Agostino, R. B. S., D’Agostino, R. B. Jr. & Vasan, R. S. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27, 157–172, doi:10.1002/sim.2929, discussion 207–112 (2008).
Pickering, J. W. & Endre, Z. H. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol 7, 1355–1364, doi:10.2215/CJN.09590911 (2012).
Acknowledgements
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (NRF-2015R1D1A1A01061217).
Author information
Authors and Affiliations
Contributions
Kim M.J. contributed to the study design, procedure, analysis, and drafting and revision of the manuscript. Kim Y.H., Sol I.S., Kim S.Y., and Kim J.D. assisted with the procedures and data analysis. Kim H.Y. contributed to analyzed data and reviewed the statistical results. Kim K.W., Sohn M.H., and Kim K.-E. supervised the design, procedures, interpretation of data, drafting, and revision of the manuscript. Kim K.W., as a corresponding author, takes responsibility for the content of the manuscript, including data and analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, M.J., Kim, Y.H., Sol, I.S. et al. Serum anion gap at admission as a predictor of mortality in the pediatric intensive care unit. Sci Rep 7, 1456 (2017). https://doi.org/10.1038/s41598-017-01681-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01681-9
This article is cited by
-
Association between albumin corrected anion gap and 30-day all-cause mortality of critically ill patients with acute myocardial infarction: a retrospective analysis based on the MIMIC-IV database
BMC Cardiovascular Disorders (2023)
-
Serum anion gap is associated with mortality in intensive care unit patients with diastolic heart failure
Scientific Reports (2023)
-
Association between anion gap and mortality of aortic aneurysm in intensive care unit after open surgery
BMC Cardiovascular Disorders (2021)
-
Relation between Baseline Total Serum Cortisol Level and Outcome in Pediatric Intensive Care Unit
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.