Abstract
We assessed the association between depression status and prevalent and incident type 2 diabetes (T2D) as well as the interaction between depression and a genetic risk score (GS) based on 20 T2D single-nucleotide polymorphisms (SNPs) in a multi-ethnic longitudinal study. We studied 17,375 participants at risk for dysglycemia. All participants had genotypic and phenotypic data collected at baseline and 9,930 participants were followed-up for a median of 3.3 years. Normal glucose tolerance (NGT), impaired fasting glucose (IFG)/impaired glucose tolerance (IGT) and T2D statuses were determined using an oral glucose tolerance test and the 2003 American Diabetes Association criteria. Depression was diagnosed at baseline using Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV). Multivariate logistic regression models were adjusted for age, sex, ethnicity and body-mass index and an interaction term GS X depression was added to the model. After appropriate Bonferroni correction, no significant association between depression and T2D-related traits (IFG/IGT, T2D and dysglycemia), and no significant interaction between the GS and depression status was observed at baseline or follow-up. Our longitudinal data do not support an association between depression and abnormal glycemic status. Moreover, depression does not modify the effect of T2D predisposing gene variants.
Similar content being viewed by others
Introduction
Type 2 diabetes (T2D) is a major public health concern as it has turned into a global epidemic1. According to the International Diabetes Federation, 382 million individuals had diabetes in 2013, and this number is projected to reach 592 million in 2035 (http://www.idf.org/). T2D is a lifelong chronic disease that is difficult to treat despite a plethora of available treatments2. In that context, an improved understanding of the etiology of T2D is urgently needed to assist future T2D prevention programs3, 4.
Two meta-analyses have showed that depression increases the risk of incident T2D by 37–60%5, 6. Disturbances in insulin resistance, glucocorticoid signaling and inflammation, adipokine synthesis and secretion, and mitochondrial respiration have been proposed to link depression and subsequent development of T2D7, 8. As such, depression has been referred as ‘metabolic syndrome II’ in the literature7. In addition, weight gain is a side effect of anti-depressant medications and may represent an additional risk factor for T2D9. However, despite these observations, the biological mechanisms that explain how depression leads to T2D are still unclear and require further research.
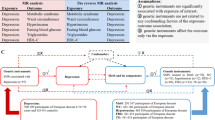
Evidence that the association between depression and T2D results from pleiotropic predisposing genes is sparse and conflicting10, 11. An alternative explanation may be that depression amplifies the effect of T2D genes in predisposed populations. Indirectly supporting this view, Rivera et al. found an interaction between the major genetic contributor to obesity FTO and depression on body mass index (BMI) in European populations12. However, no interaction study between T2D predisposing gene variants and depression has been reported so far. We used a genetic risk score (GS) based on 20 SNPs identified by genome-wide association studies for T2D to investigate the modifying effect of depression on the genetic predisposition to T2D-related traits in a multi-ethnic longitudinal study.
Results
Baseline and follow-up characteristics of depression in cases and controls
Table 1 shows participants characteristics at baseline and follow-up by depression status in the EpiDREAM study. At baseline, a total of 17,375 individuals were included from which 3203 (18.4%) were diagnosed as depressed cases and 14,172 individuals (81.4%) were not. Sixty-one percent of the study participants were female and 75.8% of depressed cases were female. Two thousand five hundred and fifty-eight (14.7%) study participants had T2D at baseline while the majority was classified as having NGT (42.8%) or IFG or IGT (42.5%). Four hundred and twenty-five patients with depression were also diagnosed with T2D (13.3%). Depressed cases were on average two years younger and had a 1.75 kg/m2 higher BMI than non-depressed controls. In terms of ethnic distribution, South Asians and East Asians reported less depression compared to Latin Americans and Native North Americans (Table 1 and Supplementary Table 2). One hundred and seventy-nine (9.7%) participants in the depression group at baseline developed incident IFG/IGT whereas 792 (9.8%) non-depressed controls converted from NGT to IFG/IGT at follow-up. Two hundred and sixty-two (14.2%) participants who reported depression at baseline and 1,008 (12.5%) in the non-depressed group had developed incident T2D at follow-up. The overall distribution of glycemic status was similar in the depression case and control groups.
Association between baseline depression and T2D-related traits at baseline and follow-up
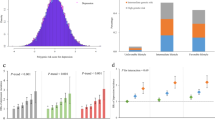
Using a logistic regression model adjusted for sex, age, BMI and ethnicity, depression was not associated with prevalent IFG/IGT (OR = 0.97 [0.89–1.06], P = 0.51), T2D (OR = 1.00 [0.88–1.13], P = 0.96) or dysglycemia (OR = 0.98 [0.90–1.06], P = 0.59) (Table 2). No association was found between baseline depression and incident IFG/IGT (OR = 0.97 [0.80–1.19], P = 0.79), T2D (OR = 1.22 [1.01–1.47], P = 0.04) or dysglycemia (OR = 1.09 [0.94–1.28], P = 0.26) after appropriate Bonferroni correction (Pcorrected = 0.0083 (0.05/6), Table 2).
Interaction between the genotype score and depression on T2D-related traits at baseline and follow-up
Using a logistic regression model adjusted for sex, age, BMI and ethnicity, the GS was associated with IFG/IGT, T2D and dysglycemia statuses at baseline (OR = 1.07 [1.06–1.08] P = 5.9 × 10−26, OR = 1.11 [1.09–1.13] P = 5.9 × 10−31, OR = 1.08 [1.07–1.09] P = 4.7 × 10−38, respectively) and follow-up (OR = 1.05 [1.03–1.08] P = 1.7 × 10−4, OR = 1.14 [1.11–1.17] P = 2.1 × 10−20, OR = 1.10 [1.07–1.12] P = 4.0 × 10−16, respectively, Table 3). No significant interaction between the GS and depression status on T2D-related traits was observed at baseline and follow-up after appropriate Bonferroni correction (Pcorrected = 0.0083 (0.05/6), (Table 3)).
Discussion
We investigated the association between depression at baseline and prevalent and incident abnormal glucose homeostasis in a longitudinal international study that enrolled up to 17,375 subjects from six ethnic groups. Our data do not support a direct association between depression and T2D-related traits at the epidemiological level, keeping in mind that our power calculations do not totally exclude the possibility of a subtle but not clinically meaningful association between depression and abnormal glucose homeostasis (Supplementary Figure 2). Studies have shown that many behaviors and physiological changes associated with depression are risk factors for insulin resistance. This includes lower physical activity levels13, weight change14, and cortisol levels15. However, evidence of association between depression and altered glucose homeostasis has been conflicting in epidemiological studies6, 16.
We also observed an absence of interaction between a T2D GS and depression on T2D-related traits at baseline and at follow up. These interaction tests add to our recent report of a lack of association of T2D predisposing SNPs with depression status at baseline in EpiDREAM10. In contrast, observational and genetic epidemiology studies by us and others have validated the existence of shared biological mechanisms between depression and obesity, an important risk factor for T2D12, 17,18,19. Consistent with this view, the nominal evidence of association observed between baseline depression and incident T2D (OR = 1.22 [1.01–1.47], P = 0.04) was strengthened when BMI was removed as a covariate in the model (OR = 1.32 [1.10–1.59], P = 0.003). Meta-analyses showing an association between depression status and T2D may have been biased by the absence of adjustment for BMI in most included studies5, 6.
Strengths of this study include a large study population including diverse ethnic backgrounds with standardized measures of glycemic and depression statuses. The selection of individuals at risk for dysglycemia increased the power for of the study. In addition, the longitudinal assessment of the glycemic status allows causal inference to be made on the results. Studying the association between depression and glycemic statuses at the epidemiological and genetic level strengthens our conclusions. Limitations of our study include a non-exhaustive list of T2D-associated SNPs that were included in the GS. The ascertainment of the study for individuals at risk for dysglycemia limits the generalization of our results to general populations. We acknowledge that our study was modestly powered for gene by environment interaction studies, especially at follow-up with reduced number of participants, and did not enable the investigation of ethnic-specific associations (Supplementary Figures 2 and 3). In addition the distribution of ethnic groups was not equally represented (Table 1). Our baseline diagnosis for depression is based on the last 12 months and as such does not represent a lifelong depression status. On the other end, a recall bias can possibly be introduced when asking participants about depressive symptoms in the past year. As the questionnaire focuses on major depressive disorder diagnosis, it does not provide a quantitative indication on the severity of the depressive episode. It also does not provide an indication of the duration of the depressive episode. We are also aware that the reliability of the major depressive disorder based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM IV) in our study is not perfect. Every method of diagnosing psychiatric disorders has its shortcomings as the core of psychopathology relies on subjective reporting of symptoms in addition to observable traits during illness episodes. As an illustration, the reliability of diagnosis of depression based on structured clinical interviews for DSM (SCID) has been questioned in literature20, 21.
In conclusion, our longitudinal data do not support an association between depression and abnormal glycemic status and genetic predisposition to T2D is not modified by the presence of depression.
Methods
Participants
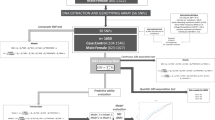
EpiDREAM is a longitudinal study that enrolled 24,872 individuals from 21 countries who were at risk for T2D, including subjects who participated in the DREAM clinical trial22. All individuals who were deemed to be at risk for dysglycemia defined by family history, ethnicity and abdominal obesity, between the ages of 18–85 years, were screened using a 75 gram oral glucose tolerance test (OGTT) from July 2001 to August 2, 2003. Detailed methods and description of the study cohort and inclusion/exclusion criteria have been described earlier23, 24. We included 17,375 subjects from six ethnic groups (East Asian, South Asian, European, African, Latin American, Native North American) who have both phenotypic and genotypic information available at baseline (Supplementary Figure 1). Self-reported ethnicity has been validated in the 17,375 individuals using the eigenstrat software (http://genepath.med.harvard.edu/~reich/Software.htm). Samples that failed to cluster with individuals of the same self-reported ethnicity were removed. Of these 17,375 individuals, 9,930 have been prospectively followed for a median of 3.3 years. The EpiDREAM and DREAM studies were approved by local ethics committees. All experimental protocols were approved by McMaster University and were performed in accordance with relevant guidelines and regulations of McMaster University. Written informed consent was obtained from each subject prior to participation in the EpiDREAM or DREAM studies, in accordance with the Declaration of Helsinki.
Genotyping
DNA was successfully extracted from buffy coats in 19,498 participants of the EpiDREAM study using the Gentra System (Supplementary Figure 1). Genotyping was performed using the Illumina cardiovascular gene-centric bead chip ITMAT Broad Care (IBC) array25. Genotyping was performed at the McGill University and Genome Quebec Innovation Centre using the Illumina Bead Studio genotyping module, version 3.2. We established a list of SNPs that reached genome-wide significance (P < 5 × 10−8) for association with T2D status in populations of European ancestry. We used three different strategies to optimize the SNP selection procedure using a key word search on i) the National Human Genome Research Institute (NHGRI) GWAS Catalog (www.genome.gov/gwastudies/) ii) the HuGE Navigator GWAS Integrator (www.hugenavigator.net/HuGENavigator/gWAHitStartPage.do) iii) the PubMed database (www.ncbi.nlm.nih.gov/pubmed). Using this strategy, we found 93 independent SNPs associated with T2D in literature. From this list, 20 autosomal SNPs were available on versions 1 and 2 of the IBC 50 K SNP array (Supplementary Table 1), as reported in our previous paper10. We double checked the availability of proxies using SNAP and their chromosomal position in the Illumina data file, as previously reported by our team26. We did not find additional SNPs for inclusion. The 20 SNPs include: rs1260326 (GCKR), rs2943634 (IRS1), rs1801282 (PPARγ2), rs1470579 (IGF2BP2), rs1801214 (WFS1), rs7754840 (CDKAL1), rs1799884 (GCK), rs13266634 (SLC30A8), rs2383208 (CDKN2A/B), rs5015480 (HHEX), rs7903146 (TCF7L2), rs231362 (KCNQ1), rs2283228 (KCNQ1), rs5219 (KCNJ11), rs10830963 (MTNR1B), rs4430796 (HNF1B), rs12454712 (BCL2), rs16996148 (GATAD2A), rs8108269 (GIPR), and rs1884614 (HNF4A). SNPs obeyed Hardy-Weinberg equilibrium (HWE) within each ethnic group in the overall sample as well as in the depressed and non-depressed subgroups (P ≥ 0.00014 (0.05/(6 × 20 × 3)), Supplementary Table 1), with the exception of one SNP. We detected a significant departure from HWE for rs1884614 (HNF4A) in the Latin American group, but not in the other ethnic groups (Supplementary Table 1). This suggests that the HWE identified in the Latin American group for this SNP likely did not result from genotyping errors but was the consequence of the admixed nature of this population. Therefore, all the SNPs were included in the study. The call rate for each of the 20 SNPs was between 99.33 and 100% (Supplementary Table 1).
Phenotyping
Demographic data as well as direct anthropometric measurements were obtained from study participants using a standardized protocol. Height (m) and weight (kg) were measured in clinical centers by trained staff. BMI was calculated as weight in kilograms (kg) divided by height in meters (m) squared. The 2003 ADA criteria were used to classify participants as having normal glucose tolerance (NGT), impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or T2D at baseline, following an oral glucose tolerance test (OGTT). Normoglycemia was defined as a fasting plasma glucose <5.6 mmol/L, IFG was defined as a fasting plasma glucose of 5.6 to 6.9 mmol/L, IGT was defined as a fasting plasma glucose below 7.0 mmol/L and a 2-h glucose between 7.8 and 11.0 mmol/L, and diabetes was defined if either the fasting plasma glucose was ≥7.0 mmol/L or the 2-h glucose was ≥11.1 mmol/L27. At follow-up, glycemic status was ascertained using i) an OGTT conducted at the final exam; or ii) a diagnosis made by a participant’s physician during the course of the study, confirmed by participant’s use of an oral hypoglycaemic agent, and an OGTT. Subjects with IFG, IGT or T2D were considered as having dysglycemia.
The assessment of a major depressive episode in the past 12 months was performed at baseline. Interviews were conducted face to face by research personnel who were not mental health specialists but were research assistants trained in the study procedures and administration of the questionnaires, including inter rater reliability to calibrate the training. A structured case report form was completed in which participants were asked: “During the past 12 months, was there ever a time when you felt sad, blue, or depressed for 2 weeks or more in a row?”. If the participant answered yes, then the following yes or no questions were asked: (a) Lose interest in most things like hobbies, work or activities that usually give you pleasure?; (b) Feel tired or low on energy?; (c) Gain or lose weight?; d) Have more trouble falling asleep than you usually do?; (e) Have more trouble concentrating than usual?; (f) Think a lot about death (either your own, someone else’s, or death in general)?; (g) Feel down on yourself, no good or worthless? These questions were compiled to fulfil DSM IV28 diagnostic criteria for a depressive episode. A depressive episode was considered present if the individual had five or more of the above symptoms including depressed mood that were present for at least 2 weeks nearly every day for most of the day in the last 12 months.
Statistical analyses
All statistical analyses were performed using the SPSS 14.0 software. The comparison of baseline characteristics between depression cases and controls was done using T-tests or Chi-square tests. We coded genotypes as 0, 1 and 2, depending on the number of copies of the T2D risk alleles. A GS was calculated by summing the alleles of the 20 T2D predisposing SNPs. We used an unweighted GS as recommended by Dudbridge29. Missing genotype values were imputed using the method of the mean for each SNP individually in each ethnicity separately using the arithmetic average of the coded genotypes observed for all the successfully genotyped individuals. The association of GS with T2D-related traits was tested using a logistic regression model. Regression models were adjusted for age, sex, BMI, ethnicity/population stratification and depression and a GS by depression status interaction term was added to the model. Ethnicity and population stratification were accounted for using the first 10 axes of variation from Principal Components Analysis, determined using EIGENSTRAT30. Applying a Bonferroni corrected P-value across all the outcomes reduces the chance of making type I errors, but increases the chance of making type II errors. Therefore, we applied a separate Bonferroni correction to each research question31. A P-value of less than 0.0083 was considered statistically significant when analyses of associations between baseline depression and T2D-related traits at baseline and follow-up were performed as well as when interactions between baseline depression, and the GS on T2D-related traits at baseline and follow-up were performed (6 analyses in total).
References
Bonnefond, A. & Froguel, P. Rare and common genetic events in type 2 diabetes: what should biologists know? Cell metabolism 21, 357–368, doi:10.1016/j.cmet.2014.12.020 (2015).
Kahn, S. E., Cooper, M. E. & Del Prato, S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383, 1068–1083, doi:10.1016/S0140-6736(13)62154-6 (2014).
Karam, J. G. & McFarlane, S. I. Update on the prevention of type 2 diabetes. Current diabetes reports 11, 56–63, doi:10.1007/s11892-010-0163-x (2011).
Alyass, A. et al. Modelling of OGTT curve identifies 1 h plasma glucose level as a strong predictor of incident type 2 diabetes: results from two prospective cohorts. Diabetologia. doi:10.1007/s00125-014-3390-x (2014).
Knol, M. J. et al. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 49, 837–845 (2006).
Mezuk, B., Eaton, W. W., Albrecht, S. & Golden, S. H. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes care 31, 2383–2390, doi:10.2337/dc08-0985 (2008).
McIntyre, R. S. et al. Should Depressive Syndromes Be Reclassified as “Metabolic Syndrome Type II”? Annals of clinical psychiatry: official journal of the American Academy of Clinical Psychiatrists 19, 257–264, doi:10.1080/10401230701653377 (2007).
Kan, C. et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 36, 480–489, doi:10.2337/dc12-1442 (2013).
Blumenthal, S. R. et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiatry 71, 889–896, doi:10.1001/jamapsychiatry.2014.414 (2014).
Samaan, Z. et al. Lack of association between type 2 diabetes and major depression: epidemiologic and genetic evidence in a multiethnic population. Transl Psychiatry 5, e618, doi:10.1038/tp.2015.113 (2015).
Kan, C. et al. Genetic overlap between type 2 diabetes and depression in Swedish and Danish twin registries. Mol Psychiatry, 10.1038/mp.2016.28 (2016).
Rivera, M. et al. Depressive disorder moderates the effect of the FTO gene on body mass index. Mol Psychiatry. Jun; 17(6), 604–11 (2012).
Steptoe, A., W, J., Fuller, R., Holte, A., Justo, J., Sanderman, R. & Wichstrom Laakso, M. Leisure-time physical exercise: Prevalence, attitudinal correlates, and behavioral correlates among young Europeans from 21 countries. Prev Med 26, 845–854 (1997).
Christensen, L. & S., S. Comparison of nutrient intake among depressed and nondepressed individuals. Int J Eat Disorder 20, 105–109 (1996).
Zahn, D. et al. Cortisol, platelet serotonin content, and platelet activity in patients with major depression and type 2 diabetes: an exploratory investigation. Psychosomatic medicine 77, 145–155, doi:10.1097/PSY.0000000000000145 (2015).
Nouwen, A. et al. Prevalence of depression in individuals with impaired glucose metabolism or undiagnosed diabetes: a systematic review and meta-analysis of the European Depression in Diabetes (EDID) Research Consortium. Diabetes Care 34, 752–762 (2011).
Samaan, Z. et al. The protective effect of the obesity-associated rs9939609 A variant in fat mass- and obesity-associated gene on depression. Mol Psychiatry 18, 1281–1286, doi:10.1038/mp.2012.160 (2013).
Samaan, Z. et al. Obesity genes and risk of major depressive disorder in a multiethnic population: a cross-sectional study. The Journal of clinical psychiatry 76, e1611–1618, doi:10.4088/JCP.14m09720 (2015).
Afari, N. et al. Depression and obesity: do shared genes explain the relationship? Depress Anxiety 27, 799–806 (2010).
Williams, J. B. et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Archives of general psychiatry 49, 630–636 (1992).
Regier, D. A. et al. DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. Am J Psychiatry 170, 59–70, doi:10.1176/appi.ajp.2012.12070999 (2013).
Dream, I. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia 47, 1519–1527 (2004).
Gerstein, H. C., Yusuf, S., Holman, R., Bosch, J. & Pogue, J. Rationale, design and recruitment characteristics of a large, simple international trial of diabetes prevention: the DREAM trial. Diabetologia 47, 1519–1527 (2004).
Anand, S. et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: the EpiDREAM cohort study. European journal of preventive cardiology 19, 755–764 (2011).
Keating, B. J. et al. Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS One 3, e3583 (2008).
Robiou-du-Pont, S., Li, A., Christie, S., Sohani, Z. N. & Meyre, D. Should we have blind faith in bioinformatics software? Illustrations from the SNAP web-based tool. PLoS One 10, e0118925, doi:10.1371/journal.pone.0118925 (2015).
ADA. Diagnosis and classification of diabetes mellitus. Diabetes Care 27, Suppl 1, S5–S10 (2004).
APA. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision DSM-IV-TR. Washington DC: American Psychiatric Association, 1–982 (2000).
Dudbridge, F. Power and predictive accuracy of polygenic risk scores. PLoS Genet 9, e1003348, doi:10.1371/journal.pgen.1003348 (2013).
Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38, 904–909, doi:10.1038/ng1847 (2006).
Feise, R. J. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2, 8 (2002).
Acknowledgements
We thank Hudson Reddon for his technical assistance in power figure generation. Sonia S. Anand holds the Heart and Stroke Foundation of Ontario, the Michael G. DeGroote endowed Chair in Population Health and a Canada Research Chair in Ethnicity and Cardiovascular Disease, Hertzel C. Gerstein holds the McMaster-Aventis Population Health Research Institute Chair in Diabetes Research and Care, and David Meyre holds a Canada Research Chair in Genetics of Obesity.
Author information
Authors and Affiliations
Contributions
S.G., Z.S. and D.M. designed research; S.G., Z.S., H.C.G., J.C.E., V.M., R.D., S.S.A. and D.M. conducted research; H.C.G., J.C.E., V.M., R.D., and S.S.A. provided essential materials; S.G. and D.M. analyzed data; S.G., Z.S. and D.M. wrote the manuscript; H.C.G., J.C.E., V.M., R.D. and S.S.A. critically reviewed the manuscript for important intellectual content; D.M. had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garasia, S., Samaan, Z., Gerstein, H.C. et al. Influence of depression on genetic predisposition to type 2 diabetes in a multiethnic longitudinal study. Sci Rep 7, 1629 (2017). https://doi.org/10.1038/s41598-017-01406-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01406-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.