Abstract
Candida species are commensals but some develop biofilms in prosthetic materials and host surfaces that may represent up to 30% of deaths related to infections, particularly in immunosuppressed patients. Tumor necrosis factor (TNF) exhibits a plethora of functions in host defense mechanisms whereas excessive release of TNF in inflammation promotes tissue damage. Cytokines released in an inflammatory milieu may influence the development of microorganisms either by promoting their growth or displaying antimicrobial activity. In protozoa, TNF may affect growth by coupling through a lectin-like domain, distinct from TNF receptors. TNF was also shown to interact with bacteria via a mechanism that does not involve classical TNF receptors. Using an in vitro C. albicans biofilm model, we show that TNF dose-dependently prevents biofilm development that is blocked by incubating TNF with N,N’-diacetylchitobiose, a major carbohydrate component of C. albicans cell wall. This finding represents a relevant and hitherto unknown mechanism that adds to the understanding of why TNF blockade is associated with opportunistic C. albicans infections.
Similar content being viewed by others
Introduction
Biofilms are the major state that microorganisms utilize in their struggle to thrive since antimicrobials act against their planktonic, free-floating, state. Development of aggregates formed by the microorganism and an extracellular coat of secreted components and even host parts leads to biofilm formation1. In biofilm development, features related to the microorganism and to the extracellular polymeric substances that compose its environment are relevant2.
Patients subjected to immunosuppression as well as those exposed to implanted devices and indwelling catheters are particularly affected by life-threatening systemic infections. Organization in biofilms after adherence to those devices offers to microorganisms an alternative to evade host defense. Candida albicans account for a major part of those opportunistic infections with a mortality rate that can reach 40% of affected individuals, with obvious health and economic impacts3.
There is extensive knowledge on the role of cytokines in human defense against microbes. Usually, cytokines are synthesized and released by host cells after being triggered by diverse stimuli. Analogous to other cytokines, TNF acts on mammalian cells via coupling to specific membrane or soluble receptors, leading to cell activation. Excessive TNF release during inflammation is associated to pain development, cell infiltration, hypotension, hepatotoxicity, and structural damage to tissues4,5,6. Thus, targeting TNF has become an alternative to treat autoimmune diseases, including rheumatoid arthritis and inflammatory bowel disease. Considering that TNF has a major protective role against microorganisms, anti-TNF treatment would render patients prone to develop life-threatening infections4.
To the best of our knowledge, TNF was never shown to directly provoke changes in fungal development without the participation of mammalian cells. However, there is evidence that TNF induces trypanosome lysis through a TNF receptor independent mechanism7. We investigated whether TNF could alter C. albicans in vitro growth. Our data, showing a previously unrecognized TNF interference with C. albicans biofilm formation, unravels a protective role of TNF against systemic, life-threatening opportunistic infections. In addition to the importance in the pathogenesis of Candida infections, this finding does also offer an alternative to prevent yeast biofilm formation.
Results
TNF alters C. albicans biofilm metabolism
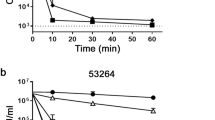
Adding increasing concentrations of TNF to the growing biofilm of two strong biofilm producer strains of C. albicans (ATCC 10.2.31; CEMM 01-005-006) significantly and dose-dependently inhibited the metabolic activity of the yeast biofilm, measured by the 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)- 2H-tetrazolium-5-carboxanilide (XTT) assay (Fig. 1a). The assay evaluates yeast metabolic ability to reduce XTT leading to a water-soluble formazan-colored product. The results, originally designed to evaluate Candida biofilms, provide a semiquantitative estimation of the metabolic activity of the biofilm8. On the other hand, TNF solutions that affected growing C. albicans biofilm did not alter mature biofilm metabolic activity (Fig. 1b), suggesting that the cytokine has no effect in an established biofilm. Further, adding TNF to the same C. albicans strains in a free-living, planktonic condition, did not influence yeast growth so that readings obtained adding TNF were similar to those after incubation solely with medium (RPMI). As a control of antifungal activity, amphotericin B significantly impaired C. albicans planktonic growth (data not shown).
Effect of TNF on biofilm formation by C. albicans. Murine tumor necrosis factor (mTNF ng/mL) or medium (−) were added to growing (a) or mature (b) biofilms of two strong biofilm producers C. albicans strains. Biofilm metabolic activity was assessed using the XTT reduction assay. Data represent the mean ± SEM (Absorbance492nm) of two independent experiments conducted in triplicate; *P < 0.001 as compared to medium.
TNF alteration of yeast morphology
Morphological evaluation and blastoconidia filamentation (yeast to hyphal development) of C. albicans TNF-treated biofilms is shown in Fig. 2a–g. TNF addition to C. albicans growing biofilm led to a decreased filamentation after 24 h of incubation. The appearance, as well as the filamentation index, after 6 h of incubation (Fig. 2a,b) is similar in TNF-treated and untreated biofilms, whereas 24 h and 48 h after incubation there was a significant reduction in the number of hyphae, thus suggesting a TNF interference with yeast filamentation and biofilm maturation (Fig. 2d,f,g). On the other hand, untreated C. albicans biofilm showed a clear formation of branched hyphae (Fig. 2c,e). Increasing TNF concentrations apparently impaired the formation of true hyphae, so that only blastoconidia and pseudohyphae were observed under scanning electron microscopy, while untreated biofilms demonstrated a typical, dense structure, with yeast, pseudohyphae, and hyphae (Fig. 3a,d).
Effect of TNF on the morphology of preformed C. albicans biofilm. Illustrations of lactophenol cotton blue dye stained C. albicans ATCC 10.2.31 growing biofilms treated with TNF (20 ng/mL) (b,d,f) or untreated (a,c,e) after 6, 24 or 48 h incubation (Original x200); (g) C. albicans ATCC 10.2.31 growing biofilms were treated with mTNF (ng/mL) or untreated (none) for 6, 24 or 48 h. Filamentation index represents the percentage of hyphae/yeast measured in 10 high power fields, under inverted optical microscopy.
Effects of anti-TNF compounds
Although Candida species have not been shown to display classic TNF receptors, we wondered whether TNF inhibitors would alter that TNF effect on Candida biofilms. Preincubation of TNF with the humanized monoclonal anti-TNF antibody adalimumab inhibited TNF effect on growing biofilms, whereas adding adalimumab without TNF actually increased biofilm metabolic activity, when compared to TNF-untreated biofilm (Fig. 4a). On the other hand, pre-incubation with the IgG coupled soluble TNF receptor etanercept did not alter TNF effect whereas adding etanercept without TNF significantly decreased biofilm metabolic activity, similar to incubation with TNF alone. Incubation with irrelevant immunoglobulin did not alter TNF effect (Fig. 4a,b).
Anti-TNF compounds effect on C. albicans biofilm. Human TNF (hTNF ng/mL), the anti-TNF monoclonal antibody Adalimumab (ADA ng/mL), the IgG coupled soluble TNF-receptor Etanercept (ETA ng/mL), human immunoglobulin G (IgG) or (−) Medium were added isolated or combined to C. albicans ATCC 10.2.31 growing biofilm. The biological activity was assessed using the XTT reduction assay. Data represent the mean ± SEM Absorbance492nm of two independent experiments conducted in triplicate; *P < 0.001 as compared to medium.
XTT results display variability between assays. However, we should call attention to the fact that adding murine or human TNF to growing C. albicans biofilms consistently and reproducibly decreased biofilm metabolic activity. We may speculate that conformational changes or steric hindrance account for adalimumab blockade of that TNF activity. As expected, incubation with etanercept, presumably blocking TNF access specifically to its classic receptor, did not interfere with Candida biofilm thus arguing that the cytokine effect depended on binding via its lectin-like domain. Similar data were shown in fluid reabsorption using in situ and in vivo flooded rat lungs where TNF activation of lung liquid clearance via its lectin-like domain was inhibited by the anti-TNF antibody infliximab but not by a soluble TNF receptor9. However, the inhibition and improvement in biofilm formation provided by etanercept and adalimumab, respectively, are hard to explain. In clinical practice, it has been shown that anti-TNF monoclonal antibodies and etanercept may differ regarding efficacy to treat acute anterior uveitis in patients with ankylosing spondylitis10. More recently, the administration of either a monoclonal anti-TNF antibody or a soluble TNF receptor increased mice susceptibility to hematogeneously disseminated candidiasis whereas only the monoclonal antibody increased susceptibility to oropharyngeal candidiasis11. Differences in the glycan pattern on therapeutic proteins, including adalimumab and etanercept, affect their binding to lectins and biological activity12, opening the possibility of such a mechanism to explain this apparently controversial result.
A specific oligosaccharide blocks TNF effect
N,N′-diacetylchitobiose, a carbohydrate present in C. albicans cell wall, was previously shown to block TNF trypanosomicidal activity7, 13. Further, β-glucan induced TNF release from macrophages was inhibited by N,N′-diacetylchitobiose showing that the lectin-like domain, which is distinct from TNF residues that bind TNF receptors, is responsible for that cytokine effect14. D-glucose, N-acetyl-D-glucosamine (GlcNAc), and D-mannose are major carbohydrates in Candida cell wall. Polymers of GlcNAc are called chitin and aggregate to glucans (glucose polymers) strengthening the fungus cell wall. A chitobiose is formed by GlcNAc molecules and provides linkage of asparagine to glucan residues in the cell wall. Those carbohydrates are very relevant to yeast metabolism. For instance, the structure of the septum during hyphae formation is formed by chitin. In addition, bioses rather than monosaccharides, as competitors, are considered more relevant to demonstrate a role of carbohydrates in yeast physiology13. Preincubation of TNF with N,N′-diacetylchitobiose abrogated the cytokine inhibition of biofilm formation, restoring yeast filamentation, a step where carbohydrates participate in Candida growth. This was a clear and significant effect shown whether using the XTT assay or biofilm morphological analysis (Fig. 5a–i). It has to be remarked that addition of isolated N,N′-diacetylchitobiose did not modify biofilm formation. Addition of cellobiose, a disaccharide that does not bind to TNF lectin-like domain7, or the monosaccharides mannose or GlcNAc, two other major components of Candida cell wall, also did not alter TNF activity (Fig. 5h,i). Similarly, incubation with other nonspecific carbohydrates (D-xylose, arabinose, glucose) did not impair TNF activity on biofilm growth (data not shown).
N,N-diacetylchitobiose blocks TNF effect on C. albicans biofilm. (a,b) N,N-diacetylchitobiose (Chi µg/mL) isolated or combined with hTNF or mTNF (ng/mL) or medium (−) was added to C. albicans ATCC 10.2.31 biofilm (metabolic activity − XTT assay); optical microscopy of biofilms grown with medium (c), medium + mTNF 20 (d), medium + mTNF (20) + Chi (100) (e), or medium + Chi (100) (f) for 48 h (Original x200); (g) filamentation index of biofilms after 48 h growth with medium, medium + hTNF (20), medium + hTNF + Chi (100) or medium + Chi (100); (i) metabolic activity after treatment with cellobiose (Cel), mannose (Man) or N-Acetylglucosamine (GlcNAc). Data represent the mean ± SEM Absorbance492nm of two independent experiments conducted in triplicate; *P < 0.001. Filamentation index represents the percentage of hyphae/blastoconidia in 10 hpf (Original x200).
Discussion
The present findings, showing an as yet unrecognized mechanism of TNF interference with C. albicans biofilm growth are relevant both as the description of a physiological, TNF receptor independent mechanism of this cytokine in host defense against C. albicans as well as the opening of a potential strategy to prevent in vivo Candida biofilm growth. TNF activity is commonly linked to specific membrane receptors coupling, triggering the activation of downstream cellular pathways thereby inducing the transcription of genes associated with the inflammatory response. Planktonic, free growing C. albicans was not affected by TNF leading us to speculate the involvement of a mechanism interfering with the extracellular biofilm matrix. This TNF mechanism is particularly relevant to dissect host barriers against life-threatening infections caused by opportunistic agents. In addition to the reduction of the metabolic activity of growing C. albicans biofilms treated with TNF, there was a marked reduction in hyphae filamentation, most likely interfering with cell division. C. albicans biofilm formation, which is under a complex gene network regulation, comprises adherence to a surface, followed by proliferation, growth of pseudohyphae and true hyphae, and a later dispersal phase3, 15. Extracellular matrix accumulates during biofilm maturation, being essential to its resistance to antimicrobials2, 15. Mechanisms involved in TNF inhibition of biofilm growth may include alteration of gene expression or direct interference with extracellular matrix formation. The fact that TNF concentrations used in the present study resemble those used in other in vitro TNF studies on mammalian cells indicates that the obtained results likely are clinically relevant.
Trypanosome species, which do also lack classic TNF receptors, are susceptible to lysis via a TNF domain with a lectin-like activity. Substitutions in the amino acid sequence encompassing the Thr104 to Glu109 positions of murine(m) TNF or Thr105 to Glu110 of human(h) TNF altered that lectin-like activity7. Additionally, a synthetic peptide bearing the specific TNF sequence that mimics the lectin domain prevented tissue damage in an in vivo lung inflammatory model16.
TNF is one of the cytokines operating both in innate and adaptive immune mechanisms and our results were similar regardless of using mTNF or hTNF. Other cytokines may also operate similarly. Interleukin(IL)-17 was recently shown to promote C. albicans and Aspergillus fumigatus planktonic and biofilm growth, activating various genes, rendering those fungi less susceptible to antimicrobials17.
Limitations of our study include the use of an in vitro biofilm model, as it may not reflect in vivo systems. However, patients exposed to treatment with etanercept were reported to have increased susceptibility to oral candidiasis18 and it was recently shown that TNF protected mice from disseminated C. tropicalis infection19. An in depth approach to possible mechanisms to explain TNF effect on yeast metabolism and biofilm structure is also needed to better evaluate the clinical relevance of the present data. Institutionalized patients as well as those needing implanted devices and prosthetic material are seriously affected by systemic, resistant, multigerm infections, commonly due to biofilm formation in implanted materials. Lining of implant materials with bioactive components is a current prophylactic strategy to prevent infections, rendering the development of compounds reproducing TNF domain with that lectin activity to halt C. albicans biofilm growth plausible.
Methods
Microorganisms
ATCC 10.2.31 and CEMM 01.05.006C. albicans strains from the collection of the Specialized Medical Mycology Center (CEMM, Federal University of Ceará, Brazil19, 20).
Reagents
TNF solutions were prepared at the time of use using RPMI 1640 supplemented with L-glutamine. Recombinant rat TNF-α (mTNF) and human Tumor Necrosis Factor-α (hTNF) were purchased from R&D Systems and Sigma-Aldrich, São Paulo, Brazil, respectively. Other reagents were purchased from Sigma-Aldrich do Brasil Ltda., SP, Brazil. Adalimumab and Etanercept were from Abbvie Farmaceutica and Laboratórios Pfizer Ltda., São Paulo, Brazil, respectively.
In vitro susceptibility testing
Susceptibility of C. albicans to TNF was determined using the broth microdilution method (Protocol M27-A3; The Clinical and Laboratory Standards Institute - CLSI). Amphotericin B was a drug control.
In vitro biofilm assay
TNF solutions were prepared using buffered RPMI 1640. Biofilms were grown on 96-well microtitre plates. Fungal suspensions containing approximately 0.25 × 103CFU mL−1 in RPMI 1640 supplemented or not with TNF solutions, added at the start of the experiment, were incubated for 48 h, at 37 °C to evaluate TNF effect in growing biofilms. In order to investigate TNF effect on mature (preformed) biofilms, fungal suspensions containing approximately 0.25 × 103CFU mL−1 in RPMI 1640 were incubated for 48 h, at 37 °C, medium was then aspirated, nonadherent cells removed by thoroughly washing with sterile buffered PBS and wells were then filled with RPMI supplemented or not with TNF solutions and incubated for an additional period of 48 h, at 37 °C. Biofim formation was calculated using XTT (2,3-Bis-(2-Methoxy-4-Nitro-5-Sulfophenyl)- 2H-Tetrazolium-5-Carboxanilide) assay8, performed after either 48 h (growing biofilm) or 96 h (preformed biofilm) of incubation. XTT was added and samples were incubated at 36 °C for 5 h using an orbital shaker. After staining, plates were read at 492 nm.
Biofilm morphological analysis
After 6, 24 or 48 h incubation, plates were stained with lactophenol cotton blue dye and photographed under an inverted light microscope (Fisher Scientific™). The number of filamentous (hyphae) and yeast (blastoconidia) in 10 high power fields (hpf; Original x200) was calculated to obtain a filamentation index: Filamentation = number hyphae/total cell counts (hyphae and blastoconidia) ×100.
Ultra structural analysis
Biofilms were fixed with Karnovsky solution, dehydrated in graded ethanol, dried in hexamethyldisilazane, coated with gold and observed in an Inspect S50™ scanning electron microscope.
Statistical analysis
Data are means ± SEM of at least 3 replicates for each treatment, analyzed using one-way ANOVA, followed by Tukey’s test.
References
Bjarnsholt, T., Ciofu, O., Molin, S., Givskov, M. & Høiby, N. Applying insights from biofilm biology to drug development - can a new approach be developed? Nat. Rev. Drug Discov. 12, 791–808 (2013).
Flemming, H. C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Nobile, C. J. & Johnson, A. D. Candida albicans biofilms and human disease. Annu. Rev. Microbiol. 69, 71–92 (2015).
Kalliolias, G. D. & Ivashkiv, L. B. TNF biology, pathogenic mechanisms and emerging therapeutic strategies. Nat. Rev. Rheumatol. 12, 49–62 (2016).
Mann, D. L. Stress-activated cytokines and the heart: from adaptation to maladaptation. Annu. Rev. Physiol. 65, 81–101 (2003).
Streetz, K. et al. Tumor necrosis factor alpha in the pathogenesis of human and murine fulminant hepatic failure. Gastroenterology 119, 446–460 (2000).
Lucas, R. et al. Mapping the lectin-like activity of tumor necrosis factor. Science 263, 814–817 (1994).
Pierce, C. G. et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 3, 1494–1500 (2008).
Braun, C., Hamacher, J., Morel, D. R., Wendel, A. & Lucas, R. Dichotomal role of TNF in experimental pulmonary edema reabsorption. J. Immunol. 175, 3402–3408 (2005).
Wu, D. et al. Efficacy of anti-tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: a meta-analysis. BMC Musculoskelet. Disord. 16, 19 (2015).
Park, H. et al. Different tumor necrosis factor α antagonists have different effects on host susceptibility to disseminated and oropharyngeal candidiasis in mice. Virulence 5, 625–629 (2014).
Zhang, L., Luo, S. & Zhang, B. The use of lectin microarray for assessing glycosylation of therapeutic proteins. MAbs. 8, 524–535 (2016).
Masuoka, J. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin. Microbiol. Rev. 17, 281–310 (2004).
Olson, E. J., Standing, J. E., Griego-Harper, N., Hoffman, O. A. & Limper, A. H. Fungal beta-glucan interacts with vitronectin and stimulates tumor necrosis factor alpha release from macrophages. Infect. Immun. 64, 3548–3554 (1996).
Finkel, J. S. & Mitchell, A. P. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 9, 109–118 (2011).
Hartmann, E. K. et al. Inhalation therapy with the synthetic TIP-like peptide AP318 attenuates pulmonary inflammation in a porcine sepsis model. BMC Pulm. Med. 15, 7–17 (2015).
Zelante, T. et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat. Commun. 3, 683–692 (2012).
Ford, A. C. & Peyrin-Biroulet, L. Opportunistic infections with antitumor necrosis factor-a therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 108, 1268–1276 (2013).
Whibley, N. et al. Delinking CARD9 and IL-17: CARD9 protects against Candida tropicalis infection through a TNF-α–dependent, IL-17–independent mechanism. J. Immunol. 195, 3781–3792 (2015).
Sidrim, J. J. et al. β-Lactam antibiotics and vancomycin inhibit the growth of planktonic and biofilm Candida spp.: an additional benefit of antibiotic-lock therapy? Int. J. Antimicrob. Agents. 45, 420–423 (2015).
Acknowledgements
This work was supported by grants 302218/2014-9 and 459334/2014-0 from CNPq (Conselho Nacional de Desenvolvimento e Tecnológico - Brasil). Authors are grateful to Central Analítica - UFC/CT-INFRA/MCTI-SISNANO/Pró-Equipamentos for support with SEM.
Author information
Authors and Affiliations
Contributions
F.A.C.R., M.F.G.R., R.A.C., R.S.M.B., V.C.C.G., J.J.C.S. - conception of the protocol; A.M.C.V.A., R.A.C., A.C.M.D.P., R.M.N. - biofilm analysis; R.A.C., R.S.M.B., V.C.C.G. – SEM; F.A.C.R., M.F.G.R., J.J.C.S. - data analysis; F.A.C.R., A.M.C.V.A., M.F.G.R., J.J.C.S. - wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rocha, F.A.C., Alves, A.M.C.V., Rocha, M.F.G. et al. Tumor necrosis factor prevents Candida albicans biofilm formation. Sci Rep 7, 1206 (2017). https://doi.org/10.1038/s41598-017-01400-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01400-4
This article is cited by
-
Biofilm-associated genes as potential molecular targets of nano-Fe3O4 in Candida albicans
Pharmacological Reports (2023)
-
Possible Molecular Targeting of Biofilm-Associated Genes by Nano-Ag in Candida albicans
Applied Biochemistry and Biotechnology (2023)
-
Mannich base limits Candida albicans virulence by inactivating Ras-cAMP-PKA pathway
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.