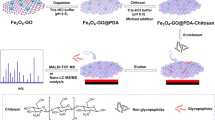
Abstract
Highly efficient enrichment of glycopeptides or phosphopeptides from complex biological samples is indispensable for high-throughput mass spectrometry analysis. In this study, for the first time, a “one for two” hydrophilic magnetic amino-functionalized metal-organic framework (MOF) was designed and synthesized for selective enrichment of both glycopeptides and phosphopeptides. A well-known solvo-thermal reaction was adopted to prepare a magnetic core Fe3O4, followed by self- polymerization of dopamine, creating a polydopamine (PDA) onto Fe3O4. Thanks to the hydroxyl and amino group of PDA, Zr3+ was easily adhered to the surface, inducing the following one-pot MOF reaction with amino ligand. After characterization of the as-prepared MOFs (denoted as Fe3O4@PDA@UiO-66-NH2), its ultrahigh surface area, excellent hydrophilicity and strong magnetic responsiveness were highly confirmed. Based on hydrophilic interaction, it was applied to glycopeptide enrichment, while based on strong binding between Zr and phosphopeptides, it was applied to phosphopeptide enrichment, both exhibiting excellent performance in standard proteins and human serum with high sensitivity and selectivity. These results showed the as-prepared MOFs had great potential in proteomics research.
Similar content being viewed by others
Introduction
Protein glycosylation and phosphorylation, two of the most important post-translational modifications (PTMs), play a critical role in many biological processes, such as signal transduction1, cell-cell interaction2, cell adhesion3, and so on. As abnormal phosphorylation or glycosylation serves as the biomarkers for several types of cancers and diseases4, 5, it is of great importance to identify the certain sites, and the corresponding proteins. To dates, Mass spectrometry (MS) has become one of the most common tools to identify glycosylation and phosphorylation because of its high throughput, fast speed and high sensitivity6,7,8. Unfortunately, due to the low abundance of peptides and poor ionization efficiency9, 10, direct analysis still remains a big challenge. Herein, enrichment of glycopeptides and phosphopeptides from the complicated bio-samples before MS analysis is an indispensable step.
Various strategies have been proposed to glycopeptide or phosphopeptide enrichment, including boronic acid affinity chromatography11, 12, hydrazine chemistry13, hydrophilic interaction chromatography (HILIC)14, 15, lectin affinity chromatography16, and size exclusion17 for glycopeptides, while immobilized metal affinity chromatography (IMAC)18, metal oxide affinity chromatography (MOAC)19, immunoprecipitation20 and ion-exchange chromatography21 for phosphopeptides. Among them, HILIC materials, including metal-organic frameworks22, monoliths23, and nanoparticles24, have been regarded as the most potential materials for glycopeptide enrichment with excellent performance, and could be applied to phosphopeptide enrichment based on IMAC techniques, which aroused great interest and popularity due to handy operation, simple enrichment process, low bias to different types of peptides, and mild conditions for MS analysis25. However, few people have thought of creating only one material, which could be used for two purposes. (“One for Two”) It brought us to view of finding a novel hydrophilic material, which could be applied to not only glycopeptide enrichment, but also phosphopeptide enrichment, combining with IMAC, that is to say “One for Two”.
In the past decades, metal-organic frameworks (MOFs), a fascinating class of porous materials, have gained much popularity as a tool of separation and enrichment due to its high surface area, adjustable pore size and easy-to-functionalize26. MOFs, which are consisted of metal ions and organic ligands, have been employed in separation27, gas adsorption, drug delivery28, and so on. Recently, metal–organic frameworks have been applied to proteomics, such as, endogenous peptide enrichment29, glycopeptide enrichment30, 31, enzyme immobilization32, etc. For this reason, a new spot hit our head to create a promising material, combining the hydrophilic interaction, IMAC techniques and MOF, further applied to glycoproteome and phosphoproteome analysis. TiO2 and Ti (IV)-IMAC materials have been reported before33, and it made a very meaningful and attractive work. With only one material, enrichment of both glycopeptides and phosphopeptides could be performed. Inspired by this beautiful research, developing a novel magnetic MOFs to enrich both glycopeptides and phosphopeptides was catchy. As reported, MOFs was endowed with huge surface area, adjustable pore size and easy-to-synthesize and functionalize so it would be meaningful to develop such a material for two purposes.
Herein, to improve the enrichment performance, reduce the trouble in synthesis and cut the time in separation, a facile route was first proposed for preparation of a “One for Two” hydrophilic magnetic amino-functionalized metal-organic framework by modifying UiO-66-NH2 (Zr-MOF) onto the polydopamine (PDA)-coated magnetic microspheres (Fe3O4@PDA@UiO-66-NH2). Zr was confirmed that it had strong binding with phosphopeptides and the ligand with amino functioned could be applied to glycopeptide enrichment via hydrophilic interaction. As a core-shell-shell structure, MOFs with 2-aminoterephthalic acid (denoted as H2BDC-NH2) as the ligand were easily synthesized and with a hydrophilic surface. The as-prepared MOFs exhibited strong magnetic responsiveness and excellent hydrophilicity and strong binding between Zr and phosphopeptides, so a promising future for excellent performance in glycopeptide and phosphopeptide enrichment could be anticipated.
Results
Characterization of Fe3O4@PDA@UiO-66-NH2
Transmission electron microscopy (TEM) and Scanning electron microscopy (SEM) were used here to confirm the microstructure of Fe3O4@PDA@UiO-66-NH2. Figure 1a shows the SEM images of Fe3O4@PDA, exhibiting a thin polymer-shell outside the spherical Fe3O4 microspheres. After modified with UiO-66-NH2, the surface of as-prepared MOFs is crystallized and distinct from the smooth surface of Fe3O4@PDA. EDX analysis of Fe3O4@PDA@UiO-66-NH2 further confirmed the existence of Zr. (Supporting Information Figure S2) TEM image (Fig. 1c) of Fe3O4@PDA@UiO-66-NH2 shows that MOF shell is grafted onto the Fe3O4@PDA nanoparticles and PDA@MOF shell-shell is around 70 nm with the Fe3O4 core unchanged. TEM image (Fig. 1d) further exhibits the tiny pore from the magnified MOF shell, which is consistent with the 3.11 nm of the pore size from the BET analysis results. The surface area is 136.36 m2/g, which is extremely large based on the rough Fe3O4 core (Supporting Information Figure S3).
Magnetic responsiveness is crucial for time-saving and simple application in the following enrichment process. Herein, magnetic core is introduced inside the hydrophilic MOFs and magnetic properties of Fe3O4, Fe3O4@PDA and Fe3O4@PDA@UiO-66-NH2 were measured via SQUID magnetometer. (Supporting Information Figure S4) As expected, although the core-shell-shell MOFs exhibited decreasing magnetization values when compared to Fe3O4, it still showed superparamagnetic properties with no coercivity. Apart from magnetic responsiveness, hydrophilicity is another merit of this MOF. After dissolving in DI water, it can maintain as a homogeneous solution for a relatively long time (Supporting Information Figure S5).
FT-IR spectra (Supporting Information Figure S6) and Raman spectra (Supporting Information Figure S7) were recorded for Fe3O4, Fe3O4@PDA, and Fe3O4@PDA@UiO-66-NH2 respectively and showed obvious characteristic peaks of Fe-O-Fe vibration (560 cm−1) and specific groups, like –COOH (3400 cm−1), aromatic rings (1500–1600 cm−1 in Raman), etc.
Wide-angle X-ray diffraction patterns of Fe3O4@PDA@UiO-66-NH2 were recorded to confirm the structure of magnetic MOFs (Supporting Information Figure S8). Diffraction peaks at 2θ = 5.2, 7.0, 12.3, 18.2 and 22.3° were from the UiO-66-MOF, while 2θ = 30.3, 35.4, 43.2, 57.2 and 63.0° were from the Fe3O4 lattice. Zeta potential measurements were operated in ethanol solution (Supporting Information Figure S9). The values dropped first and then went up, which was ascribed to the negative acidic catechol hydroxyl groups and MOFs modification.
Enrichment of glycopeptides and phosphopeptides from standard proteins using Fe3O4@PDA@UiO-66-NH2
The workflow of glycopeptide or phosphopeptide enrichment is illustrated in Figure S1. The ability of the as-prepared hydrophilic magnetic MOFs for glycopeptide enrichment was investigated by using HRP digests and human IgG digests. As shown in Fig. 2a, before enrichment, the signals of N-glycopeptides were overwhelmed by the non-glycopeptides. Notably, after selective enrichment, the intensity of glycopeptides was significantly increased and 19 glycopeptides could be observed, anticipating an excellent enrichment performance. What’s more, enrichment of IgG digests also exhibited excellent performance with 21 glycopeptides identified. (Figure 2c,d) Detailed information of glycopeptide of HRP and IgG digests could be found in the Supporting Information Table S1 and Table S2. The ability of the as-prepared hydrophilic magnetic MOFs for phosphopeptide enrichment was investigated by using β-Casein digest. As shown in Fig. 2e,f, after enrichment, the peaks of phosphopeptides appeared with high intensities and a clear background. Detailed information of phosphopeptide of β-Casein digests could be found in the Supporting Information Table S3.
MALDI-TOF mass spectra for the glycopeptide enrichment from 250 fmol/μL HRP tryptic digest: (a) before enrichment, and (b) after treatment; from 1 pmol/μL IgG: (c) before enrichment, and (d) after treatment; from 200 fmol/μL β-Casein: (e) before enrichment, and (f) after treatment, where glycopeptides were marked with Arabic Numerals in red, phosphopeptides were marked with Arabic Numerals in green, and Δ indicates the losses of phosphoric acid.
HRP (β-Casein) was chosen as the standard glycoprotein (phosphoprotein) to prove the properties of Fe3O4@PDA@UiO-66-NH2 in the following analysis. The reusability (Supporting Information Figure S10) and stability (Supporting Information Figure S11) of the hydrophilic magnetic MOFs were also investigated. The spectrum of 250 fmol/μL HRP tryptic digest and 200 fmol/μL β-Casein using the five-times-recycled MOFs and the MOFs stored at −20 °C for a month showed almost the same enrichment performance as that using the first-time freshly-prepared MOFs, exhibiting the excellent reusability and stability.
To further confirm the excellent capacity and sensitivity of the MOFs, different concentrations of HRP digests and β-Casein digests were applied to the MOFs. As shown in Fig. 3, with the decreasing concentration (25, 5, 1, and 0.2 fmol/μL) of HRP and (25, 1, 0.2, 0.02 fmol/μL) of β-Casein, several glycopeptides or phosphopeptides could still be identified after enrichment. Even though the concentration of HRP was as low as 0.2 fmol/μL, two characteristic peaks of glycopeptides dominated the spectrum (Fig. 3d), exhibiting a relatively lower detection limit while compared to previous reports30. Through the different concentrations from high to ultra-low, by calculation, the maximum capacity of MOFs towards HRP (glycoproteins) was 4 mg/g, down to 0.8 μg/g; while the maximum capacity of MOFs towards β-Casein (phosphoproteins) was 0.8 mg/g, down to 0.08 μg/g.
MALDI-TOF mass spectra for the glycopeptide enrichment from HRP tryptic digest: (a) 25 fmol/μL, (b) 5 fmol/μL, (c) 1 fmol/μL, and (d) 0.2 fmol/μL; for the phosphopeptide enrichment from β-Casein tryptic digest: (e) 25 fmol/μL, (f) 1 fmol/μL, (g) 0.2 fmol/μL, and (h) 0.02 fmol/μL, where glycopeptides were marked with Arabic Numerals in red, phosphopeptides were marked with Arabic Numerals in green, and Δ indicates the losses of phosphoric acid.
The selectivity of Fe3O4@PDA@UiO-66-NH2 toward glycopeptides and phosphopeptides was investigated via the mixture of HRP (β-Casein) and BSA tryptic digests. With a mass ratio of 1:50 (HRP:BSA), as shown in Fig. 4a, glycopeptides could hardly be identified after direct analysis. However, after enrichment, most glycopeptides dominated the spectra with a relatively high signal intensity. With an increasing ratio as 1:100, although the absolute intensity decreased, the relative intensity of glycopeptides showed the high selectivity of the MOFs for capturing glycopeptides. For phosphopeptides, the ratio could be as high as 1:500 (β-Casein:BSA) with outstanding performance. Due to the instability of MALDI TOF 5800, all the experiments should be done at the same time in theory. However, it was unrealistic so all the experiments were performed under same experimental conditions. The recovery was confirmed here and almost no non-glycopeptides appeared after enrichment. (Supporting Information Figure S12) What’s more, the recovery of the MOFs was further confirmed through a parallel test, around 94% (Supporting Information Figure S13).
MALDI-TOF mass spectra for the glycopeptide enrichment from a mixture of HRP and BSA at a mass ratio of 1:50: (a) before enrichment, and (b) after enrichment; 1:100: (c) before enrichment, and (d) after enrichment; for the phosphopeptide enrichment from a mixture of β-Casein and BSA at a mass ratio of 1:500: (e) before enrichment, and (f) after enrichment, where glycopeptides were marked with Arabic Numerals in red, phosphopeptides were marked with Arabic Numerals in green, and Δ indicates the losses of phosphoric acid.
Enrichment of glycopeptides and phosphopeptides from human serum using Fe3O4@PDA@UiO-66-NH2
Inspired by the excellent enrichment performance above, the as-prepared MOFs were applied to the enrichment of glycopeptides and phosphopeptides from human serum digest and analyzed by LC-MS/MS and MALDI respectively. According to the procedure, the eluted glycopeptides were deglycosylated by PNGase F and analyzed by Nano-HPLC-MS/MS. The human Uniprot-SwissProt database (release 2015_03_11, with 20199 entries) was chosen as a database, and a total of 307 N-glycosylation peptides from 121 different glycoproteins were identified, showing excellent and promising potential in glycoproteome research. Detailed information could be found in Supporting Information Table S4. For phosphopeptides, four endogeneous phosphopeptides and one peak of loss of phosphoric acid could be identified from the spectrum (Fig. 5) and the detailed information of phosphopeptides from human serum was listed in Supporting Information Table S5. From the human serum digests, a total of 33 phosphopeptides from 16 different phosphoproteins were identified, apart from the endogenous phosphopeptide enrichment from human serum without additional pretreatment, it became a breakthrough of phosphopeptide enrichment from human serum digests. Detailed information could be found in Supporting Information Table S6.
Discussion
To dates, Mass spectrometry (MS) has become one of the most common tools to identify protein glycosylation because of its high throughput, fast speed and high sensitivity. Unfortunately, due to the low abundance of glycopeptides and phosphopeptides and poor ionization efficiency, direct analysis of certain peptides still remains a big challenge. Herein, enrichment of glycopeptides and phosphopeptides from the complicated bio-samples before MS analysis is an indispensable step.
A new spot hit our head to create a promising hydrophilic MOF material, which could be applied to not only glycopeptide enrichment, but also phosphopeptide enrichment, combining with IMAC, that is to say “One for Two”.
Herein, a facile route was first proposed for preparation of a “One for Two” hydrophilic magnetic amino-functionalized metal-organic framework by modifying UiO-66-NH2 (Zr-MOF) onto the polydopamine (PDA)-coated magnetic microspheres (Fe3O4@PDA@UiO-66-NH2). Based on hydrophilic interaction, it was applied to glycopeptide enrichment, while based on strong binding between Zr and phosphopeptides, it was applied to phosphopeptide enrichment. Fe3O4 (strong magnetic responsiveness), PDA (excellent biological amphiphilicity) and MOFs (high surface area) are combined here so the as-prepared material is deemed to enrich glycopeptides and phosphopeptides with high sensitivity and selectivity.
A magnetic core Fe3O4 was easily prepared, followed by self- polymerization of dopamine, creating a polydopamine (PDA) onto Fe3O4. Thanks to the hydroxyl and amino group of PDA, Zr3+ was easily adhered to the surface, inducing the following one-pot MOF reaction with amino ligand. Owing to its large surface area, strong magnetic responsiveness, high selectivity and sensitivity, it showed great potential in glycoproteome and phosphoproteome research, exhibiting excellent performance with high sensitivity (0.2 fmol/μL) and selectivity (1:100) for glycopeptide, while 0.02 fmol/μL and 1:500 for phosphopeptide. In addition, the novel as-prepared MOFs was successfully applied to complex biological samples, like human serum (with 307 N-glycosylation peptides from 121 different glycoproteins, 33 phosphopeptides from 16 different phosphoproteins and four endogenous phosphopeptides identified), revealing its promising application in glycopeptide and phosphopeptide enrichment.
Methods
Synthesis of Fe3O4@PDA@UiO-66-NH2
A facile route for Fe3O4@PDA@UiO-66-NH2 is proposed in Fig. 6. The magnetic microspheres were prepared via a well-known solvo-thermal reaction34. Then, the magnetic particles were coated with a PDA layer through the polymerization of dopamine in basic atmosphere. The obtained PDA coated magnetic particles (denoted as Fe3O4@PDA) were dispersed in N,N-dimethylformamide (DMF), containing ZrCl4 and 2-aminoterephthalic acid as MOFs precursors. Then the mixed solution was heated under 120 °C for 45 min. Finally, Fe3O4@PDA@UiO-66-NH2 was successfully prepared by this simple one-pot reaction.
Characterization and Measurements
Scanning electron microscopy (SEM), energy dispersive X-ray (EDX), transmission electron microscopy (TEM), fourier transform infrared spectra (FT-IR), raman spectra (Raman), magnetization measurement, powder X-ray diffraction patterns (XRD), magnetization measurements and nitrogen sorption isotherms and zeta potential measurements were measured and detailed information were illustrated in SI.
Enrichment of glycopeptides from standard glycoproteins and human serum
In brief, 200 μg of Fe3O4@PDA@UiO-66-NH2 was added into 100 μL standard peptide mixture and the solution was incubated at 37 °C for 30 min. 90%ACN/1%TFA was chosen as the loading buffer here to establish a hydrophobic environment. After washing by the loading buffer and 80%ACN/1%H3PO4 to remove the non-glycopeptides30, the captured glycopeptides were eluted by 30%ACN/0/1%FA at 37 °C for 20 min and then analyzed by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). For human serum, after the same enrichment and elution process, the elution was lyophilized first and then redissolved in 25 mM NH4HCO3 solution and incubated with 1 μL PNGase F at 37 °C overnight. After lyophilization, it was transferred to nano high performance liquid chromatography- mass spectrometry (Nano-HPLC-MS/MS) analysis. The protocol of LC-MS/MS analysis could be found in Supporting Information in detail.
Enrichment of phosphopeptides from standard phosphoproteins and human serum
The enrichment steps are the same as glycopeptide enrichment except for the loading and washing buffer were replaced by 50%ACN/0.1%TFA and the elution was replaced by 0.4 M NH3·H2O. For human serum, after the same enrichment and elution process, the elution was lyophilized first and then transferred to nano high performance liquid chromatography- mass spectrometry (Nano-HPLC-MS/MS) analysis.
References
Ohtsubo, K. & Marth, J. D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 126, 855–867, doi:10.1016/j.cell.2006.08.019 (2006).
Lowe, J. B. Glycosylation, Immunity, and Autoimmunity. Cell 104, 809–812, doi:10.1016/S0092-8674(01)00277-X (2001).
Gudelj, I. et al. Changes in total plasma and serum N-glycome composition and patient-controlled analgesia after major abdominal surgery. Sci Rep 6, 31234, doi:10.1038/srep31234 (2016).
Hang, Q. et al. N-Glycosylation of integrin α5 acts as a switch for EGFR-mediated complex formation of integrin α5β1 to α6β4. Sci Rep 6, 33507, doi:10.1038/srep33507 (2016).
Jansen, B. C. et al. MALDI-TOF-MS reveals differential N-linked plasma- and IgG-glycosylation profiles between mothers and their newborns. Sci Rep 6, 34001, doi:10.1038/srep34001 (2016).
Min, Q. et al. Magnetite/Ceria-Codecorated Titanoniobate Nanosheet: A 2D Catalytic Nanoprobe for Efficient Enrichment and Programmed Dephosphorylation of Phosphopeptides. Acs Appl Mater Inter 7, 9563–9572, doi:10.1021/acsami.5b01006 (2015).
Schulz, B. L., Packer, N. H. & Karlsson, N. G. Small-Scale Analysis of O-Linked Oligosaccharides from Glycoproteins and Mucins Separated by Gel Electrophoresis. Anal. Chem. 74, 6088–6097, doi:10.1021/ac025890a (2002).
Yan, Y., Zhang, X. & Deng, C. Designed Synthesis of Titania Nanoparticles Coated Hierarchially Ordered Macro/Mesoporous Silica for Selective Enrichment of Phosphopeptides. Acs Appl Mater Inter 6, 5467–5471, doi:10.1021/am500412v (2014).
Li, F. et al. GlycoMinestruct: a new bioinformatics tool for highly accurate mapping of the human N-linked and O-linked glycoproteomes by incorporating structural features. Sci Rep 6, 34595, doi:10.1038/srep34595 (2016).
Prinz, B. et al. Hierarchical phosphorylation of apical membrane antigen 1 is required for efficient red blood cell invasion by malaria parasites. Sci Rep 6, 34479, doi:10.1038/srep34479 (2016).
Xu, Y. et al. Highly Specific Enrichment of Glycopeptides Using Boronic Acid-Functionalized Mesoporous Silica. Anal. Chem. 81, 503–508, doi:10.1021/ac801912t (2009).
Zhang, L. et al. Boronic Acid Functionalized Core–Satellite Composite Nanoparticles for Advanced Enrichment of Glycopeptides and Glycoproteins. Chem. Eur. J 15, 10158–10166, doi:10.1002/chem.v15:39 (2009).
Patel, T. et al. Use of hydrazine to release in intact and unreduced form both N- and O-linked oligosaccharides from glycoproteins. Biochem 32, 679–693, doi:10.1021/bi00053a037 (1993).
Ruhaak, L. R. et al. Hydrophilic Interaction Chromatography-Based High-Throughput Sample Preparation Method for N-Glycan Analysis from Total Human Plasma Glycoproteins. Anal. Chem. 80, 6119–6126, doi:10.1021/ac800630x (2008).
Seo, P. W., Bhadra, B. N., Ahmed, I., Khan, N. A. & Jhung, S. H. Adsorptive Removal of Pharmaceuticals and Personal Care Products from Water with Functionalized Metal-organic Frameworks: Remarkable Adsorbents with Hydrogen-bonding Abilities. Sci Rep 6, 34462, doi:10.1038/srep34462 (2016).
Wang, Z.-G., Lv, N., Bi, W.-Z., Zhang, J.-L. & Ni, J.-Z. Development of the Affinity Materials for Phosphorylated Proteins/Peptides Enrichment in Phosphoproteomics Analysis. Acs Appl Mater Inter 7, 8377–8392, doi:10.1021/acsami.5b01254 (2015).
Alvarez-Manilla, G. et al. Tools for Glycoproteomic Analysis: Size Exclusion Chromatography Facilitates Identification of Tryptic Glycopeptides with N-linked Glycosylation Sites. J Proteome Res 5, 701–708, doi:10.1021/pr050275j (2006).
Sun, W. et al. Attenuation of synaptic toxicity and MARK4/PAR1-mediated Tau phosphorylation by methylene blue for Alzheimer’s disease treatment. Sci Rep 6, 34784, doi:10.1038/srep34784 (2016).
Yao, J., Sun, N., Deng, C. & Zhang, X. Designed synthesis of Graphene @titania @mesoporous silica hybrid material as size-exclusive metal oxide affinity chromatography platform for selective enrichment of endogenous phosphopeptides. Talanta 150, 296–301, doi:10.1016/j.talanta.2015.12.050 (2016).
Zhang, G. & Neubert, T. A. Use of detergents to increase selectivity of immunoprecipitation of tyrosine phosphorylated peptides prior to identification by MALDI quadrupole-TOF MS. Proteomics 6, 571–578, doi:10.1002/pmic.200500267 (2006).
Miquel, E., Alegría, A., Barberá, R. & Farré, R. Speciation analysis of calcium, iron, and zinc in casein phosphopeptide fractions from toddler milk-based formula by anion exchange and reversed-phase high-performance liquid chromatography-mass spectrometry/flame atomic-absorption spectroscopy. Anal. Bioanal. Chem. 381, 1082–1088, doi:10.1007/s00216-004-3002-6 (2005).
Ji, Y. et al. Efficient enrichment of glycopeptides using metal-organic frameworks by hydrophilic interaction chromatography. Analyst 139, 4987–4993, doi:10.1039/c4an00971a (2014).
Chen, M., Lu, Y., Ma, Q., Guo, L. & Feng, Y.-Q. Boronate affinity monolith for highly selective enrichment of glycopeptides and glycoproteins. Analyst 134, 2158–2164, doi:10.1039/b909581k (2009).
Oh, E. et al. Nanoparticle-Based Energy Transfer for Rapid and Simple Detection of Protein Glycosylation. Angew. Chem. Int. Ed. 45, 7959–7963, doi:10.1002/anie.200601948 (2006).
Wan, S. et al. The “Sweet” Side of the Protein Corona: Effects of Glycosylation on Nanoparticle–Cell Interactions. ACS Nano 9, 2157–2166, doi:10.1021/nn506060q (2015).
Song, W.-C., Cui, X.-Z., Liu, Z.-Y., Yang, E.-C. & Zhao, X.-J. Light-triggered Supramolecular Isomerism in a Self-catenated Zn(II)-organic Framework: Dynamic Photo-switching CO2 Uptake and Detection of Nitroaromatics. Sci Rep 6, 34870, doi:10.1038/srep34870 (2016).
Li, J.-R., Kuppler, R. J. & Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 38, 1477–1504, doi:10.1039/b802426j (2009).
Lee, J. et al. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 38, 1450–1459, doi:10.1039/b807080f (2009).
Xie, Y. & Deng, C. Highly efficient enrichment of phosphopeptides by a magnetic lanthanide metal-organic framework. Talanta 159, 1–6, doi:10.1016/j.talanta.2016.05.075 (2016).
Zhang, Y.-W. et al. A facilely synthesized amino-functionalized metal-organic framework for highly specific and efficient enrichment of glycopeptides. Chem. Commun. 50, 11504–11506, doi:10.1039/c4cc05179c (2014).
Zhu, X. et al. Metal–Organic Frameworks with Boronic Acid Suspended and Their Implication for cis-Diol Moieties Binding. Adv. Funct. Mater. 25, 3847–3854, doi:10.1002/adfm.v25.25 (2015).
Zhao, M., Zhang, X. & Deng, C. Rational synthesis of novel recyclable Fe3O4@MOF nanocomposites for enzymatic digestion. Chem. Commun. 51, 8116–8119, doi:10.1039/C5CC01908G (2015).
Wang, J., Wang, Y., Gao, M., Zhang, X. & Yang, P. Facile synthesis of hydrophilic polyamidoxime polymers as a novel solid-phase extraction matrix for sequential characterization of glyco- and phosphoproteomes. Anal Chim Acta 907, 69–76, doi:10.1016/j.aca.2015.12.015 (2016).
Sun, S. & Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 124, 8204–8205, doi:10.1021/ja026501x (2002).
Acknowledgements
This work was supported by the National Basic Research Priorities Program (2012CB910602, 2013CB911201), the National Natural Science Foundation of China (21425518, 21075022, 21275033, 21105016), Research Fund for the Doctoral Program of Higher Education of China (20110071110007 and 20100071120053).
Author information
Authors and Affiliations
Contributions
Yiqin Xie and Chunhui Deng designed the experiments. Yiqin Xie performed the experiments and analysed the data. Yiqin Xie wrote the manuscript, and Chunhui Deng contributed with comments.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41598_2017_1341_MOESM1_ESM.pdf
Designed synthesis of a “One for Two” hydrophilic magnetic amino-functionalized metal-organic framework for highly efficient enrichment of glycopeptides and phosphopeptides
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, Y., Deng, C. Designed synthesis of a “One for Two” hydrophilic magnetic amino-functionalized metal-organic framework for highly efficient enrichment of glycopeptides and phosphopeptides. Sci Rep 7, 1162 (2017). https://doi.org/10.1038/s41598-017-01341-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01341-y
This article is cited by
-
Metal organic frameworks as advanced extraction adsorbents for separation and analysis in proteomics and environmental research
Science China Chemistry (2022)
-
Effect of shell structure of Ti-immobilized metal ion affinity chromatography core-shell magnetic particles for phosphopeptide enrichment
Scientific Reports (2019)
-
Recent applications of metal–organic frameworks in matrix-assisted laser desorption/ionization mass spectrometry
Analytical and Bioanalytical Chemistry (2019)
-
Nanoparticle-based surface assisted laser desorption ionization mass spectrometry: a review
Microchimica Acta (2019)
-
Metal–organic framework-based affinity materials in proteomics
Analytical and Bioanalytical Chemistry (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.