Abstract
Recent studies have implicated long non-coding RNA, AC079767.4, as a highly susceptible gene in tuberculosis. The aim of the study was to preliminarily explore the possible association of single nucleotide polymorphisms (SNPs) in AC079767.4 gene with clinical phenotypes and TB susceptibility in Western Chinese Han population. The improved multiplex ligation detection reaction (iMLDR) method was employed to genotype 4 SNPs in AC079767.4 in 554 tuberculosis patients and 561 healthy individuals. In subgroup analysis, only the C allele for rs12477677 was associated with the decreased susceptibility to pulmonary TB with a p-value of 0.026, but p-value was 0.103 after Bonferroni correction. In total samples, haplotype [ACAC], representing four AC079767.4 variants, was found to slightly decrease TB risk (p = 0.045). Furthermore, patients with the CC genotype of rs12477677 were correlated with fewer occurrences of fever (p = 0.016), while patients carrying the T allele were associated with lower levels of ESR in the dominant model of rs1055229 (p = 0.021). For the first time, we reported the potential susceptibility and clinical traits of tuberculosis with lncRNA variants in the Western Han Chinese population. Our data indicate AC079767.4 polymorphisms may potentially act as novel biomarkers for tuberculosis diagnostic and therapeutic purposes.
Similar content being viewed by others
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (MTB) infection presents a major threat all over the world. According to the WHO Global Tuberculosis (TB) report in 2015, 9.6 million incident cases of TB and 1.5 million TB-related deaths are estimated to occur worldwide1. China ranks second among the 27 high TB-burden countries issued by WHO, which accounts for nearly 11% of the global burden. It is estimated that approximately a fifth of the population is infected with MTB across China; however, only 5–10% of infected individuals will develop active TB disease during their lifetime2. Abundant evidence from the investigations of case observations, twin studies and mouse models suggests that host genetic factors are of particular importance for determining susceptibility to TB disease3. For years, many genetic loci have been identified to be associated with TB development, including genetic variants of the Wnt signaling pathway4, cytokines and chemokines5, and toll-like receptors6. Recently, there has been considerable work revolving around genetic determinants of disorder clinical phenotypes. Pan G. et al. have previously shown that known multiple sclerosis (MS) risk single nucleotide polymorphisms (SNPs) could influence conversion to MS, relapse and disability progression, suggesting MS genetic risk variants may significantly influence the clinical course of MS7. Therefore, better understanding of the roles of genetic polymorphisms in mechanisms underlying TB disease and how these variants affect the clinical phenotypes of TB may benefit diagnosis as well as the development of treatments and vaccines.
Systemic genomic analysis has demonstrated that most of the human genome is transcribed; however, very little of it has protein-coding capacity, thus producing a broad range of non-coding RNA transcripts (named as ncRNA)8. Long non-coding RNAs (lncRNAs) are the largest class of ncRNA transcripts, which are defined as transcripts more than 200 nucleotides in length9. Recently, there has been an increase in attention around lncRNAs and their roles in human development and disease. LncRNAs have been known to be important regulatory players of gene expression through functioning as protein scaffolds, transcription co-activators or inhibitors, and mRNA decoys or microRNA sponges at transcriptional or post-transcriptional levels10, 11. By dysregulating the target gene, lncRNA could participate in the development and progression of many human diseases, including cancer12, cardiovascular disorders13, neurodegenerative diseases14, and autoimmune diseases15. Previous reports have shown that lncRNAs are also essential in human infectious diseases such as TB. A study by Pawar K. et al. revealed that down-regulated lncRNA MEG3 caused enhanced eradication of intracellular infected mycobacteria in Mycobacterium bovis BCG-infected macrophages16. Importantly, according to microarray analyses conducted by Yi Z. et al., the long non-coding RNA transcript AC079767.4 (Ensembl ID ENST00000429730) encoded by gene AC079767.4 (Ensembl ID ENSG00000224137) was differentially expressed in the active tuberculosis group, latent tuberculosis infection (LTBI) group and healthy control group. AC079767.4 transcript was down-regulated in both the active TB group and LTBI group compared with healthy controls, and its expression level was elevated in the LTBI group when compared to the active TB group17. These researchers subsequently verified these microarray data using RT-qPCR method and the results had excellent concordance with the microarray data. It is possible that the lncRNA AC079767.4 gene may be involved in the establishment and progression of TB infection. Inspired by the aforementioned information, we assume that AC079767.4 would be a highly promising susceptibility gene in TB.
With the rapid growth of identifications of cancer-associated lncRNAs, considerable efforts have concentrated on studying the contribution of lncRNA genetic variants in susceptibilities to many types of human cancers. Liu Y et al.18 found that the rs7763881 variant in lncRNA HULC was highly up-regulated in hepatocellular carcinoma and may confer susceptibility to HBV-related hepatocellular carcinoma in Chinese populations. In addition to disorder risk, several studies focused on the potential association between lncRNA polymorphisms and both disease clinical phenotype and treatment response. For example, lncRNA CASC8 polymorphism rs10505477 has been identified to be greatly correlated with platinum-based chemotherapy response in lung cancer in the dominant model19. Bayram S. et al. found that the rs12826786 TT genotype of well-characterized lncRNA HOTAIR was markedly related with multiple clinic-pathological features of breast cancer, such as larger tumor size (T3 and T4), distant metastasis (M1), advanced TNM stage (III and IV), and poor histological grade (III), which are all related to worse cancer progression20. These finding strongly indicate that lncRNA polymorphisms can act as new molecular biomarkers in the diagnosis and therapeutic areas of human diseases. However, to date there is a lack of genetic evidence describing the effect of lncRNA polymorphisms on clinical traits and predisposition to TB in the Han Chinese population. Thus, our study genotyped a set of SNPs within AC079677.4 among 554 tuberculosis cases and 561 healthy controls and systematically analyzed the relationship between risk as well as the clinical characteristics of active tuberculosis disease and selected lncRNA polymorphisms.
Results
General characteristics of the study subjects
The demographic and clinical features of the study participants are described in Table 1. Overall, there was no significant difference in age and gender between cases and controls, and both groups mainly involved middle-aged males. Among patients with tuberculosis, smoker and BCG scar were 53.97% and 52.17%, respectively, while smoker and BCG scar of healthy controls were 39.93% and 42.78%, respectively (p all <0.001). The mean values of body mass index (BMI) for the control and TB groups were 23.53 and 20.74 kg/m2, respectively (p < 0.001). For laboratory examinations, when compared to control subjects, patients with active tuberculosis showed an obvious increase in the levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and absolute leucocyte, platelet and monocyte counts (p all <0.001). TB patients had reduced levels of albumin (Alb), hemoglobin (Hb) and erythrocyte counts (p all <0.001) compared to healthy controls.
A series of TB-associated laboratory indices were also collected in the current study, mainly including tuberculosis subtype and etiological examinations. Amongst the TB cases enrolled in the study, 49.64% (275/554) were pulmonary tuberculosis (PTB), 11.19% (62/554) were extra-pulmonary tuberculosis (EPTB) and 39.17% (217/554) were PTB combined with EPTB (PTB&EPTB). Among patients having EPTB, the proportion of tuberculosis pleurisy was the largest with 25.09% (70/279), the followings were bronchial tuberculosis and tuberculous meningitis, with the ratios of 22.22% (62/279) and 14.34% (40/279), respectively. The positive rate for TB-DNA was 38.26% (212/554), 29.24% (162/554) for the smear microscopy and 24.18% (44/182) for culture, implying that TB-DNA testing was more sensitive than both smear microscopy and culture for diagnosing TB (38.26% versus. 29.24% and 24.18%, respectively). In a cohort of patients with pulmonary tubercular lesion, most of which received chest radiographic examinations read by professional physicians on admission, we found that lung cavitation lesions accounted for 20.77% (70/337) of all patients enrolled. In terms of the clinical presentations of TB patients, fever was the most common constitutional clinical symptom (53.25%) in our studied cases, followed by complaint of poor appetite (41.88%). Meanwhile, the most frequent local symptom was cough (56.32%).
LncRNA genetic polymorphisms in relation to tuberculosis risk
Genotyping results. Genotyping of selected SNPs was successfully completed for all 554 TB patients and 561 healthy controls. Genotype distributions of 4 SNPs within the AC079767.4 gene in the control group did not deviate from Hardy-Weinberg equilibrium (HWE) (p > 0.05 for all loci). Detailed information with respect to genotyped SNPs, including chromosomal locations, functional consequences, minor allele frequencies and p-values for HWE test in controls is summarized in Table S1.
Single SNP association analysis
Table 2 displays the genotype distributions and allelic frequencies of the four SNPs in the AC079767.4 gene between all tuberculosis cases and controls. The minor allele (C allele) frequency of rs12477677 was 46.48% in case group and 50.53% in controls, and the p-value of 0.056 after adjusting for age and gender was borderline significant (OR = 0.85, 95% CI = 0.72–1.00), but the p value was 0.224 after adjustment with Bonferroni correction. The frequency of genotype rs12477677 CC in cases was 22.02% and 25.13% in controls. The presence of allele C in homozygosis was more common in controls, although the difference did not reach statistical significance (p > 0.05). On the basis of results shown in Table 2, we surprisingly found that polymorphism rs10178277 was in perfect linkage disequilibrium with rs1055228 (r 2 > 0.99). The other three loci (rs10178277, rs1055228 and rs1055229) showed no significant differences between cases and controls in either genotype or allele frequencies (all p greater than 0.05). We next carried out a genetic model analysis, including dominant and recessive models, to further explore the difference in genotype distributions. The particular data are exhibited in Table 3. No significant differences in the genetic pattern analysis were demonstrated. However, we observed that rs12477677 was associated with non-significant declined risk for TB infection in the dominant model (CC + CT versus TT), with an estimated OR of 0.77 (95% CI = 0.59–1.01, p = 0.059 after adjusting for age and sex, p = 0.237 after Bonferroni correction).
Considering the correlation between age of onset of tuberculosis and genetic variants, described by Hijikata M. et al.21, we performed an age-stratified analysis in our study. Unfortunately, we did not see any association of all 4 SNPs with predisposition to TB in our age-subgroup analysis (data not shown).
To examine whether these four candidate SNPs were preferentially correlated with specific tubercular subtype according to Fernando S.L. et al.’s study22, we classified all tuberculosis patients into 2 subgroups: 275 patients with PTB and 217 with PTB&EPTB. Due to the low number of samples, EPTB was excluded from the subgroup analysis. The results are summarized in Table 4. Rs12477677 seemed to have a stronger magnitude of decreased risk for PTB in comparison to all forms of TB, and subjects carrying the C allele were correlated with decreased susceptibility to PTB relative to T allele carriers (p = 0.026, OR = 0.79, 95% CI = 0.64–0.97). But the relationship was also non-significant via Bonferroni correction with p = 0.103. However, the remaining three SNPs were not substantially different in the PTB subgroup analysis (data not shown). Additionally, genotypes of 4 polymorphisms showed no significant association with PTB susceptibility by applying dominant and recessive patterns. When comparing genotype distributions between PTB&EPTB and healthy controls, no any significant results were obtained (data not shown).
Haplotype analysis
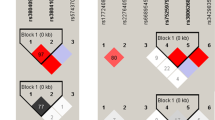
The linkage disequilibrium measurement and haplotype construction of variants of the AC079767.4 gene were conducted in the present study. Figure 1 presents that four loci in the AC079767.4 gene were in one linkage disequilibrium (LD) block based on the threshold of pairwise r 2 > 0.80. Three haplotypes, ACAC, GTGC, and ATAC, were constructed for AC079767.4 and those consisted of rs10178277, rs12477677, rs1055228, and rs1055229. Table 5 summarizes the haplotype frequencies as well as their associations with tuberculosis predisposition. The results revealed that the haplotype ACAC was significantly associated with reduced susceptibility to TB at a p-value of 0.045 with an OR of 0.84 (95% CI = 0.71–0.99).
Association between polymorphisms and clinical phenotypes
Early reports showed that the clinical manifestation and progression of active tuberculosis might be impacted by the given genetic polymorphism23. We therefore analyzed whether the four candidate SNPs influenced patients’ clinical manifestations of the disease. Common TB-associated clinical traits were evaluated, including clinical symptoms (including typically systemic and local symptoms), albumin and hemoglobin concentrations, absolute leukocyte counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) level and lung cavitation lesion. Regarding the possible TB susceptibility locus, rs12477677, our results revealed that patients homozygous for the minor allele (C allele) were less likely to suffer from fever, the most frequent symptom in our study patients, than genotype TT and TC carriers (p = 0.016, as shown in Table 6). Due to the small numbers of the homozygous minor genotypes of SNPs rs1055229 and rs10178277, these two loci were stratified using the dominant model. The results are listed in Tables 7 and 8. Although the SNP rs1055229 was not found to be significantly associated with TB susceptibility in individual SNP analysis, it was significantly associated with ESR levels routinely used to assess the disease activity for TB. As presented in Fig. 2 and Table 7, ESR levels were clearly higher among patients carrying homozygous CC genotype (median = 49.50, IQR = 24.25–77.75 mm/h) in the rs1055229 locus compared to those with TC and TT genotypes (median = 41.00, IQR = 15.50–70.00 mm/h) with a p value of 0.021. An increased trend for patients with the CC genotype of rs1055229 in CRP levels was found but did not reach statistical significance (p = 0.076). For rs10178277, none of the clinical phenotypes were correlated with the genotypes (Table 8).
Discussion
Our study aimed to preliminarily investigate the possible associations of susceptibility and disease traits in active tuberculosis with four candidate SNPs in lncRNA AC079767.4 within the Western Chinese Han population. The main findings of our study are that not only target lncRNA polymorphisms (rs12477677) are possibly related with risk for TB development but also that SNPs (rs12477677 and rs1055229) may influence clinical TB disease presentations. This study strongly suggests that genetic variants in AC079767.4 could become promising novel biomarkers for diagnosis and therapeutic applications in TB.
Currently, more non-coding genetic loci and protein-coding genes associated with TB have been determined. Non-protein coding RNA transcripts are diverse and, with a cutoff value of 200 nucleotides, can be roughly classified into small non-coding RNAs and long non-coding RNAs (lncRNAs). The idea that non-coding RNAs can be regulatory molecules of gene expression has been well established for many years, especially for small non-coding RNAs like microRNAs (miRNAs). In contrast to miRNAs, the roles of lncRNAs in diverse human physiological and pathological processes remain largely elusive. Recently, compelling studies have already shown that many lncRNAs are implicated in the host response against Mycobacterium tuberculosis (MTB) infection. For example, a study by Yang W. et al. uncovered that lncRNA-CD244, up-regulated by CD244 signaling, could inhibit IFN-γ/TNF-α expression by mediating repressive chromatin states at infg/tnfa loci through epigenetic programming in CD8+ cells infected with active tuberculosis24. The critical roles of lncRNA genetic variants in development and prognosis of various human cancers have been adequately studied. However, there is a limited amount of data supporting the effects of lncRNA genetic polymorphisms on predisposition and clinical phenotype for TB, which has an especially high prevalence in China. Given this concern, we performed the case-control study in the Western Chinese Han population to preliminarily explore potential TB-associated SNPs within lncRNA AC079767.4 and to assess whether these loci influence clinical manifestations of tuberculosis.
According to single locus analysis, there were no significant associations of TB risk with all 4 SNPs within AC079767.4 between tuberculosis cases and control subjects in the Western Chinese Han population. Nonetheless, the differences in both C allele frequencies (p = 0.056) and genotype distributions under the dominant model (p = 0.059) in the rs12477677 locus neared statistical significance. In subsequent subgroup analysis, we discovered the C allele for rs12477677 was statistically associated with reduced susceptibility to pulmonary tuberculosis (PTB) (p = 0.026) when comparing patients with only PTB to healthy individuals. However, statistical results showed that the allele and genotypic distributions were similar when cases with PTB & EPTB were compared to PTB patients and control individuals, respectively. Although the differences found in the rs12477677 locus did not remain statistical significant after Bonferroni correction, based on our results, we speculate that the C allele of rs12477677 within the lncRNA AC079767.4 gene seems to possibly pose a relatively weak protective effect for the development of tuberculosis. However, this genetic component by itself may not be a determining factor for severe clinical types of TB, such as PTB and EPTB combined. Subsequently, we conducted haplotype analysis for these genetic variants and revealed that the ACAC haplotype, formed by 4 AC079767.4 polymorphisms and the possible beneficial C allele in the rs12477677 locus, was statistically associated with decreased susceptibility to TB disease (p = 0.045). This finding was consistent with the individual SNP rs12477677 analysis. Our data indicate the potentially protective effect of the C allele in rs12477677 in TB development.
Although statistical analyses of the single locus of other 3 AC079767.4 variants failed to yield significant correlations, the investigations of these polymorphisms supplemented our knowledge on the association of lncRNA polymorphisms with TB susceptibility. In our study, the detected SNPs were mainly concentrated on intrinsic regions. Thus, polymorphisms in exons and regulatory genetic sequences might have been ablated, which may further the need for more comprehensive and systematic variants association studies in the future. Consequently, the correlation between this lncRNA gene and TB should be interpreted cautiously.
The lncRNA AC079767.4, aberrantly expressed among the LTBI, active TB and healthy control groups, is from an intergenic region adjacent to the protein-coding gene methyltransferase like 21 A (METTL21A). The polymorphic locus rs12477677 was found to lie in the AC079767.4 intron region and reside in the METTL21A downstream regulatory interval by the bioinformatics software HaploReg v4.1 (http://www.broadinstitute.org/), as shown in Fig. 3. It was reported that lncRNAs and nearby protein-coding genes might share local transcriptional and up-stream regulation effects25, 26. A possible explanation is that rs12477677 might serve as a protective SNP through influencing the expression or function of the AC079676.4 and METTL21A genes. Although there is currently a lack of report regarding the biologic functions of lncRNA AC079767.4, the protein product METTL21A of the METTL21A gene is well-described and defined as a novel human protein methyltransferase (MTase) responsible for trimethylation of the conserved lysine residue found in several Hsp70 (heat shock protein of ~70 kDa)27. Additionally, it has been established that several members of the Hsp70 family are closely associated with tuberculosis28. Together, our findings provide useful information for the future investigation of potential biological functions of SNP rs12477677 in tuberculosis development.
Using the cohorts of tuberculosis patients with clinical data, we investigated whether the four candidate SNPs in AC079767.4 were associated with clinical presentations of active TB. We found that rs12477677 and rs1055229 loci significantly contributed to differences in the clinical manifestations of active tuberculosis. Our analysis showed that patients carrying the homozygous CC genotype of rs12477677 had a highly significant association with fewer frequency of fever (p = 0.016) among patients with TB, which to a certain extent reflects the host inflammatory response to pathogen invasion. This is consistent with the result from the genetic susceptibility association analysis, further supporting the possibly favorable impact of the C allele in rs12477677 on TB risk as well as clinical presentation form. One interesting observation in our study was that the effect of SNP rs1055229 on TB clinical features and its insignificant effect on TB susceptibility differed. The TB non-susceptibility locus rs1055229 was linked to ESR levels in patients with TB within the current dataset. The elevated ESR levels were strongly associated with the CC genotype carriers when compared to those with the T allele, including the homozygous and heterozygous genotypes (p = 0.021). Hence, we consider that the variants in lncRNA AC079767.4 are most likely related to inter-individual differences in host defense response to MTB infection. Previous studies have shown that rs11465802 and rs1884444 in IL-23R were significantly associated with the drug-resistance of the infected MTB strain and cavitary lesion among pulmonary tuberculosis patients, respectively, and were unassociated with susceptibility to pulmonary TB in Chinese Uygurs29. The differences between TB susceptibility SNPs and genetic loci related to TB clinical phenotype indicate that TB development, progression and disease manifestation may be independently impact by different genetic loci and different signaling pathways. Although the detailed molecular mechanisms are poorly understood, these results may support the argument that lncRNA genetic variants may influence host defense responses against MTB infection and lead to different clinical manifestations in patients with the active disease.
This investigation represents the first exploration of the association between lncRNA genetic variations and active TB in a Han Western Chinese population. In our disease association study, we recruited all participants from the same geographic area during the same study period to minimize the bias of MTB pathogen exposure. However, our study has several limitations. First, we did not further carry out the gene-environment or gene-gene interaction analyses in our study because of the uncorrelated-TB risk among these SNPs. Second, we only studied a small amount of SNPs within lncRNA AC079767.4. Third, the relatively small sample size restricts the possibility of determining a significant genetic association. Lastly, we did not distinguish the specific population with latent tuberculosis infection (LTBI) among the disease-free controls, and thus the correlation of lncRNA variations with LTBI remains uncharacterized. Therefore, replication studies in other large independent populations and ethnicities, along with functional experiment studies, are urgently needed to explain the mechanisms of genetic polymorphisms in lncRNA AC079767.4 in TB development and clinical manifestation.
In conclusion, the potentially TB-associated protective effect was first identified for the C allele of SNP rs12477677 in lncRNA AC079767.4 in our study. Furthermore, we observed that the rs12477677 CC genotype may protect patients with TB from bearing fever, while the rs1055229 CC genotype was associated with greater ESR levels in a dominant model. Our findings strongly indicate that lncRNA AC079767.4 polymorphisms may serve as novel molecular biomarkers to diagnose tuberculosis and even as potential therapeutic targets.
Methods
Study subjects
This case-control association study included 554 tuberculosis patients and 561 healthy controls. All subjects were recruited from West China Hospital of Sichuan University between November 2011 and September 2015. All enrolled patients were newly diagnosed with TB, confirmed by two independent experienced respiratory physicians. The active TB diagnosis was made based on the following criteria: typical symptoms and signs of active TB disease, positive smear/culture/TB-DNA results, TB-associated radiological and histopathological findings as well as appropriate response to anti-TB treatment. Patients with HIV-infection, immunodeficiency disease, hepatitis virus infection, and other lung diseases were excluded from this study. The flow diagram of enrollment of TB patients was delineated in Fig. 4. Healthy controls were recruited from a pool of a health physical examination population. To be a healthy individual, people needed to be asymptomatic with no history of TB and negative sputum smear and culture. All controls have normal chest radiograph. Control groups were frequency-matched with case groups regarding age and gender, and all participants were of unrelated Han ethnicity.
Demographic data and clinical information of the studied population were reviewed from the medical record system of West China Hospital of Sichuan University. Three milliliter (ml) EDTA-anticoagulated whole blood samples were collected from each subject for genotyping. This study was approved by the Clinical Trials and Biomedical Ethics Committee of West China Hospital, Sichuan University (reference no. 198; 2014) and written informed consent was obtained from all the participants. All methods in this study were carried out in accordance with the approved guidelines.
Single nucleotide polymorphisms selection and genotyping
The human AC079767.4 gene is located on chromosome 2q34 and is approximately 4.6 kb nucleotides long with two exons. The genetic polymorphism data of the entire sequence of AC079767.4 was obtained from the dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP/). First, all SNPs were initially filtered according to the minor allele frequency (MAF) of 0.05 or more in Han Chinese in Beijing (CHB). Subsequently, we preferentially selected the SNPs that were located in potentially functional regions, including promoter, exon, untranslated region (UTR), intron and potential regulatory regions. Combined with experimental conditions required for genotyping, four SNPs in AC079767.4 (rs10178277, rs12477677, rs1055228 and rs1055229) were eventually included in our analysis.
Genomic DNA was isolated from peripheral blood samples by using QIAamp® DNA blood mini kit (Qiagen, Germany) according to the manufacturer’s instructions. The improved multiplex ligation detection reaction (iMLDR) method (Genesky Biotechnologies Inc., Shanghai, China) was used to genotype candidate SNPs, as described elsewhere30. The primer information for the multiplex PCR reaction is described in Table S2. The oligos and probes used in the ligation reaction are presented in Table S3. Briefly, this genotyping assay consisted of an initial multiplex PCR reaction that was designed to amplify fragments containing all 4 SNP loci, followed by the highly specific improved ligation reaction. Finally, a 0.5 μl ligation product was fractionated and loaded in ABI 3730XL, and the GeneMapper v4.1 software was employed to analyze the raw data. The different SNPs were identified according to different extended lengths at the 3′ terminal site, and the specific allele of each SNP was distinguished by different fluorescent labels of allele-specific oligo probe pairs.
To guarantee the genotyping quality, the ddH2O was considered to be the negative control used in each reaction, and the genotyping experiment was blinded so that the experimentt staff was unaware of the status of case-control. Furthermore, approximately 10% of the total samples were selected at random to genotype in duplicate and the concordance rate was perfect (100%).
Statistical analysis
The needed sample size was determined using PASS Statistical Software version 11 before data collection31 using the following parameters: a = 0.05, 80% power, a polymorphism prevalence of 5%, an allelic odds ratio for TB of 2 compared with the control, and a match ratio of 1:1. The Chi-square test and the Mann-Whitney U test were applied for categorical variables and continuous variables analyses, respectively. Hardy-Weinberg equilibrium (HWE) for all SNPs in the controls was assessed using the goodness-of-fit Chi-square test. Statistical methods above were performed by SPSS version 19.0 (IBM, Chicago, USA). Associations between candidate SNPs and TB risk were determined based on the distributions of allele and genotypic frequencies along with two different genetic models (dominant and recessive models). The strength of association was estimated with odds ratios (ORs) and 95% confidence intervals (CIs) using unconditional logistic regression analysis adjusted for age and gender by PLINK v1.0732. The Bonferroni method was further employed to correct for multiple testing. Additionally, we employed Haploview software version 4.2 to establish the linkage disequilibrium block with a threshold of the pairwise r 2 coefficient ≥0.80 and to construct haplotypes by using the expectation-maximization clustering algorithm. The correlations of each genetic variant with clinical features of tuberculosis were evaluated. All statistical tests were two-sided, and p < 0.05 was considered statistically significant.
References
WHO, Global tuberculosis report. http://www.who.int/tb/publications/global_report/en/, (Date of access: 25/12/2015) (2015).
Gao, L. et al. Latent tuberculosis infection in rural China: baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect Dis 15, 310–319, doi:10.1016/S1473-3099(14)71085-0 (2015).
Möller, M. & Hoal, E. G. Current findings, challenges and novel approaches in human genetic susceptibility to tuberculosis. Tuberculosis (Edinb) 90, 71–83, doi:10.1016/j.tube.2010.02.002 (2010).
Hu, X. et al. Pathway analyses identify novel variants in the WNT signaling pathway associated with tuberculosis in Chinese population. Sci Rep 6, 28530, doi:10.1038/srep28530 (2016).
Lindenau, J. D. et al. Cytokine gene polymorphisms are associated with susceptibility to tuberculosis in an Amerindian population. Int J Tuberc Lung Dis 18, 952–957, doi:10.5588/ijtld.14.0060 (2014).
Velez, D. R. et al. Variants in toll-like receptors 2 and 9 influence susceptibility to pulmonary tuberculosis in Caucasians, African-Americans, and West Africans. Hum Genet 127, 65–73, doi:10.1007/s00439-009-0741-7 (2010).
Pan, G. et al. Role of genetic susceptibility variants in predicting clinical course in multiple sclerosis: a cohort study. J Neurol Neurosurg Psychiatry 313722 (2016).
Dinger, M. E., Amaral, P. P., Mercer, T. R. & Mattick, J. S. Pervasive transcription of the eukaryotic genome: functional indices and conceptual implications. Brief Funct Genomic Proteomic 8, 407–423, doi:10.1093/bfgp/elp038 (2009).
Heward, J. A. & Lindsay, M. A. Long non-coding RNAs in the regulation of the immune response. Trends in immunology 35, 408–419, doi:10.1016/j.it.2014.07.005 (2014).
Elling, R., Chan, J. & Fitzgerald, K. A. Emerging role of long noncoding RNAs as regulators of innate immune cell development and inflammatory gene expression. Eur J Immunol 46, 504–512, doi:10.1002/eji.v46.3 (2016).
Rinn, J. L. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol 6, a018614–a018614, doi:10.1101/cshperspect.a018614 (2014).
Bartonicek, N., Maag, J. L. & Dinger, M. E. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer 15, 43, doi:10.1186/s12943-016-0530-6 (2016).
Lorenzen, J. M. & Thum, T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol 12, 360–373, doi:10.1038/nrneph.2016.51 (2016).
Wan, P., Su, W. & Zhuo, Y. The role of long noncoding RNAs in neurodegenerative diseases. Mol Neurobiol. (2016).
Mirza, A. H., Kaur, S., Brorsson, C. A. & Pociot, F. Effects of GWAS-associated genetic variants on lncRNAs within IBD and T1D candidate loci. PLoS One 9, e105723, doi:10.1371/journal.pone.0105723 (2014).
Pawar, K. et al. Down regulated lncRNA MEG3 eliminates mycobacteria in macrophages via autophagy. Sci Rep 6, 19416, doi:10.1038/srep19416 (2016).
Yi, Z., Li, J., Gao, K. & Fu, Y. Identifcation of differentially expressed long non-coding RNAs in CD4+ T cells response to latent tuberculosis infection. J Infect 69, 558–568, doi:10.1016/j.jinf.2014.06.016 (2014).
Liu, Y. et al. A genetic variant in long non-coding RNA HULC contributes to risk of HBV-related hepatocellular carcinoma in a Chinese population. PLoS One 7, e35145, doi:10.1371/journal.pone.0035145 (2012).
Hu, L. et al. Clinical significance of long non-coding RNA CASC8 rs10505477 polymorphism in lung cancer susceptibility, platinum-based chemotherapy response, and toxicity. Int J Environ Res Public Health 13, 6, doi:10.3390/ijerph13060545 (2016).
Bayram, S., Sümbül, A. T. & Dadaş, E. A functional HOTAIR rs12826786 C > T polymorphism is associated with breast cancer susceptibility and poor clinicopathological characteristics in a Turkish population: a hospital-based case-control study. Tumour Biol 37, 5577–5584, doi:10.1007/s13277-015-4430-y (2016).
Hijikata, M. et al. Association of IFNGR2 gene polymorphisms with pulmonary tuberculosis among the Vietnamese. Hum Genet 131, 675–682, doi:10.1007/s00439-011-1112-8 (2012).
Fernando, S. L. et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Respir Crit Care Med 175, 360–366, doi:10.1164/rccm.200607-970OC (2007).
Zhao, Z. et al. SFRP1 variations influence susceptibility and immune response to Mycobacterium tuberculosis in a Chinese Han population. Infect Genet Evol 37, 259–265, doi:10.1016/j.meegid.2015.11.031 (2016).
Yang, W. et al. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc Natl Acad Sci USA 112, E3883–3892, doi:10.1073/pnas.1501662112 (2015).
Guttman, M. et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature 458, 223e7–227, doi:10.1038/nature07672 (2009).
Giakountis, A. et al. A Positive regulatory loop between a Wnt-Regulated non-coding RNA and ASCL2 controls intestinal stem cell fate. Cell Rep 15, 2588–2596, doi:10.1016/j.celrep.2016.05.038 (2016).
Jakobsson, M. E. et al. Identification and characterization of a novel human methyltransferase modulating Hsp70 protein function through lysine methylation. J Biol Chem 288, 27752–27763, doi:10.1074/jbc.M113.483248 (2013).
Kumar, G. S. et al. Gene Expression Profiling of Tuberculous Meningitis Co-infected with HIV. Proteomics Bioinform 5, 235–244, doi:10.4172/jpb.1000243 (2012).
Jiang, D. et al. The variations of IL-23R are associated with susceptibility and severe clinical forms of pulmonary tuberculosis in Chinese Uygurs. BMC Infect Dis 15, 550, doi:10.1186/s12879-015-1284-2 (2015).
Hu, X. et al. No significant effect of ASAP1 gene variants on the susceptibility to tuberculosis in Chinese population. Medicine (Baltimore) 95, e3703, doi:10.1097/MD.0000000000003703 (2016).
Hintze, J. PASS 11. NCSS, LLC. Kaysville, Utah, USA http://www.ncss.com/ (2011).
Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81, 559–575, doi:10.1086/519795 (2007).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China [81472026, 81672095] and the Projects in the Science and Technology Department of Sichuan Province pillar program [2014SZ0208]. The authors are grateful to Shanghai Genesky Bio-Tech Genetic Core Lab for providing assistance in genotyping techniques.
Author information
Authors and Affiliations
Contributions
Z.Z., M.Z. and J.Y. wrote the main manuscript text and participated in the experiment all the way. X.H. and J.Z. participated in modifying the manuscript; Z.Z. and M.Z. participated in the analysis of data and prepared Tables and figures; Y.Z., X.S. and Y.Z. engaged in the acquisition of data (laboratory or clinical); B.Y. and Y.Z. designed the study. All authors have reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Z., Zhang, M., Ying, J. et al. Significance of genetic polymorphisms in long non-coding RNA AC079767.4 in tuberculosis susceptibility and clinical phenotype in Western Chinese Han population. Sci Rep 7, 965 (2017). https://doi.org/10.1038/s41598-017-01163-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01163-y
This article is cited by
-
Integrated analysis of lncRNA, miRNA and mRNA profiles reveals potential lncRNA functions during early HIV infection
Journal of Translational Medicine (2021)
-
Long Noncoding RNA HULC Promoter Polymorphism rs1041279 Is Associated with an Increased Risk of Cervical Squamous Cell Carcinoma
Reproductive Sciences (2020)
-
The dominant model analysis of Sirt3 genetic variants is associated with susceptibility to tuberculosis in a Chinese Han population
Molecular Genetics and Genomics (2020)
-
Association Study of ANRIL Genetic Variants and Multiple Sclerosis
Journal of Molecular Neuroscience (2018)
-
Screening and identification of lncRNAs as potential biomarkers for pulmonary tuberculosis
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.