Abstract
RNA silencing constitutes an important antiviral mechanism in plants. Small RNA guided Argonaute proteins fulfill essential role in this process by acting as executors of viral restriction. Plants encode multiple Argonaute proteins of which several exhibit antiviral activities. A recent addition to this group is AGO2. Its involvement in antiviral responses is established predominantly by studies employing mutants of Arabidopsis thaliana. In the virological model plant, Nicotiana benthamiana, the contribution of AGO2 to antiviral immunity is much less certain due to the lack of appropriate genetic mutants. Previous studies employed various RNAi based tools to down-regulate AGO2 expression. However, these techniques have several disadvantages, especially in the context of antiviral RNA silencing. Here, we have utilized the CRISPR/Cas9 technology to inactivate the AGO2 gene of N. benthamiana. The ago2 plants exhibit differential sensitivities towards various viruses. AGO2 is a critical component of the plants’ immune responses against PVX, TuMV and TCV. In contrast, AGO2 deficiency does not significantly influence the progression of tombusvirus and CMV infections. In summary, our work provides unequivocal proof for the virus-specific antiviral role of AGO2 in a plant species other than A. thaliana for the first time.
Similar content being viewed by others
Introduction
The discovery of nucleic acid guided nucleases (NGNs) has fundamentally influenced molecular biology in the last two decades. Directing nucleases to their substrates using the simple rules of Watson-Crick base pairing serves the basis for a number of gene regulatory and defensive mechanisms employed by both prokaryotes and eukaryotes. In eukaryotes, one of the best-studied NGN-based processes is RNA silencing (or RNA interference, RNAi), which is also a major antiviral defense mechanism in plants1,2,3,4,5,6,7. Antiviral RNA silencing is triggered by double-stranded RNA (dsRNA) replication intermediates of viruses or intramolecular fold-back structures within their genomes. These structures are processed by RNase III-like enzymes (DCLs), generating primary viral small interfering RNAs (vsiRNAs). Endogenous RNA-dependent RNA polymerases (RDRs) can also use single-stranded (ss) viral RNAs to synthesize dsRNAs, which serve as substrates for the production of secondary vsiRNAs. Both classes of vsiRNAs are eventually incorporated into Argonaute (AGO) protein containing RNA-induced silencing complexes (RISCs). RISCs can inhibit virus amplification either by directly slicing the viral genome or by limiting the expression of virus-encoded proteins in a cleavage-dependent or -independent fashion. While primary vsiRNAs limit virus replication predominantly locally, secondary vsiRNAs appear to play an important role in restricting systemic infection. Many viruses have evolved various means to evade detection and neutralization by the above mechanisms. One of their most effective countermeasures is the deployment of proteins - viral suppressors of RNA silencing (VSRs) - that interferes with RNA silencing at different steps8.
NGN-based mechanisms are also employed by prokaryotes to protect the integrity of their genome9,10,11. Adaptive immune systems, based on the concerted actions of clustered regularly interspaced short palindromic repeat (CRISPR) loci and CRISPR associated (Cas) endonucleases, help the elimination of invading genetic parasites such as viruses and plasmids in many species of archaea and eubacteria. At the end of 2012 a breakthrough paper reported a repurposed version of the type II CRISPR system from Streptococcus pyogenes 9. In the modified system, the Cas9 endonuclease is programmed with an engineered single guide RNA (sgRNA), allowing the cleavage of any 20 nt DNA sequence that lies 5′ to an NGG motif (PAM, protospacer adjacent motif). Soon after, it was shown that the SpCas9 protein could find and cleave its target sequence not only in bacteria but in the chromatin context of higher eukaryotes as well12, 13. The introduced double strand DNA break can be repaired either by homologous recombination (HR), if appropriate template is present, or more frequently by the error-prone non-homologous end-joining (NHEJ) pathway. Since then, due to its simplicity and robustness, the system has been successfully adapted for gene inactivation and genome editing in many organisms, including a number of plant and animal species14,15,16.
The genome of the model plant Arabidopsis thaliana encodes 10 AGO proteins, of which the antiviral roles of AGO1, AGO2, AGO4, AGO5, AGO7 and AGO10 have been demonstrated17,18,19. These analyses have been greatly facilitated by the availability of extensive collections of mutants. Although, A. thaliana is an excellent model organism for studying many aspects of plant physiology, it is a non-host for many viruses, considerably limiting its use for investigating plant-virus interactions. In stark contrast, Nicotiana benthamiana is exceptionally susceptible to a wide array of plant pathogens, including viruses, bacteria, oomycetes and fungi20. However up until now, its widespread use as a model organism has been greatly hampered by the fact that like many members of the Nicotiana genus, N. benthamiana has an amphidiploid genome. This feature significantly hiders the generation of genetic mutants of this plant. Importantly, the advent of the CRISPR/Cas9 technology can offer a solution for the above problem by allowing efficient production of mutants of this genetically intractable species.
In addition to AGO1 and AGO4, recent data have also implicated AGO2 in antiviral defenses of N. benthamiana 21,22,23,24,25. These findings however, were made in plants where AGO2 expression was down-regulated by RNAi (VIGS, shRNA). The effectiveness of these techniques can sometimes be uncertain (especially in virus infected plants) and other issues may also complicate the interpretation of the obtained results (see later in discussion). To avoid these problems, we set out to generate ago2 mutants of N. benthamiana, using the CRISPR/Cas9 system of S. pyogenes. The created mutant plants were challenged with a number of viruses. The susceptibility of the ago2 plants to different viruses varied significantly. For the first time, conclusive proof was provided for the involvement of AGO2 in antiviral immunity in a plant species other than A. thaliana.
Materials and Methods
Plasmid construction
Plasmid construction was performed using conventional techniques26. The bi-functional Cas9-sgRNA targeting plasmid was created as follows: (1) Plant codon optimized SpCas9 gene was cloned into pENTR11. (2) The resulting plasmid was used to transfer the SpCas9 gene into the pK7WG2D binary plasmid using LR clonase to yield pK7WG2D-SpCas9. (3) A. thaliana U6 promoter driven sgRNA expression cassette was assembled by over-lapping PCRs27. (4) After digesting with AatII-XhoI, the expression cassette was ligated into pK7WG2D-SpCas9, which was also cleaved with the same restriction enzymes. The N. benthamiana genome was searched for potential off target sites of the selected sgRNA using the CCTop-CRISPR/Cas9 target online predictor (http://crispr.cos.uni-heidelberg.de/28.
Agroinfiltration, protein analysis
Agroinfiltration of N. benthamiana leaves and western blot analysis of protein lysates were carried out as described24.
Plant transformation
Agrobacterium mediated leaf disc transformation of N. benthamiana was performed essentially as described29. Briefly, N. benthamiana plants were cultured under sterile conditions on MS medium for 2–3 months. Leaf discs were cut from the plants and infected with a C58C1 A. tumefaciens strain carrying the binary Cas9-sgRNA targeting construct. The infected discs were incubated on MS104 agar plates for two days. Next, the discs were transferred onto MS104 agar plates supplemented with 300 μg/ml kanamycin and 500 μg/ml cefotaxime. Every month the discs were passaged onto fresh selective plates. Approximately after three month, the appearing shoots were transferred onto rooting medium. Finally, plantlets with fully developed root system were transplanted into appropriate soil mix and reared under standard greenhouse conditions.
Evaluation of CRISPR/Cas9 mediated target modification, identification of ago2 mutant N. benthamiana plants
Efficiency of the selected AGO2-specific sgRNA was tested in transient agroinfiltration assays. Briefly, N. benthamiana leaves were infiltrated with a suspension of Agrobacteria carrying the binary Cas9-sgRNA targeting construct. Three days later, genomic DNA was extracted from the infiltrated leaf tissue using a DNeasy Plant Kit (Qiagen) according to the manufacturer’s instructions. The targeted region of the AGO2 gene was PCR amplified using Phusion polymerase (Thermo). Subsequently, the 564 bp PCR product was cloned into pGEM-T easy plasmid vector (Promega). To assess target modification efficiency, 20 plasmid clones containing the AGO2 fragment was sequenced.
To identify stable N. benthamiana transformant lines carrying CRISPR/Cas9 modified AGO2 alleles, the targeted segment of the AGO2 gene was PCR amplified using Phire Plant Direct PCR kit (Thermo). The PCR fragments were cloned into pGEM T easy plasmid. At least ten independent insert containing plasmid clones were obtained and sequenced from each transformant line to appraise their AGO2 status.
RNA analysis
Preparation of total RNA from leaf tissue and subsequent northern blot analyses were performed as described24.
Virus inoculation
Cymbidium ringspot virus (CymRSV), carnation italian ringspot virus (CIRV) and turnip crinkle virus (TCV) inoculations were performed using in vitro transcribed full-length viral transcripts as described before25. Infections by GFP expressing turnip mosaic virus (TuMV-GFP) and potato virus X (PVX-GFP) were initiated either by infiltrating the appropriate Agrobacterium strains30, 31 into N. benthamiana leaves or by rubbing them with sap prepared from TuMV-GFP or PVX-GFP infected plants. PVX-GFP could also be transmitted via the root system by shared irrigation water. Cucumber mosaic virus (CMV) inoculations were carried out as described before32. Viral infection experiments were repeated at least three times. In every experiment the treatment groups consisted of three plants. The results were highly reproducible and representative data are shown. The protein and RNA samples, used for northern and western blots, were also prepared and pooled from the three plants, which constituted the given treatment group.
Results
Generation of ago2 mutant N. benthamiana
To examine the antiviral role of the AGO2 protein of N. benthamiana, we decided to generate ago2 mutant plants using the type II CRISPR/Cas9 system of S. pyogenes. We created a single plasmid system to express the Cas9 protein and the appropriate sgRNA (Fig. 1A). With the use of such system, we hoped to achieve higher gene modification efficiency compared to multi-plasmid systems, where efficient co-delivery of the various components could be problematic. Despite its amphidiploid genome, transcriptome analyses have indicated that N. benthamiana expresses only a single AGO2 homologue33, 34. An sgRNA, targeting the functionally essential PIWI domain of the expressed AGO2 gene was designed. The CCTop plant CRISPR analysis online tool did not detect off target sites for this sgRNA in coding regions of N. benthamiana. The pK7WG2D binary plasmid vector was modified to co-express the sgRNA and the plant codon optimized SpCas9 nuclease27. Next, we tested the efficiency of the selected sgRNA in a transient assay system. Agrobacteria carrying the binary targeting construct was infiltrated into N. benthamiana leaves. Three days later, genomic DNA was extracted from the infiltrated leaf tissue and the targeted region of the AGO2 gene was PCR amplified. The product was cloned into pGEM-T easy vector and the DNA fragments were sequenced. We detected Cas9 introduced mutations at the expected position in 6,25% of the analyzed DNA fragments (data not shown). The observed relatively low targeting efficiency was obviously an underestimate, since the leaf tissue used for genomic DNA purification comprised of a mixture of Agrobacteria infected and non-infected cells. Nonetheless, our results demonstrated that the CRISPR/Cas9 system was suitable to specifically target the AGO2 gene of N. benthamiana using the designed sgRNA.
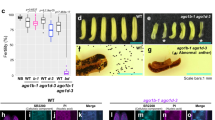
Generation of ago2 mutant N. benthamiana. (A) Schematic structure of the bi-functional Cas9-sgRNA expression plasmid used for the targeted inactivation of the AGO2 gene of N. benthamiana. (B) Structure of the isolated ago2 alleles. The targeted sequence of the wild-type AGO2 gene is highlighted with blue and the adjacent PAM motif with orange. In the isolated mutant alleles the insertions are highlighted with green and the deletions are with yellow. The nucleotide changes accompanying some of the deletions are indicated by red letters. (C) Summary of the mutations identified in the T0 and T1 transformant plants. (D) The mutations in the ago2 alleles of transformant lines 1.3 and 1.4 result in premature stop codons. Both alleles encode truncated, dysfunctional AGO2 proteins, which lack three out of the four catalytically essential amino acids of the PIWI domain.
Next, we proceeded to generate stably transformed N. benthamiana plants using the well-established Agrobacterium mediated leaf disc transformation protocol29. Nine T0 transformant plants were regenerated on kanamycin containing medium and tested for the desired genetic modification. The targeted segment of the AGO2 gene was PCR amplified, the products were cloned into pGEM-T easy plasmid and sequenced. Mutations in the AGO2 gene were detected at the expected position in all transformants (Fig. 1B,C). Wild-type AGO2 allele was also found in seven of the nine T0 plants. In five transformants more than two different AGO2 alleles were found, indicating that these plants were chimeric. The identified mutations were mostly deletions starting at the predicted Cas9 cleavage site (3 nt upstream of the PAM sequence). The deletions ranged from 2 to 65 nt and extended in both 5′ and 3′ directions. Sometimes single nucleotide changes also accompanied the deletions. Less frequently insertions were also observed. These were mostly single nucleotide insertions at the Cas9 cleavage site. In one instance, insertion of a 59 nt DNA segment was also detected. BLAST search of this sequence revealed an identical region in the N. benthamiana genome. The DNA segment neither seemed to be a part of a functional coding region nor possessed any recognizable functional motif. Nonetheless, this finding indicated that in addition to the more conventional NHEJ and HR pathways, the double strand breaks generated by the Cas9 endonuclease could be sealed via the acquisition of pieces of DNA from distant parts of the genome.
Exclusively mutant ago2 alleles were found in only two transformant lines (#1 and #9), however one of them (#9) carried three different ago2 alleles. In transformant line #1 only two mutant ago2 alleles were detected in our initial screen. Both the 5 nt deletion and the single C insertion (both at the Cas9 cleavage site) resulted in frame-shifts and as a consequence premature stop codons. Both alleles had the capacity to encode truncated AGO2 proteins, which lack three out of the four catalytically essential amino acid residues of the PIWI domain (E875, D907, D1037) (Fig. 1D). Earlier, we have demonstrated that the D907A mutation alone was sufficient to totally abolish substrate cleavage, gene silencing and antiviral activities of AGO224. Consequently, the truncated AGO2 proteins encoded by the above ago2 alleles were most likely dysfunctional. Transformant #1 was allowed to self-pollinate and T1 plants were generated. The targeted region of the AGO2 gene was sequenced in five T1 plants, as described above. Three of the examined plants carried wild-type AGO2 alleles, in addition to the mutant ones, indicating that the parental T0 plant was indeed chimeric. Importantly however, in lines #1.3 and #1.4 only two of the above-described ago2 mutant alleles were detected. These biallelic plants were again allowed to self-pollinate and the resulting T2 progenies were used in all subsequent experiments as ago2 mutant plants. The phenotype of these plants was indistinguishable from the parental wild-type N. benthamiana plants, under the growth conditions used in our experiments.
ago2 mutant N. benthamiana plants are hyper-susceptible to PVX infection
PVX is a type member of the Potexvirus genus (Alphaflexiviridae), which can predominantly infect Solanaceous hosts. Plants belonging to the Brassicaceae family are generally considered to be non-hosts for PVX. Paradoxically, most of the information on the mechanisms involved in restricting PVX infection has been derived from studies employing A. thaliana, which is naturally a non-host for this virus. Indeed, wild-type A. thaliana can not be infected by PVX. Efficient infection requires either the inactivation of DCLs (DCL2 and/or DCL4) or AGO2 35,36,37. Recently, a synergistic role of AGO5 in systemic PVX infection has also been reported38. Even though N. benthamiana is a natural host for PVX, almost nothing is known about the AGOs involved in the anti-PVX defenses of this plant. So far, we and others have only shown that in a transient over-expression system, the AGO2 protein (either from N. benthamiana or A. thaliana) has the capacity to locally limit the replication of a VSR and movement deficient form of PVX (PVX-ΔTGB)24, 38. However, the role of AGO2 in a bona fide PVX infection in N. benthamiana has not been studied, due to the lack of the appropriate mutant. Thus, to investigate this question, we challenged our ago2 and control wild-type N. benthamiana plants with a GFP expressing version of PVX30. The use of this virus allowed us to monitor the progression of infection in vivo. In most experiments, we initiated the infection from a leaf-infiltrated Agrobacterium strain carrying a PVX-GFP encoding binary vector. Inoculation of the plants by PVX virions either via the root system (irrigation) or the leaves (rubbing) provided essentially the same results.
Five days post-inoculation (dpi), signs of systemic infection could be observed on both wild-type and ago2 plants. By 8–10 dpi, significant differences could be noted between the two cohorts (Fig. 2A). In wild-type plants, as a sign of recovery, new leaves were starting to emerge. With decreasing age, these leaves exhibited gradually reduced GFP signals, the youngest ones having only a few small fluorescent spots. In contrast, the mutant plants usually did not show any sign of recovery. Instead, necrosis of the apical stem region was visible, which generally culminated in the death of the plants by 17–21 dpi. Using a GFP specific probe, levels of virus RNA were also monitored in the inoculated plants by northern blot analysis (Fig. 2B). Despite the dramatically different outcome of infection, PVX genomic RNA (gRNA) levels were only slightly elevated in systemically infected leaves of the ago2 mutants compared to wild-type plants. In contrast, subgenomic transcripts (sgRNAs) accumulated strongly in the mutant plants and the virally encoded GFP protein levels also largely followed the sgRNA levels (as assessed by western blot).
ago2 mutant N. benthamiana plants are hyper-susceptible to PVX infection. (A) Photographs of PVX-GFP infected plants. As a sign of recovery new leaves were starting to emerge in wild-type plants, which were generally absent in ago2 plants. On the top left panel white arrowheads point to newly emerging leaves, with higher numbers indicating younger leaves. Picture of the same plants was taken under UV light (top right panel). In the wild-type plants the new leaves exhibited gradually reduced GFP signals with decreasing age. At 17 dpi, wild-type plants show recovery, while in the ago2 plants the apical necrosis usually results in plant death (bottom panel). (B) PVX infections of wild-type and ago2 N. benthamiana plants were initiated from a leaf-infiltrated Agrobacterium strain carrying a PVX-GFP encoding binary vector. Different dilutions of the bacterium suspension were infiltrated into the leaves. Concentrations of the suspensions are given as optical densities measured at 600 nm (OD600). At 5 dpi, total RNA and protein lysates were prepared from the first symptomatic non-inoculated leaves. Samples were collected from three plants and pooled. Viral RNA levels were monitored in northern blot using a GFP probe. Viral genomic and subgenomic RNAs are indicated by arrowheads (top panel). Ethidium-bromide (EtBr) stained gel is shown as loading control (second panel). Virus encoded GFP protein levels were analyzed by western blot (third panel). The same blot was developed with an actin antibody to verify equal loading (bottom panel). (C) Induction of defense related genes in PVX infected plants were monitored by northern blot. RNA samples were prepared from systemically infected leaves at 7 dpi. Samples were pooled from three plants. Actin probe hybridized filter and Ethidium-bromide stained gel are shown as loading controls. Arrowheads indicate PVX genomic and subgenomic RNAs.
Virus induced systemic necrosis (SN) is accompanied by the up-regulation of defense-related genes39. In non-inoculated symptomatic leaves of PVX infected ago2 mutants, northern blot analysis revealed robust induction of PR-1a and Hsr203J genes (both are reliable markers for virus induced SN), while their levels did not change significantly in wild-type plants (Fig. 2C). These results confirmed that PVX infection elicited a strong necrotic response in ago2 plants.
AGO2 is required for the recovery of N. benthamiana from TuMV infection
In A. thaliana, genetic analysis has identified the involvement of several AGO proteins in defense against TuMV (Potyviridae), in a modular and organ-specific manner40. Though highly susceptible, N. benthamiana can efficiently recover from TuMV infection. The genetic background of this recovery is largely unexplored. Nonetheless, two sets of observations have suggested that RNA silencing plays an essential role in the defense against potyviruses in this species as well:
-
(1)
Silencing of RDR6 causes hyper-accumulation of potato virus Y (PVY) and plum pox virus (PPV) in N. benthamiana 41, 42. This indicates that RDR6-derived vsiRNAs are necessary for curtailing replication of potyviruses in this host.
-
(2)
Co-infection of N. benthamiana with PVX and PVY results in increased systemic symptoms (synergism) and eventually plant death. This synergism can be recapitulated by infecting the plants with a PVX chimera expressing the silencing suppressor of potyviruses (helper component proteinase, HC-Pro)43.
To evaluate the role of AGO2 in TuMV infection, ago2 mutant and wild-type N. benthamiana plants were inoculated with a GFP expressing form of TuMV (TuMV-GFP). The infection was usually initiated from a leaf infiltrated Agrobacterium strain, which carries a TuMV-GFP transgene encoding binary vector31. Inoculation of the plants with virion-containing sap was also performed and gave essentially the same results. As a sign of systemic infection, GFP signals were starting to emerge in apical non-inoculated leaves at 7–8 dpi in plants of both genetic backgrounds. By 12 dpi, the symptoms exhibited by the mutant and wild-type plants became clearly distinguishable (Fig. 3A). In wild-type plants, new leaves were starting to emerge, which exhibited fewer and fewer fluorescent foci. In contrast, in the mutants the apical regions became necrotic, with no signs of recovery. After four weeks, the wild-type plants almost completely recovered from the infection, while the ago2 mutants died by that time. The amounts of TuMV-derived RNA and GFP protein were also monitored in the two groups of plants (Fig. 3B). In general, both accumulated to higher quantities in the inoculated and systemically infected leaves of ago2 mutants compared to wild-type plants. In summary, the symptomatologies of PVX and TuMV infections were quite similar in N. benthamiana though, the latter proceeded with a somewhat delayed kinetics. The AGO2 status of the plants critically influenced the outcome of infections by both viruses.
ago2 mutant N. benthamiana plants are hyper-susceptible to TuMV infection. (A) Photographs of TuMV-GFP infected plants. On the top left panel white arrowheads indicate newly emerging leaves in the recovering wild-type plants. Higher numbers correspond to younger leaves. Top right panel shows picture of the same plants taken under UV light. Younger apical leaves exhibit decreased GFP accumulation. No signs of recovery were detectable in ago2 plants. By 29 dpi, wild-type plants almost completely recovered, while ago2 plants died (bottom panel). (B) TuMV infection was initiated in N. benthamiana plants from a leaf-infiltrated Agrobacterium strain carrying a TuMV-GFP encoding binary expression vector. At 8 dpi, total RNA and protein lysates were prepared from the infiltrated and first symptomatic systemically infected leaves. Samples were collected from three plants and pooled. Accumulation of TuMV viral RNA was analyzed in infiltrated and systemically infected leaves by northern blot using the indicated probes. Ethidium-bromide stained gels are shown as loading controls. Virus encoded GFP levels were monitored by western blot. The partially processed TuMV polyprotein species were detected by GFP antibody as high molecular weight signals in the infiltrated lysates. Equal loading was verified by actin antibody staining.
AGO2 deficiency exacerbates symptoms of TCV infection in N. benthamiana
The Carmovirus TCV has a broad host range, which predominantly includes members of the Brassicaceae. It can also infect N. benthamiana as an experimental host and is one of the relatively few viruses that are highly virulent on A. thaliana. An earlier study has demonstrated that ago1 and ago7 mutants are hypersusceptible to TCV infection44. Recently, it has also been shown that AGO2 plays a critical role in the survival of TCV infected A. thaliana 45, 46. The role of various AGOs in TCV infection in N. benthamiana is unexplored. RNA silencing is most likely involved in the process, since infection of this host with a chimeric PVX construct, expressing the VSR of TCV (the coat protein of TCV, TCV-CP), elicits severe systemic tissue necrosis and subsequent death by 7–10 dpi47. Interestingly, the symptoms of TCV infection are relatively mild on N. benthamiana, which include vein clearing, leaf curling and shortened internodes. Generally, no lethality is associated with the infection. The apparent contradiction between the above observations can be reconciled by the finding that the essential determinant of the silencing suppressor activity of TCV (the N terminal 25 amino acids of TCV-CP) is inaccessible within the native virion capsid structure.
To assess the effect of AGO2 deficiency on TCV infection in N. benthamiana, ago2 and wild-type control plants were inoculated with in vitro transcribed infectious TCV RNA. gRNA levels were monitored in the inoculated and non-inoculated symptomatic leaves of the infected plants by northern blotting (Fig. 4A). At 7 dpi, similar buildups of gRNAs were detected in both cohorts. Symptoms of infection were starting to diverge between the two groups at 14 dpi. In the mutant plants, extension of the apical internodes was strongly reduced compared to the wild-type ones, however unlike in PVX or TuMV infected ago2 plants, no apical necrosis was detectable. By 28 dpi, the mutant plants became severely chlorotic and usually died, while the wild-type controls only exhibited the moderate symptoms of TCV infection (vein clearing, leaf curling and shortened internodes) (Fig. 4B).
AGO2 deficiency exacerbates symptoms of TCV infection in N. benthamiana. (A) Wild-type and ago2 N. benthamiana plants were infected with in vitro transcribed full-length TCV viral transcripts. As controls, mock infections were also performed. Accumulation of TCV genomic RNA was analyzed in inoculated and systemically infected leaves at 7 dpi by northern blot. As a loading control the Ethidium-bromide stained gel is shown. (B) Photographs of TCV and mock infected wild-type and ago2 plants were taken at 28 dpi.
AGO2 has a modulatory effect on tombusvirus infection
Tombusviruses are well suited to study antiviral RNA silencing because they produce large quantities of vsiRNAs during infection. In addition, they encode a 19 kD protein (P19), which is one of the most potent and best studied suppressors of RNA silencing25, 48,49,50. Although, a wealth of biochemical data is available on the antiviral mechanisms elicited by tombusviruses, genetic dissection of the process is greatly hampered by the fact that A. thaliana is a non-host for these pathogens. N. benthamiana AGO2 has been implicated in the defense against tomato bushy stunt virus (TBSV). However, these data have been obtained on plants, in which AGO2 expression was only partially knocked-down by VIGS or shRNA22, 23. Thus, we tested the sensitivity of our ago2 mutants against various tombusviruses. Wild-type CymRSV and CIRV caused very severe systemic symptoms in both wild-type and mutant plants, which generally resulted in their death by 7–10 dpi (data not shown). The genetic backgrounds of the plants had no effect on the progression of the disease. This was also reflected in the comparable accumulation of viral gRNAs in the inoculated and systemically infected leaves of the two groups of plants (Fig. 5).
Effect of AGO2 on tombusvirus infection. Wild-type and ago2 N. benthamiana plants were infected with in vitro transcribed wild-type and ΔP19 mutant CymRSV (B) and CIRV (C) viral transcripts. Mock infections were also performed (A). At 5 dpi, total RNA was prepared from the inoculated and the first symptomatic non-inoculated leaves. RNA samples were purified from recovered apical leaves at 21 dpi. Samples were collected from three plants and pooled. Viral gRNA accumulation was monitored by northern blot. Ethidium-bromide stained gels are shown as loading controls. Photographs of virus infected and mock infected N. benthamiana plants are also shown at 21 dpi.
Tombusviral P19 is a strong suppressor of RNA silencing and may be able to mask the antiviral activity of AGO2. To avoid the interference by P19, the above infections were repeated with VSR deficient forms of CymRSV and CIRV (CymRSV-ΔP19 and CIRV-ΔP19). These viruses elicited much milder symptoms in both wild-type and ago2 plants. No lethality was detected in either groups and signs of recovery became obvious by 2 weeks after inoculation. By 21 dpi, wild-type plants mostly recovered. Although somewhat stunted, clear signs of recovery were also exhibited by the ago2 mutants. Interestingly however, some reproducible differences could be noted between the infection kinetics of CymRSV-ΔP19 and CIRV-ΔP19. The recovery of the ago2 plants infected with the former virus proceeded faster than the ones infected with the latter. The patterns of gRNA accumulation were also different between the two viruses (Fig. 5). CymRSV-ΔP19 gRNA reached similar levels in the inoculated and systemically infected leaves of both wild-type and mutant plants. At 21 dpi, gRNAs were barely detectable in either groups. In contrast, CIRV-ΔP19 gRNA accumulated to higher levels in ago2 mutants than in wild-type plants, which was especially pronounced in systemically infected leaves. The difference persisted at least until 3 weeks after infection.
Recently, a critical role for AGO2 has been reported during the progression of TBSV-ΔP19 infection in N. benthamiana 22, 23. Thus, we inoculated ago2 and wild-type plants with in vitro transcribed infectious TBSV-ΔP19 viral transcript. Symptom development and viral RNA levels were followed as above. The pattern of TBSV-ΔP19 RNA accumulation was similar to that of CIRV-ΔP19. Likewise, the recovery of ago2 plants was only slightly delayed compared to wild-type controls (Suppl. Fig. 1). The difference between our results and the ones reported earlier, was likely due to the different methods employed for the down-regulation of AGO2 (RNAi vs. CRISPR mediated mutagenesis).
Progression of CMV infection is not significantly affected by AGO2 deficiency in N. benthamiana
CMV (Bromoviridae) is a tripartite plus stranded RNA virus with a wide host range. Study of the infection process by CMV provided one of the first examples of the antiviral role of RNA silencing in plants51, 52. The involvements of AGO1, AGO2 and AGO4 in anti-CMV defenses have been extensively demonstrated in A. thaliana 19, 45, 53,54,55,56. To evaluate the role of AGO2 in CMV infection in N. benthamiana, ago2 and wild-type plants were inoculated with the Y-sat strain of CMV. Y-sat (Y satellite RNA) is a noncoding subviral RNA, which can modify the symptoms of CMV infection in certain hosts32, 57. It generates siRNAs, which down-regulate the mRNA of a key enzyme of chlorophyll biosynthesis (magnesium protoporphyrin chelatase subunit I, ChlI) in N. tabacum and N. benthamiana, thereby resulting in a characteristic yellow phenotype. The use of this virus-satellite combination allowed us to easily monitor the progression of virus infection. Signs of systemic infection (yellowing of the apical leaves) started to appear at the same time (12–14 dpi) in both groups of plants and even at 28 dpi, no significant difference could be noted between the two genotypes (Fig. 6A). RNA3 of CMV exhibited comparable accumulation in the inoculated leaves of both wild-type and ago2 plants. In the systemically infected leaves, only slightly higher viral RNA level could be detected in ago2 mutants relative to the wild-type plants. Y-sat was present in similar quantities in the inoculated leaves of both groups. However, in the systemically infected leaves of ago2 mutants, Y-sat accumulated to considerably higher level than in the equivalent leaves of wild-type plants. The significance of this observation remains to be investigated.
CMV infection is not affected by AGO2 deficiency in N. benthamiana. (A) Wild-type and ago2 N. benthamiana plants were infected with sap produced from Y-sat CMV infected N. benthamiana. Photographs of infected plants were taken at 28 dpi (top panel). At 14 dpi, RNA was prepared from the inoculated and the first systemically infected leaves. Samples were prepared from three plants and pooled. Accumulations of CMV RNA3 and sgRNA4 were monitored by northern blot using an RNA3-specific probe (second panel). Arrowheads indicate CMV RNA3 and sgRNA4. Y-sat levels were also analyzed by northern blot (third panel). The same filter was also hybridized with an actin probe as a loading control (bottom panel). (B) Wild-type and ago2 N. benthamiana plants were infected with sap produced from Fny CMV infected N. benthamiana. Mock infections were also performed with sap of uninfected plants. To monitor the progression of CMV infection, photographs of infected plants were taken at 14 dpi, 22 dpi and 28 dpi. At 7 dpi, RNA was prepared from the inoculated and the first symptomatic non-inoculated leaves. Samples were taken from three plants and pooled. Accumulations of CMV RNA3 and sgRNA4 were monitored by northern blot using an RNA3-specific probe. Arrowheads indicate CMV RNA3 and sgRNA4. Ethidium-bromide stained gel is shown as loading control.
Virus associated satellite and defective interfering (DI) RNAs can significantly modify symptom manifestation during virus infection58. Therefore, the surprising lack of effect of AGO2 on the progression of CMV infection might be due to the presence of the Y satellite. To inspect this question more closely, the highly aggressive Fny strain of CMV (which is not associated with any satellite RNA) was used to infect wild-type and ago2 plants, instead of the temperate Y-sat strain. Again, the two cohorts exhibited nearly identical symptoms until 28 dpi (Fig. 6B). No difference in the accumulation of RNA3 could be noted either between the two plant genotypes. In summary, the progression of CMV infection did not seem to be significantly influenced by AGO2 in N. benthamiana.
Discussion
Programmable DNA cleavage by CRISPR/Cas9 enables targeted inactivation of genes and it also serves the basis for more sophisticated genome engineering. This system has been demonstrated to work efficiently not only in single cells, but in whole organisms as well. In genetically intractable species, like the amphidiploid N. benthamiana, the use of this technology opens up avenues to generate mutants, which can be used for the genetic dissection of various biological phenomena. Thus far, in species like N. benthamiana largely RNAi based methodologies (antisense RNA, VIGS, shRNA) have been available to interrupt the functions of genes of interest. Although widely accepted, skeptics often point out that these techniques suppress gene expression indirectly and the knockdown is often only partial. Additionally, studying components of RNA silencing by using RNAi techniques presents the paradoxical situation, where one relies on the activity of those molecules, which he wishes to disable. Viral vectors applied for VIGS experiments (TRV, PVX etc.) may also interfere with various endogenous cellular functions and cause unintended changes in the expression of non-target genes, making it difficult to unambiguously interpret the results59. This can become especially problematic for studying host-virus interactions, since viruses often exhibit synergistic or antagonistic relationships with each other. Further complicating the issue is that viruses often encode proteins, which can inhibit RNA silencing (VSRs). To circumvent this problem, VSR deficient forms of viruses are frequently employed. However, the immense amounts of vsiRNAs generally produced during viral infections can themselves saturate and thereby neutralize the silencing effectors.
To circumvent the above problems, we used the CRISPR/Cas9 system to generate ago2 mutants of N. benthamiana. Using a specific sgRNA, mutations were introduced into the catalytically essential PIWI domain of AGO2. In a single transformation, we were able to generate plants with both alleles of the expressed AGO2 gene disrupted. During plant regeneration, chimera formation was frequently observed. However, repeated self-pollination rapidly produced plants with exclusively mutant ago2 alleles (T2 generation). The mutated alleles contained premature stop codons and as a consequence encoded truncated, dysfunctional AGO2 proteins. Stable inheritance of the mutations in subsequent generations was demonstrated (followed up to T3, Suppl. Fig. 2). Under normal growth conditions, the ago2 plants did not exhibit any obvious phenotypic alteration. However, AGO2 deficiency had a significant impact on the plant’s antiviral immunity, a feature reproducibly observed in successive generations of independent transformant lines (#1 and #9). Segregation of the Cas9 transgene from the induced mutation did not alter the phenotype either. Additionally, a transformant line carrying the Cas9 transgene on its own behaved indistinguishably from the non-transformed, parental N. benthamiana plants (Suppl. Fig. 3). Combined, these data confirmed that the observed phenotype of the ago2 plants was the consequence of the inactivation of the AGO2 gene and independent of the integration site of the T-DNA or the presence of the Cas9 transgene.
The lack of AGO2 had differential effects on the plants’ antiviral responses, upon which the viruses could be categorized into two groups:
-
(1)
AGO2 was a critical component of the antiviral response. The viruses that belonged to this group (PVX, TuMV and TCV) were quite dissimilar and the symptoms they elicited were also diverse. The lack of AGO2 resulted in apical necrosis and eventual lethality in both PVX and TuMV infected plants. Contrary, during TCV infection no localized necrosis was evident, however after 4 weeks general exacerbation of symptoms resulted in the death of the ago2 plants.
-
(2)
AGO2 had no or only a minor, modulatory effect on virus infection. This group included various tombusviruses and CMV. The AGO2 status of the plants did not influence the infection process caused by wild-type tombusviruses and only slightly affected the rate of recovery from infections elicited by their VSR deficient forms. These results are in agreement with a recent report suggesting that AGO1 is the main antiviral AGO involved in tombusvirus infection25. The symptoms of CMV infection on wild-type and ago2 mutant N. benthamiana plants were essentially indistinguishable.
How one can explain the differential need for AGO2 in the plant’s defensive measures elicited by various viruses? We hypothesize that the importance of AGO2 in a specific antiviral response may reflect its reliance on the use of secondary vsiRNAs. The critical contribution of RDR6-derived secondary vsiRNAs in PVX, potyvirus and TCV provoked antiviral responses has already been demonstrated41, 42, 44. Contrary, their role in tombusvirus infection seems to be negligible60. The lack of effect of AGO2 on CMV infection is however unexpected, as numerous studies have reported on the sensitizing effect of AGO2 mutation on CMV infection and symptom manifestation in A. thaliana 19, 45, 55. A possible explanation can be related to the observation that in N. benthamiana the down-regulation of RDR6 has not aggravated CMV infection41. This either suggests that secondary vsiRNAs do not play a critical role in anti-CMV immunity or more likely, other RDRs and/or AGOs (RDR2, AGO1, AGO5 etc.) may have overtaken the above functions of AGO2 and RDR6 in this plant species. Regardless, the surprising lack of effect of AGO2 on CMV infection in N. benthamiana, cautions against the simple extension of findings made in A. thaliana and emphasizes the need to employ additional model organisms to study host-virus interactions.
In summary, by using the CRISPR/Cas9 system we were able to generate an ago2 mutant line of N. benthamiana, and for the first time provided unequivocal proof for the essential role of AGO2 in antiviral immunity in a plant species other than A. thaliana. Most viruses are able to efficiently overcome the defense responses of their host plants. Thus, antiviral activities of components of RNA silencing can usually be studied using mutant viruses, which lack their cognate VSRs. However, VSRs are often multifunctional proteins involved not only in suppressing RNA silencing, but in many other aspects of the viral lifecycle. Therefore, their inactivation may result in viruses that are unable to carry out normal infection. The defenses that are effective against such crippled viruses may not be able to restrict the replication of wild-type ones. In this respect, it is especially significant that we were able to demonstrate the antiviral activity of AGO2 against a collection of highly diverse wild-type viruses including PVX, TuMV and TCV.
References
Wilson, R. C. & Doudna, J. A. Molecular mechanisms of RNA interference. Ann Rev Biophys 42, 217–239, doi:10.1146/annurev-biophys-083012-130404 (2013).
Voinnet, O. Origin, biogenesis, and activity of plant microRNAs. Cell 136, 669–687, doi:10.1016/j.cell.2009.01.046 (2009).
Martinez de Alba, A. E., Elvira-Matelot, E. & Vaucheret, H. Gene silencing in plants: a diversity of pathways. Biochim Biophys Acta 1829, 1300–1308, doi:10.1016/j.bbagrm.2013.10.005 (2013).
Ding, S. W. RNA-based antiviral immunity. Nat Rev Immunol 10, 632–644, doi:10.1038/nri2824 (2010).
Carthew, R. W. & Sontheimer, E. J. Origins and Mechanisms of miRNAs and siRNAs. Cell 136, 642–655, doi:10.1016/j.cell.2009.01.035 (2009).
Baulcombe, D. RNA silencing in plants. Nature 431, 356–363, doi:10.1038/nature02874 (2004).
Csorba, T., Kontra, L. & Burgyan, J. viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 479–480, 85–103, doi:10.1016/j.virol.2015.02.028 (2015).
Burgyan, J. & Havelda, Z. Viral suppressors of RNA silencing. Trends Plant Sci 16, 265–272, doi:10.1016/j.tplants.2011.02.010 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821, doi:10.1126/science.1225829 (2012).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096–1258096, doi:10.1126/science.1258096 (2014).
Hochstrasser, M. L. & Doudna, J. A. Cutting it close: CRISPR-associated endoribonuclease structure and function. Trends Biochem Sci 40, 58–66, doi:10.1016/j.tibs.2014.10.007 (2015).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823, doi:10.1126/science.1231143 (2013).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826, doi:10.1126/science.1232033 (2013).
Harrison, M. M., Jenkins, B. V., O’Connor-Giles, K. M. & Wildonger, J. A CRISPR view of development. Genes Dev 28, 1859–1872, doi:10.1101/gad.248252.114 (2014).
Barrangou, R. & Doudna, J. A. Applications of CRISPR technologies in research and beyond. Nat Biotech 34, 933–941, doi:10.1038/nbt.3659 (2016).
Wang, H., La Russa, M. & Qi, L. S. CRISPR/Cas9 in Genome Editing and Beyond. Ann Rev Biochem 85, 227–264, doi:10.1146/annurev-biochem-060815-014607 (2016).
Mallory, A. & Vaucheret, H. Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22, 3879–3889, doi:10.1105/tpc.110.080671 (2010).
Poulsen, C., Vaucheret, H. & Brodersen, P. Lessons on RNA silencing mechanisms in plants from eukaryotic argonaute structures. Plant Cell 25, 22–37, doi:10.1105/tpc.112.105643 (2013).
Carbonell, A. & Carrington, J. C. Antiviral roles of plant ARGONAUTES. Curr Opin Plant Biol 27, 111–117, doi:10.1016/j.pbi.2015.06.013 (2015).
Goodin, M. M., Zaitlin, D., Naidu, R. A. & Lommel, S. A. Nicotiana benthamiana: its history and future as a model for plant-pathogen interactions. Mol Plant Microbe Interact 21, 1015–1026, doi:10.1094/mpmi-21-8-1015 (2008).
Bhattacharjee, S. et al. Virus resistance induced by NB-LRR proteins involves Argonaute4-dependent translational control. Plant J 58, 940–951, doi:10.1111/j.1365-313X.2009.03832.x (2009).
Scholthof, H. B. et al. Identification of an ARGONAUTE for antiviral RNA silencing in Nicotiana benthamiana. Plant Physiol 156, 1548–1555, doi:10.1104/pp.111.178764 (2011).
Odokonyero, D. et al. Transgenic down-regulation of ARGONAUTE2 expression in Nicotiana benthamiana interferes with several layers of antiviral defenses. Virology 486, 209–218, doi:10.1016/j.virol.2015.09.008 (2015).
Fatyol, K., Ludman, M. & Burgyan, J. Functional dissection of a plant Argonaute. Nucleic Acids Res 44, 1384–1397, doi:10.1093/nar/gkv1371 (2016).
Kontra, L. et al. Distinct Effects of p19 RNA Silencing Suppressor on Small RNA Mediated Pathways in Plants. PLoS Pathog 12, e1005935, doi:10.1371/journal.ppat.1005935 (2016).
Maniatis, T., Fritsch, E. F. & Sambrook, J. Molecular Cloning: A Laboratory Manual. (Cold Spring Harbor Laboratory Press, 1982).
Li, J. F. et al. Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotech 31, 688–691, doi:10.1038/nbt.2654 (2013).
Stemmer, M., Thumberger, T., Del Sol Keyer, M., Wittbrodt, J. & Mateo, J. L. CCTop: An Intuitive, Flexible and Reliable CRISPR/Cas9 Target Prediction Tool. PloS One 10, e0124633, doi:10.1371/journal.pone.0124633 (2015).
Clemente, T. in Agrobacterium Protocols Vol. 1 Methods in Molecular Biology (ed K. Wang) 143–154 (Humana Press, 2006).
Peart, J. R., Cook, G., Feys, B. J., Parker, J. E. & Baulcombe, D. C. An EDS1 orthologue is required for N-mediated resistance against tobacco mosaic virus. Plant J 29, 569–579, doi:10.1046/j.1365-313X.2002.029005569.x (2002).
Garcia-Ruiz, H. et al. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection. Plant Cell 22, 481–496, doi:10.1105/tpc.109.073056 (2010).
Shimura, H. et al. A viral satellite RNA induces yellow symptoms on tobacco by targeting a gene involved in chlorophyll biosynthesis using the RNA silencing machinery. PLoS Pathog 7, e1002021, doi:10.1371/journal.ppat.1002021 (2011).
Nakasugi, K. et al. De novo transcriptome sequence assembly and analysis of RNA silencing genes of Nicotiana benthamiana. PloS One 8, e59534, doi:10.1371/journal.pone.0059534 (2013).
Nakasugi, K., Crowhurst, R., Bally, J. & Waterhouse, P. Combining transcriptome assemblies from multiple de novo assemblers in the allo-tetraploid plant Nicotiana benthamiana. PloS One 9, e91776, doi:10.1371/journal.pone.0091776 (2014).
Andika, I. B. et al. Differential contributions of plant Dicer-like proteins to antiviral defences against potato virus X in leaves and roots. Plant J 81, 781–793, doi:10.1111/tpj.12770 (2015).
Andika, I. B. et al. Different Dicer-like protein components required for intracellular and systemic antiviral silencing in Arabidopsis thaliana. Plant Signal Behav 10, e1039214, doi:10.1080/15592324.2015.1039214 (2015).
Jaubert, M., Bhattacharjee, S., Mello, A. F., Perry, K. L. & Moffett, P. ARGONAUTE2 mediates RNA-silencing antiviral defenses against Potato virus X in Arabidopsis. Plant Physiol 156, 1556–1564, doi:10.1104/pp.111.178012 (2011).
Brosseau, C. & Moffett, P. Functional and Genetic Analysis Identify a Role for Arabidopsis ARGONAUTE5 in Antiviral RNA Silencing. Plant Cell 27, 1742–1754, doi:10.1105/tpc.15.00264 (2015).
Komatsu, K. et al. Viral-induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol Plant Microbe Interact 23, 283–293, doi:10.1094/mpmi-23-3-0283 (2010).
Garcia-Ruiz, H. et al. Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip mosaic virus infection. PLoS Pathog 11, e1004755, doi:10.1371/journal.ppat.1004755 (2015).
Schwach, F., Vaistij, F. E., Jones, L. & Baulcombe, D. C. An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol 138, 1842–1852, doi:10.1104/pp.105.063537 (2005).
Vaistij, F. E. & Jones, L. Compromised virus-induced gene silencing in RDR6-deficient plants. Plant Physiol 149, 1399–1407, doi:10.1104/pp.108.132688 (2009).
Pruss, G., Ge, X., Shi, X. M., Carrington, J. C. & Bowman Vance, V. Plant viral synergism: the potyviral genome encodes a broad-range pathogenicity enhancer that transactivates replication of heterologous viruses. Plant Cell 9, 859–868, doi:10.1105/tpc.9.6.859 (1997).
Qu, F., Ye, X. & Morris, T. J. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci USA 105, 14732–14737, doi:10.1073/pnas.0805760105 (2008).
Harvey, J. J. et al. An antiviral defense role of AGO2 in plants. PloS One 6, e14639, doi:10.1371/journal.pone.0014639 (2011).
Zhang, X., Zhang, X., Singh, J., Li, D. & Qu, F. Temperature-dependent survival of Turnip crinkle virus-infected arabidopsis plants relies on an RNA silencing-based defense that requires dcl2, AGO2, and HEN1. J Virol 86, 6847–6854, doi:10.1128/jvi.00497-12 (2012).
Thomas, C. L., Leh, V., Lederer, C. & Maule, A. J. Turnip crinkle virus coat protein mediates suppression of RNA silencing in Nicotiana benthamiana. Virology 306, 33–41, doi:10.1016/S0042-6822(02)00018-1 (2003).
Vargason, J. M., Szittya, G., Burgyan, J. & Hall, T. M. Size selective recognition of siRNA by an RNA silencing suppressor. Cell 115, 799–811, doi:10.1016/S0092-8674(03)00984-X (2003).
Lakatos, L., Szittya, G., Silhavy, D. & Burgyan, J. Molecular mechanism of RNA silencing suppression mediated by p19 protein of tombusviruses. EMBO J 23, 876–884, doi:10.1038/sj.emboj.7600096 (2004).
Scholthof, H. B. The Tombusvirus-encoded P19: from irrelevance to elegance. Nat Rev Microbiol 4, 405–411, doi:10.1038/nrmicro1395 (2006).
Mourrain, P. et al. Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101, 533–542, doi:10.1016/S0092-8674(00)80863-6 (2000).
Vaucheret, H., Beclin, C. & Fagard, M. Post-transcriptional gene silencing in plants. J Cell Sci 114, 3083–3091 (2001).
Zhang, X. et al. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev 20, 3255–3268, doi:10.1101/gad.1495506 (2006).
Gonzalez, I. et al. Cucumber mosaic virus 2b protein subcellular targets and interactions: their significance to RNA silencing suppressor activity. Mol Plant Microbe Interact 23, 294–303, doi:10.1094/mpmi-23-3-0294 (2010).
Wang, X. B. et al. The 21-nucleotide, but not 22-nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in Arabidopsis thaliana. Plant Cell 23, 1625–1638, doi:10.1105/tpc.110.082305 (2011).
Hamera, S., Song, X., Su, L., Chen, X. & Fang, R. Cucumber mosaic virus suppressor 2b binds to AGO4-related small RNAs and impairs AGO4 activities. Plant J 69, 104–115, doi:10.1111/j.1365-313X.2011.04774.x (2012).
Smith, N. A., Eamens, A. L. & Wang, M. B. Viral small interfering RNAs target host genes to mediate disease symptoms in plants. PLoS Pathog 7, e1002022, doi:10.1371/journal.ppat.1002022 (2011).
Palukaitis, P. Satellite RNAs and Satellite Viruses. Mol Plant Microbe Interact 29, 181–186, doi:10.1094/mpmi-10-15-0232-fi (2016).
Olah, E., Pesti, R., Taller, D., Havelda, Z. & Varallyay, E. Non-targeted effects of virus-induced gene silencing vectors on host endogenous gene expression. Arch Virol 161, 2387–2393, doi:10.1007/s00705-016-2921-9 (2016).
Szittya, G. et al. Structural and functional analysis of viral siRNAs. PLoS Pathog 6, e1000838, doi:10.1371/journal.ppat.1000838 (2010).
Acknowledgements
We thank Jen Sheen for providing the plant codon optimized SpCas9 expression vector, Peter Moffett for the PVX-GFP binary plasmid and James Carrington for the TuMV-GFP binary plasmid. We also thank Anita Schamberger for helpful discussions and critically reading the manuscript. This work was funded by Agricultural Ministry of Hungary [BJ032], [BJ045]; Hungarian Scientific Research Fund [NK105850], [K112737].
Author information
Authors and Affiliations
Contributions
M.L. and K.F. designed the experiments, performed the experimental work and analyzed data. J.B. advised the work and provided financial support. K.F. wrote the manuscript and prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ludman, M., Burgyán, J. & Fátyol, K. Crispr/Cas9 Mediated Inactivation of Argonaute 2 Reveals its Differential Involvement in Antiviral Responses. Sci Rep 7, 1010 (2017). https://doi.org/10.1038/s41598-017-01050-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01050-6
This article is cited by
-
CRISPR/Cas9-mediated knockout of the DCL2 and DCL4 genes in Nicotiana benthamiana and its productivity of recombinant proteins
Plant Cell Reports (2022)
-
Targeted genome editing of plants and plant cells for biomanufacturing
Transgenic Research (2021)
-
CRISPR/Cas9-mediated knockout of the RDR6 gene in Nicotiana benthamiana for efficient transient expression of recombinant proteins
Planta (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.