Abstract
High concentrations of hydrogen sulfide (H2S) are toxic to plants and inhibit their growth. Previous research indicated that high concentrations of H2S modulate the root system architecture (RSA) by affecting auxin transport; however, the signaling pathway underlying this process remains unclear. Here, we investigated the effects of exogenous sodium hydrosulfide (NaHS), an H2S donor, on primary root (PR) growth in Arabidopsis using pharmacological, physiological, and genetic approaches. H2S toxicity repressed PR growth by triggering a signal transduction pathway involving reactive oxygen species (ROS) accumulation, MITOGEN-ACTIVATED PROTEIN KINASE 6 (MPK6) activation, and nitric oxide (NO) production. Respiratory burst oxidase homolog mutants and an NO synthase mutant were less sensitive to NaHS, suggesting that both ROS and NO mediate the inhibitory effects of H2S on PR growth. We found that exogenous H2S-activated ROS production was required for NO generation and that MPK6 mediated H2S-induced NO production. MPK6 was shown to function downstream of ROS and upstream of NO. Finally, we demonstrated that exogenous H2S repressed the distribution of auxin and reduced the meristematic cell division potential in root tips, and NO was involved in this process.
Similar content being viewed by others
Introduction
Hydrogen sulfide (H2S) is a colorless gas with a characteristic odor of rotten eggs. Low concentrations of H2S improve the tolerance of plants to pathogens1, osmotic stress, salt stress, heat shock, and heavy metal stresses2,3,4,5,6. In plants, H2S is predominantly produced by L-cysteine desulfhydrase (DES; EC 4.4.1.1)7. Endogenous H2S plays a role in modulating plant growth and development, including seed germination, root organogenesis, stomatal closure, plant maturation and flower senescence8,9,10,11. Although low concentrations of H2S improve the tolerance of plants to abiotic and biotic stresses, high concentrations are toxic to plant growth. H2S toxicity-induced primary root (PR) growth inhibition has been reported11; however, the signaling pathway underlying H2S toxicity-mediated root growth and development is still unclear.
Nitric oxide (NO) is a small gas molecule that mediates lateral root (LR) formation, adventitious root growth, and root hair development12, 13. Our previous work indicated that NO inhibits PR growth, whereas it promotes LR development14. In animals, several studies have identified possible crosstalk between H2S and NO15. H2S physiologically modifies the cysteines in a large number of proteins via S-sulfhydration. Thus, sulfhydration appears to be a physiological posttranslational modification of proteins16. H2S increases NO production by inducing the S-sulfhydration of endothelial NO synthase (eNOS), promoting its phosphorylation, inhibiting its S-nitrosylation, and increasing eNOS dimerization (the activated form of eNOS)17. In plants, an interaction between H2S and NO in modulating plant growth and development has been reported9, 10, 18. H2S promotes NO production and acts upstream of NO to modulate abscisic acid (ABA)-dependent stomatal closure10. H2S acts upstream of indole-3-acetic acid (IAA) and NO to regulate root growth and development9; however, the signaling modulation mechanisms involved are largely unclear.
Mitogen-activated protein kinase (MAPK) cascades, which consist of MAPKKK (MEKK), MAPKK (MKK), and MAPK (MPK), are highly conserved signaling transduction pathways found in animals, plants and microbes19, 20. In plants, MAPK pathways are implicated in the regulation of growth and development and in responses to many environmental cues. The activation of MPKs alters their subcellular localization and their interactions with and phosphorylation of transcription factors, thereby reprogramming gene expression20, 21. Arabidopsis MPK3/6 are the most extensively studied MPKs in plants. Previous studies have revealed that MPK3/6 modulate plant growth, development, and stress tolerance by interacting with the ABA, ethylene, jasmonate, phosphatidic acid, Ca2+, and reactive oxygen species (ROS) pathways21,22,23,24,25,26.
Root growth and development are largely influenced by plant hormones, especially auxin27. Auxin is a central regulator of root formation. Auxin flux is essential for auxin to regulate of stem cell differentiation and root development28, 29. Auxin is an important phytohormone involved in controlling the balance between cell division and differentiation in the root meristem30. H2S-mediated root formation is alleviated by the IAA transport inhibitor N-1-naphthylphthalamic acid (NPA) and the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), suggesting that both IAA and NO are involved in H2S-mediated root system development9. Auxin-induced H2S generation is involved in LR formation in tomato31. Recently, Jia et al.11 showed that high levels of H2S inhibit auxin transport and result in alterations in root system development by modulating the polar subcellular distribution of PIN proteins11.
Another important pathway for regulating root system development independent of plant hormones is ROS signaling. The transcriptional regulation of ROS by the UPBEAT1 (UPB1) transcription factor modulates root development by regulating cell proliferation and differentiation in root tips30.
In this study, using pharmacological and genetic approaches, we analyzed the possible involvement of ROS, NO, and MPK6 in exogenous H2S-mediated PR growth. Our results indicated that H2S toxicity-inhibited PR growth via the ROS-MPK6-NO signaling pathway. The potential mechanisms involved in this process are discussed.
Results
H2S toxicity inhibits PR growth by reducing the meristematic cell division potential
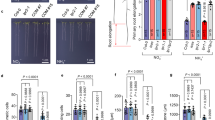
Five-day-old seedlings germinated on 1/2 MS medium were transferred to fresh medium with 0–800 μM NaHS, an H2S donor, and PR growth was measured 2 d after transfer to determine how exogenous H2S affects PR growth. With increasing NaHS concentrations of 200 μM to 800 μM, PR growth was inhibited by 34% to 51% (Fig. 1a, Fig. S1). To examine the inhibitory effects of H2S on PR growth, we also measured the length of the meristem zone in H2S-treated roots. As shown in Fig. 1b, the lengths of the meristematic zones decreased by 27% and 36.6% in roots treated with 200 μM and 800 μM exogenous NaHS, respectively. Because treatment with 500 μM NaHS induced an approximately 50% decrease in PR growth, we selected this concentration for use in subsequent experiments.
NaHS treatment inhibited PR growth. (a,b) Five-day-old wild-type seedlings grown in 1/2 MS medium were treated with 100–800 μM NaHS for 2 d, and (a) PR growth and (b) the length of the meristem zone were measured after treatment. ck, untreated control. n = 60. Error bars represent ± SD. Different letters indicate significantly different values (P < 0.05 by Tukey’s test).
ROS are involved in H2S toxicity-induced PR growth inhibition
Two independent signaling pathways regulate root growth and development: auxin signaling and the ROS signaling pathway30. Jia et al.11 found that H2S toxicity inhibits auxin transport and results in alterations in root system development11. To investigate whether ROS are also involved in H2S toxicity-induced PR growth inhibition, we assessed ROS production in the roots using the ROS-specific-fluorescent probe 2,7-dichlorofluorescin diacetate (DCFH-DA). Exogenous H2S increased ROS levels (Fig. 2a and b) in the roots; this increase was sustained for 24 h, after which ROS production gradually declined.
NaHS induces the accumulation of ROS. (a,b) Detection of ROS production in the roots of 5-d-old Col-0 seedlings exposed to 500 μM NaHS for periods of up to 2 d using the ROS-specific fluorescent probe DCFH-DA (a) and quantification of ROS-specific fluorescence intensities (b) in plants treated as described in (a). The fluorescence intensity of the untreated roots was set to 100. Bars, 100 μm. n = 30. (c) Relative root growth of Col-0 seedlings treated with 500 μM NaHS in the presence or absence of 1 μM DPI, 1 mM KI, and 1 mM H2O2 for 2 d compared with untreated seedlings. (d) Relative root growth of col-0, rbohF, and rbohD/F seedlings treated with 500 μM NaHS for 2 d compared with untreated seedlings. n = 45. Error bars represent ± SD. Different letters indicate significantly different values (P < 0.05 by Tukey’s test).
To further assess whether ROS participate in the H2S-mediated inhibition of root growth, we next observed the effects of diphenylene iodonium (DPI), an inhibitor of NADPH oxidase, and potassium iodide (KI), an ROS scavenger, on the H2S-mediated inhibition of root growth in the roots of wild-type plants. DPI and KI alleviated, whereas exogenous H2O2 increased he H2S-induced inhibition of root growth (Fig. 2c, Fig. S2). To corroborate these pharmacological data, we further used the Arabidopsis ROS-biosynthesis-related respiratory burst oxidase homologue (Rboh) NADPH oxidase single and double mutants rbohF and rbohD/F and found that PR growth was 31% and 20% greater in rbohF and rbohD/F than in wild-type plants subjected to exogenous H2S treatment (Fig. 2d and e). These data confirm the essential role of ROS in the modulation of root growth by H2S.
NO is also required for the inhibition of PR growth by exogenous H2S
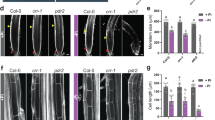
NO is another important gaseous signaling molecule that mediates root development12, 13. We therefore investigated NO production in the roots using the NO-specific fluorescent probe, 4,5-diaminofluorescein diacetate (DAF-2DA). Similar to its effects on ROS production, exogenous H2S triggered an increase in NO production in the roots; this increase was sustained for 24 h, after which NO production gradually declined (Fig. 3a and b). We further examined whether NO is involved in H2S-mediated PR growth inhibition. As shown in Fig. 3c and Fig. S3, supplementation with the NO synthase (NOS) inhibitor N G-nitro-L-Arg-methyl ester (L-NAME), or the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) alleviated the NaHS-induced inhibition of root growth by 54.7% and 21.9%, respectively. Consistent with these pharmacological results, the NO biosynthesis-related nia1-2/2-5 double mutant and the noa1 mutant were less sensitive to exogenous H2S treatment than were wild-type plants (Fig. 3d and e).
NO is involved in the NaHS-mediated inhibition of PR growth. (a,b) Detection of NO production in the roots of 5-d-old wild-type seedlings exposed to 500 μM NaHS for periods of up to 2 d using the NO-specific fluorescence probe DAF-2 DA (a) and quantification of NO-specific fluorescence intensities (b) in plants treated as described in (a). The fluorescence intensity of the untreated roots was set to 100. Bars, 100 μm. n = 30. (c) Relative root growth of Col-0 seedlings treated with 500 μM NaHS in the presence or absence of 500 μM L-NAME and 200 μM cPTIO for 2 d compared with untreated seedlings. (d) Relative root growth of Col-0, nia1/2, and noa1 seedlings treated with 500 μM NaHS for 2 d compared with untreated seedlings. n = 45. Error bars represent ± SD. Different letters indicate significantly different values (P < 0.05 by Tukey’s test).
We found that supplementation with KI or DPI markedly inhibited NO production compared with that of the exogenous H2S treatment alone in the roots (Fig. 4a and b), whereas supplementation with L-NAME did not alter ROS levels in exogenous H2S-treated roots (Fig. 4c and d). To further confirm the relationship between ROS and NO in the exogenous H2S-mediated inhibition of root growth, we assessed the rbohD/F, nia1-2/2-5, and noa1 mutants. We found that exogenous H2S-induced NO production was abolished in the rbohD/F double mutant, while supplementation with H2O2 markedly induced NO production in both the Col-0 and the rbohD/F double mutant (Fig. 4a and b). Meanwhile, H2S-induced ROS production was similar in the col-0, nia1-2/2-5, and noa1 lines (Fig. 4c and d). These data indicate that ROS is involved in exogenous H2S-induced NO production in the roots.
(a,b) Detection of NO production in the roots of 5-d-old wild-type Col-0 and rbohD/F seedlings exposed to 500 μM NaHS with or without 1 μM DPI, 1 mM KI, and 1 mM H2O2 for 24 h using the NO-specific fluorescent probe DAF-2 DA (a) and quantification of NO-specific fluorescence intensities (b) in plants treated as described in (a). (c,d) Detection of H2O2 production in the roots of 5-d-old wild-type Col-0, nia1/2, and noa1 seedlings exposed to 500 μM NaHS with or without 500 μM L-NAME for 24 h using the ROS-specific fluorescent probe DCFH-DA (c) and quantification of H2O2-specific fluorescence intensities (d) in plants treated as described in (c). The fluorescence intensity of the untreated roots was set to 100. Bars, 100 μm. n = 30. ck, untreated control. Error bars represent ± SD.
We also found that supplementation with the NO donor SNAP completely reversed the insensitivity of the rbohF, rbohD/F, and noa1 mutants to exogenous H2S (Fig. 5a and b). Additionally, supplementation with H2O2 completely reversed the insensitivity of the rbohF and rbohD/F mutants to exogenous H2S (Fig. 5a), but did not rescue the insensitivity of the noa1 mutant (Fig. 5b). Taken together, these data suggest that exogenous H2S-induced ROS production functions upstream of NO accumulation in the roots.
(a) Relative root growth of Col-0, rbohF, and rbohD/F seedlings exposed to 500 μM NaHS with or without 1 mM H2O2, 100 μM SNAP, and 500 μM L-NAME for 2 d compared with untreated seedlings. (b) Relative root growth of Col-0 and noa1 seedlings exposed to 500 μM NaHS with or without 1 mM H2O2 and 100 μM SNAP for 2 d compared with untreated seedlings. n = 45. Error bars represent ± SD. Different letters indicate significantly different values (P < 0.05 by Tukey’s test).
MPK6 mediates the inhibition of PR growth by exogenous H2S
Previous studies have indicated that MAPK is involved in the ROS signaling pathway21, 24, 25. To determine whether MAPK proteins mediate exogenous H2S-induced PR growth inhibition, we first observed the effects of PD98059, an inhibitor of MAPK, on PR growth in wild-type Col-0 plants. As shown in Fig. 6a and Fig. S4, supplementation with PD98059 alleviated the exogenous H2S-induced inhibition of PR growth by 29.6%, suggesting that MAPK is involved in the modulation of PR growth by exogenous H2S in Arabidopsis. To confirm the role of MAPK in the exogenous H2S-mediated inhibition of root growth, we used the Arabidopsis mutant mpk6. As shown in Fig. 6b, the inhibition of PR growth by exogenous H2S was alleviated by 37% in the mpk6 mutant compared with Col-0 seedlings. These data provide genetic evidence showing that MPK6 plays an important role in the inhibition of PR growth by exogenous H2S. Together with the pharmacological data described above, these results clearly indicate that MPK6 is a positive regulator that mediates the exogenous H2S-induced inhibition of PR growth in Arabidopsis.
MPK6 mediates PR growth inhibition by NaHS. (a) Relative root growth of Col-0 seedlings treated with 500 μM NaHS in the presence or absence of 150 μM PD98059 for 2 d compared with untreated seedlings. (b) Relative root growth of Col-0 and mpk6 seedlings exposed to 500 μM NaHS with or without 1 mM H2O2, 100 μM SNAP, 1 mM KI, and 500 μM L-NAME for 2 d compared with untreated seedlings. n = 45. (c,d) Detection of ROS production in the roots of 5-d-old wild-type col-0 and mpk6 seedlings exposed to 500 μM NaHS with or without 150 μM PD98059 for 24 h by the ROS-specific fluorescent probe DCFH-DA (c) and the quantification of the ROS-specific fluorescence intensities (d) in plants treated as described in (c). (e,f) Detection of NO production in the roots of 5-d-old wild-type col-0 and mpk6 seedlings exposed to 500 μM NaHS with or without 1 mM H2O2 or 150 μM PD98059 for 24 h using the NO-specific fluorescence probe DAF-2 DA (e) and quantification of NO-specific fluorescence intensities (f) in plants treated as described in (e). n = 30. Bars, 100 μm. ck, untreated control. Error bars represent ± SD. Different letters indicate significantly different values (P < 0.05 by Tukey’s test).
MPK6 promotes NO production downstream of ROS in the exogenous H2S-mediated inhibition of PR growth
The above results suggested that ROS, NO, and MPK6 are important intermediate signaling molecules in the modulation of PR growth by H2S. We then examined the relationship between ROS, NO, and MPK6 in the exogenous H2S-mediated inhibition of PR growth. For this purpose, we measured the production of ROS and NO in roots treated with exogenous H2S. In H2S-treated roots, the MAPK inhibitor PD98059 did not affect ROS production, but it markedly inhibited NO production (Fig. 6c–f). Additionally, in the roots of the mpk6 mutant compared with those of Col-0, H2S treatment did not affect ROS production, but NO production was abolished (Fig. 6c–f). ROS-induced NO production was also repressed in the mpk6 mutant (Fig. 6e and f). Supplementation with KI alleviated the H2S-mediated inhibition of PR growth by 16% in Col-0 roots but could not further alleviate the H2S-mediated inhibition of PR growth in mpk6 roots. In contrast, supplementation with H2O2 increased the H2S-mediated inhibition of PR growth by 51% in the Col-0 roots; however, the inhibitory effects of H2O2 on PR growth were weaker in mpk6 seedlings than in Col-0 seedlings exposed to exogenous H2S (Fig. 6b). These weaker effects may have occurred because the loss of MPK6 in the mpk6 mutant resulted in the inhibition of the ROS signaling transduction pathway.
We next analyzed the effects of NO on PR growth inhibition in the mpk6 mutant and found that supplementation with the NO donor SNAP increased the inhibitory effects of H2S on PR growth, while supplementation with L-NAME reduced these effects in both mpk6 and Col-0 seedlings (Fig. 6b). Compared with Col-0 plants, supplementation with SNAP or L-NAME rescued the insensitivity of the mpk6 mutant to exogenous H2S-induced PR growth inhibition (Fig. 6b). These data indicated that MPK6 mediated inhibition of PR growth downstream of ROS and upstream of NO.
NO is involved in exogenous H2S-mediated inhibition of PR growth by regulating auxin distribution in root tips
Auxin plays a central role in modulating root growth and development32. Previous work indicated that H2S toxicity reduced auxin accumulation in root tips11. We therefore investigated whether H2S-induced NO production is involved in the regulation of auxin distribution. For this purpose, we used an auxin-perceptive DII-VENUS line to monitor the possible changes in auxin distribution in H2S-treated plants in the presence or absence of the NOS inhibitor L-NAME. Consistent with the result of Jia et al.11, our data here showed that the DII-VENUS fluorescence were higher in NaHS-treated roots than in untreated plants11. Supplementation with L-NAME decreased the DII-VENUS fluorescence in H2S-treated seedlings (Fig. 7a), suggesting that H2S-induced NO production affects the distribution of auxin.
Discussion
At low concentrations, H2S is an important regulator of the stress response, which is essential for stress tolerance and survival in plants3, 5, 6. However, high concentrations of H2S are toxic to plants and inhibit their growth. A recent investigation indicated that high concentrations of H2S inhibit root growth by regulating auxin accumulation in root tips11; however, the molecular mechanisms underlying this process are largely unclear. In the present study, we elucidated a signaling pathway that controls H2S toxicity-induced PR growth inhibition in Arabidopsis. We showed that the activation of MPK6, NADPH oxidase-dependent H2O2 synthesis, Nia1/NOA1-dependent NO production, and the regulation of auxin perception are all required for the inhibition of PR growth by exogenous H2S. Furthermore, we found that exogenous H2S-induced NO production is mediated by ROS and that the modulation of NO production by ROS required the activation of MPK6. We also showed that exogenous H2S-mediated changes in auxin distribution were regulated by NO. Therefore, our study elucidates an H2S toxicity-mediated root growth signaling pathway that involves a cascade of NADPH oxidase-dependent ROS production, which, in turn, leads to MPK6- and Nia1/NOA1-dependent NO production.
H2S toxicity-stimulated ROS production inhibits PR growth
Previous reports have shown that ROS are involved in the modulation of PR growth and LR formation by phytohormones or environmental cues10, 14, 18, 33. There are different ROS sources in plants, including cell wall peroxidases, NADPH oxidases, and amine oxidase-type enzymes34,35,36. In this study, we found that exogenous H2S-induced ROS production was markedly inhibited by the NADPH oxidase inhibitor DPI and that the NADPH oxidase null mutants rbohF and rbohD/F were defective in exogenous H2S-induced ROS generation and showed a smaller reduction in PR growth than did wild-type plants. In contrast, supplementation with H2O2 further increased H2S-induced inhibition of root growth. These results indicated that exogenous H2S induces ROS generation in the roots and inhibits subsequent PR growth.
ROS-activated MPK6 promotes NO production in H2S-treated plants
In plants, MPKs are important signaling molecules that are activated in response to a variety of environmental and developmental cues21, 26. In this study, we showed that MPK6 plays an important role in the regulation of the H2S toxicity-mediated inhibition of root growth. The MAPK inhibitor PD98059 alleviated the H2S-induced inhibition of PR growth, indicating that the activation of MAPK is required for this inhibition. The mpk6 mutant showed reduced inhibition of PR growth following exogenous H2S treatment, providing genetic evidence for the essential role of MPK6 in H2S toxicity-mediated root growth.
Previous studies have indicated that MPK6 modulates plant growth and the response to stimuli by interacting with ROS and/or NO26. NO promots Cd2+-induced programmed cell death (PCD) by enhancing MPK6-mediated caspase-3-like activation in Arabidopsis 37. MPK6 controls H2O2-induced root growth by mediating the Ca2+ influx in Arabidopsis 26. The mpk6 mutant produces more and LRs than do wild-type plants after application of the NO donor sodium nitroprusside (SNP) or H2O2 38. These studies indicate that MPK6 modulates NO production and the response to ROS during root development; however, it is unknown whether and how the interactions between MPK6, ROS, and NO mediate the H2S toxicity-induced inhibition of PR growth. In this study, we found that the H2S toxicity-induced production of ROS and NO and inhibition of PR growth were markedly impaired in the rbohF and rbohD/F mutants. Moreover, the H2S-induced increase in NO and inhibition of PR growth were also impaired in the mpk6, nia1-2/2-5, and noa1 mutants. ROS rescued the defect in the H2S-induced inhibition of PR growth in the rbohF, rbohD/F, mpk6, nia1-2/2-5, and noa1 mutants, while only NO could rescue the defect in the H2S-induced inhibition of PR growth in the nia1-2/2-5 and noa1 mutants. These data confirmed that ROS-activated MPK6 promoted NO production and thereby reduced PR growth in H2S-treated plants. Our results show that ROS-activated MPK6 and NO production are involved in the H2S toxicity-mediated inhibition of PR growth and suggest that the ROS-MPK6-NO pathway is a general signaling transduction pathway that regulates plant responses to abiotic stress. The phosphorylation of NIA2 by MPK6 leads to increases in nitrate reductase (NR) activity and NO production38. However, how MPK6 mediates NO production via the NO synthase-associated (NOA) pathway remains unclear.
H2S toxicity-induced NO production inhibits PR growth
Similar to its effect on ROS, we found that H2S toxicity triggered NO accumulation in root tips within 24 h, which gradually decreased after 48 h of treatment, suggesting that both ROS and NO could act as early signaling molecules to modulate downstream gene expression, ultimately leading to PR growth inhibition.
It has been proposed that there are multiple sources of NO generation in plant cells, including NR39, the NO synthase-like/NO synthase-associated (NOS-like/NOA) enzyme40, and non-enzymatic pathways41. The roles of the NOA pathway and the NR pathway in NO generation in vivo have been well studied. Previous reports have shown that L-NAME, an L-arginine analog, inhibits NOS activity in plants42. In this study, we found that supplementation with the NOS inhibitor L-NAME markedly repressed the production of NO in H2S-treated roots. Our genetic experiments also showed that H2S-induced NO production and PR growth inhibition were markedly impaired in the NOA1-defective mutant noa1 and the NR-defective nia1-2/2-5 double mutant. These results suggested that NO production in H2S-treated seedlings may be catalyzed through both L-Arg-dependent and NR-dependent routes. Interestingly, Lisjak et al.43 found that the exogenous H2S donor NaHS repressed the ABA-induced accumulation of NO in guard cells43. The differences in these effects between studies may be due to the different tissues examined.
Previous studies have indicated that NO acts as a second messenger to regulate root growth via the auxin pathway44, 45. This prompted us to assess the possible involvement of NO in the H2S-mediated auxin distribution. Jia et al.11 reported that H2S reduces auxin accumulation in root tips by disrupting the expression of auxin carriers and subsequent polar auxin transport (PAT)11. Consistent with these findings, our data indicated that H2S toxicity increased DII-VENUS fluorescence in roots, while supplementation with L-NAME decreased DII-VENUS fluorescence. These data suggest that NO accumulation is responsible for the H2S-induced inhibition of the distribution of auxin in root tips.
Taken together, our data indicate that in addition to the auxin pathway, ROS and NO are also key players in the plant response to H2S toxicity. ROS production in roots induced by a high concentration of H2S inhibited PR growth, while ROS accumulation in roots activated MPK6. MPK6 then promoted NO production through both L-Arg- and NR-dependent routes. Elevated NO repressed the distribution of auxin in root tips, thereby reducing PR growth (Fig. 8). These results indicate that H2S toxicity inhibits PR growth via both the ROS pathway and the auxin signaling pathway. These findings show that the ROS-MPK6-NO signaling pathway mediates plant responses to H2S toxicity through morphological and physiological changes in the roots.
H2S toxicity inhibits PR growth via the ROS-MPK6-NO signaling pathway. High-concentration H2S induced ROS production via the NADPH oxidase pathway, which directly inhibited PR growth and activated MPK6. MPK6 then promoted NO production through both L-Arg-dependent and NR-dependent routes. Elevated NO repressed auxin distribution, ultimately inhibiting PR growth.
Methods
Plant growth and chemical treatments
The Arabidopsis thaliana ecotype Columbia-0 was used in this study. The transgenic and mutant lines employed in this work included DII-YFP, nia1/2, noa1, rbohF, rbohD/F, and mpk6. Seeds were surface sterilized with 5% bleach for 5 min, then washed five times with sterile water, incubated for 2 d at 4 °C in the dark and plated onto agar medium containing half-strength MS (Sigma), pH5.70, supplemented with 1% agar and 10% sucrose. Seedlings were grown in a growth chamber maintained at 22 °C under a 16/8 h light/dark cycle. Seeds were grown in the vertical position. Five-day-old seedlings were transferred to plates supplemented with various chemicals, including sodium hydrosulfide (NaHS), cPTIO, L-NAME, DPI, KI, SNAP, H2O2, and PD98059, and grown for an additional 2 d. All chemicals were obtained from Sigma-Aldrich.
Phenotypic analysis
Seeds were grown in the vertical position. After the seedlings were transferred to plates supplemented with various chemicals, root growth was measured at the same time every day. After 5 d of treatment, the PR length was measured and statistically analyzed. The length of the meristematic zone was measured from the quiescent center (QC) to the beginning of the root elongation zone as described by Liu et al.32. At least 15 replicate plants were measured for each treatment.
Measurement of NO and ROS production and fluorescence microscopy
The endogenous NO levels in root meristems were visualized using the specific NO fluorescent probe DAF-2 DA. Seedlings were incubated at 37 °C in a 5 μM staining solution for 1 h. The endogenous ROS levels in root meristems were visualized using the specific ROS fluorescent probe DCFH- DA. Seedlings were incubated at 37 °C in 10 μM staining solution for 5 min. Then, they were washed twice and viewed under a microscope. Quantitative measurement of fluorescence intensity was performed using ImageJ.
Roots were treated with DAF-2 DA and DCFH-DA and analyzed using fluorescence microscopy (Zeiss; NO: excitation 495 nm, emission 515 nm; ROS: excitation 488 nm, emission 525 nm). DII-VENUS fluorescence was observed using confocal laser scanning microscopy (Zeiss) according to the manufacturer’s instructions. The excitation and emission wavelengths were 488 to 520 nm for YFP.
Statistical analysis
For the PR growth and fluorescence microscopy analysis, the experiments were repeated three times with 15–20 seedlings in each repeat. The data were analyzed using Image-Pro Plus software (version 4.5.1.29; Media Cybernetics, Carlsbad, CA) and SPSS (Statistical Package for the Social Sciences) software. The results are presented as the mean ± SD. For statistical analysis, we used Tukey’s test (P < 0.05)46, 47.
References
Bloem, E. et al. Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. J. Exp. Bot. 55, 2305–2312, doi:10.1093/jxb/erh236 (2004).
Zhang, H. et al. Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J. Integr. Plant Biol. 50, 1518–1529, doi:10.1111/jipb.2008.50.issue-12 (2008).
Christou, A., Manganaris, G. A., Papadopoulos, I. & Fotopoulos, V. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 64, 1953–1966, doi:10.1093/jxb/ert055 (2013).
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X. & Shen, Z. Z. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 36, 1564–1572, doi:10.1111/pce.2013.36.issue-8 (2013).
Chen, J. et al. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362, 301–318, doi:10.1007/s11104-012-1275-7 (2013).
Sun, J. et al. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 65, 67–74, doi:10.1016/j.plaphy.2013.01.003 (2013).
Papenbrock, J., Reimenschneider, A., Kamp, A., Schulz-Vogt, H. N. & Schmidt, A. Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants - From the field to the test tube and back. Plant Biol. 9, 582–588, doi:10.1055/s-2007-965424 (2007).
Mancardi, D. et al. Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. BBA-Bioenergetics 1787, 864–872 (2009).
Zhang, H. et al. Hydrogen Sulfide Promotes Root Organogenesis in Ipomoea batatas, Salix matsudana and Glycine max. J. Integr. Plant Biol. 51, 1086–1094, doi:10.1111/j.1744-7909.2009.00885.x (2009).
Scuffi, D. et al. Hydrogen Sulfide Generated by L-Cysteine Desulfhydrase Acts Upstream of Nitric Oxide to Modulate Abscisic Acid-Dependent Stomatal Closure. Plant Physiol. 166, 2065–2076, doi:10.1104/pp.114.245373 (2014).
Jia, H., Hu, Y., Fan, T. & Li, J. Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci. Reports 5, 10.1038/srep08251 (2015).
Lombardo, M. C., Graziano, M., Polacco, J. & Lamattina, L. Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav. 1, 28–33, doi:10.4161/psb.1.1.2398 (2006).
Tewari, R. K., Kim, S. Y., Hahn, E. J. & Paek, K. Y. Involvement of nitric oxide-induced NADPH oxidase in adventitious root growth and antioxidant defense in Panax ginseng. Plant Biotechnol. Rep. 2, 113–122, doi:10.1007/s11816-008-0052-9 (2008).
Xu, J., Yin, H. X., Li, Y. L. & Liu, X. J. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol. 154, 1319–1334, doi:10.1104/pp.110.162982 (2010).
Wang, R. The gasotransmitter role of hydrogen sulfide. Antioxid. Redox Sign. 5, 493–501, doi:10.1089/152308603768295249 (2003).
Mustafa, A. K. et al. H2S Signals Through Protein S-Sulfhydration. Sci. Signaling 2, ra72 (2009).
Altaany, Z., Ju, Y. J., Yang, G. D. & Wang, R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci Signaling 7, ra87 (2014).
Jin, Z. P. et al. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Biochem. 62, 41–46, doi:10.1016/j.plaphy.2012.10.017 (2013).
Robinson, M. J. & Cobb, M. H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9, 180–186, doi:10.1016/S0955-0674(97)80061-0 (1997).
Liu, J. Z. et al. Positive and Negative Roles for Soybean MPK6 in Regulating Defense Responses. Molecular Plant-Microbe Interactions 27, 824–834, doi:10.1094/MPMI-11-13-0350-R (2014).
Li, G. et al. Dual-level regulation of ACC synthase activity by MPK3/MPK6 cascade and its downstream WRKY transcription factor during ethylene induction in Arabidopsis. PLoS Genetics 8, e1002767, doi:10.1371/journal.pgen.1002767 (2012).
Liu, Y. & Zhang, S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16, 3386–3399, doi:10.1105/tpc.104.026609 (2004).
Takahashi, F. et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19, 805–818, doi:10.1105/tpc.106.046581 (2007).
Xing, Y., Jia, W. S. & Zhang, J. H. AtMKK1 and AtMPK6 are involved in abscisic acid and sugar signaling in Arabidopsis seed germination. Plant Mol. Biol. 70, 725–736, doi:10.1007/s11103-009-9503-0 (2009).
Yu, L. et al. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytol. 188, 762–773, doi:10.1111/j.1469-8137.2010.03422.x (2010).
Han, S. A., Fang, L., Ren, X. J., Wang, W. L. & Jiang, J. MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol. 205, 695–706, doi:10.1111/nph.12990 (2015).
Overvoorde, P., Fukaki, H. & Beeckman, T. Auxin Control of Root Auxin control of root development, Development. Cold Spring Harb. Perspect. Boil. 2, a001537, doi:10.1101/cshperspect.a001537 (2010).
Gray, W. M., Kepinski, S., Rouse, D., Leyser, O. & Estelle, M. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414, 271–276, doi:10.1038/35104500 (2001).
Nakamura, A. et al. Arabidopsis Aux/IAA genes are involved in brassinosteroid-mediated growth responses in a manner dependent on organ type. Plant J. 45, 193–205, doi:10.1111/tpj.2006.45.issue-2 (2006).
Tsukagoshi, H., Busch, W. & Benfey, P. N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143, 606–616, doi:10.1016/j.cell.2010.10.020 (2010).
Fang, T., Cao, Z. Y., Li, J. L., Shen, W. B. & Huang, L. Q. Auxin-induced hydrogen sulfide generation is involved in lateral root formation in tomato. Plant Physiol. Biochem. 76, 44–51, doi:10.1016/j.plaphy.2013.12.024 (2014).
Liu, W. et al. Salt Stress Reduces Root Meristem Size by Nitric Oxide-Mediated Modulation of Auxin Accumulation and Signaling in Arabidopsis. Plant Physiol. 168, 343–356, doi:10.1104/pp.15.00030 (2015).
Shi, C. et al. Ethylene mediates brassinosteroid-induced stomatal closure via Galpha protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J. 82, 280–301, doi:10.1111/tpj.2015.82.issue-2 (2015).
An, Z. F., Jing, W., Liu, Y. L. & Zhang, W. H. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba. J. Exp. Bot. 59, 815–825, doi:10.1093/jxb/erm370 (2008).
Li, J. H. et al. A Signaling Pathway Linking Nitric Oxide Production to Heterotrimeric G Protein and Hydrogen Peroxide Regulates Extracellular Calmodulin Induction of Stomatal Closure in Arabidopsis. Plant Physiol. 150, 114–124, doi:10.1104/pp.109.137067 (2009).
Khokon, M. A. R. et al. Yeast Elicitor-Induced Stomatal Closure and Peroxidase-Mediated ROS Production in Arabidopsis. Plant Cell Physiol. 51, 1915–1921, doi:10.1093/pcp/pcq145 (2010).
Ye, Y., Li, Z. & Xing, D. Nitric oxide promotes MPK6-mediated caspase-3-like activation in cadmium-induced Arabidopsis thaliana programmed cell death. Plant Cell Environ. 36, 1–15, doi:10.1111/pce.2013.36.issue-1 (2013).
Wang, P. C., Du, Y. Y., Li, Y. A., Ren, D. T. & Song, C. P. Hydrogen Peroxide-Mediated Activation of MAP Kinase 6 Modulates Nitric Oxide Biosynthesis and Signal Transduction in Arabidopsis. Plant Cell 22, 2981–2998, doi:10.1105/tpc.109.072959 (2010).
Desikan, R., Griffiths, R., Hancock, J. & Neill, S. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 99, 16314–16318, doi:10.1073/pnas.252461999 (2002).
Guo, F. Q., Okamoto, M. & Crawford, N. M. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302, 100–103, doi:10.1126/science.1086770 (2003).
Stohr, C. & Ullrich, W. R. Generation and possible roles of NO in plant roots and their apoplastic space. J. Exp. Bot. 53, 2293–2303, doi:10.1093/jxb/erf110 (2002).
Neill, S. J., Desikan, R., Clarke, A. & Hancock, J. T. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 128, 13–16, doi:10.1104/pp.010707 (2002).
Lisjak, M. et al. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol. Biochem. 48, 931–935, doi:10.1016/j.plaphy.2010.09.016 (2010).
Fernandez-Marcos, M., Sanz, L., Lewis, D. R., Muday, G. K. & Lorenzo, O. Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc. Natl. Acad. Sci. USA 108, 18506–18511, doi:10.1073/pnas.1108644108 (2011).
Terrile, M. C. et al. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 70, 492–500, doi:10.1111/tpj.2012.70.issue-3 (2012).
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472, doi:10.1016/S0092-8674(00)81535-4 (1999).
Sanz, L. et al. Nitric oxide plays a role in stem cell niche homeostasis through its interaction with auxin. Plant Physiol. 166, 1972–1984, doi:10.1104/pp.114.247445 (2014).
Acknowledgements
The authors gratefully acknowledge the Central Laboratory of the Xishuangbanna Tropical Botanical Garden for providing research facilities. This work was supported by the National Key Research and Development Program of China (2016YFC0501901), China National Natural Sciences Foundation (31272239, 31170228), Hebei Province National Natural Sciences Foundation for Distinguished Young Scientists (C2013503042), and Yunnan Province Foundation for academic leader (2014HB043).
Author information
Authors and Affiliations
Contributions
J.X. designed research; Q.L., P.Z., J.P.W., and R.L.W. performed research; Q.L., P.Z., J.P.W., and J.X. analyzed data; and J.X. wrote the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, P., Luo, Q., Wang, R. et al. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway. Sci Rep 7, 868 (2017). https://doi.org/10.1038/s41598-017-01046-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-01046-2
This article is cited by
-
The role of exogenous hydrogen sulfide in mitigating cadmium toxicity in plants: A comprehensive meta-analysis
Environmental Science and Pollution Research (2024)
-
Modulations of functional traits of Spinacia oleracea plants exposed to cadmium stress by using H2S as an antidote: a regulatory mechanism
Physiology and Molecular Biology of Plants (2023)
-
Phytotoxicity of trihalomethanes and trichloroacetic acid on Vigna radiata and Allium cepa plant models
Environmental Science and Pollution Research (2023)
-
Elucidating the role of exogenous iron (Fe) in regulation of hydrogen sulphide (H2S) biosynthesis and its concomitant effect on seedling growth, pigment composition and antioxidative defense in NaCl stressed tomato seedlings
Acta Physiologiae Plantarum (2023)
-
Cellular messengers involved in the inhibition of the Arabidopsis primary root growth by bacterial quorum-sensing signal N-decanoyl-L-homoserine lactone
BMC Plant Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.