Abstract
In this study, the effects of apple pomace (AP) addition (0%, 5%, 10%, and 20% on a dry weight basis, named as control, AP1, AP2, and AP3) and citric acid (CA) addition on nitrogen conservation were investigated during aerobic composting of pig manure. Gaseous emissions of NH3 and N2O were inhibited by AP and CA addition, with AP’s effect greater. The inhibition improved with increasing AP addition. The AP3 treatment was the most effective on NH3 adsorption and transformation to \({{\bf{NH}}}_{{\bf{4}}}^{{\boldsymbol{+}}}\)-N, improved with subsequent transformation to \({{\bf{NO}}}_{{\bf{3}}}^{{\boldsymbol{-}}}\)-N, and inhibition of N2O and \({{\bf{NO}}}_{{\bf{2}}}^{{\boldsymbol{-}}}\) production. Compared with control, AP3 showed the highest inhibition of accumulated NH3 and N2O emission, by 57% and 24%, respectively, and with a 19% increase of total Kjeldahl nitrogen in the compost. The further pot experiment proved the application of the AP amendment compost could improve the yield and trace element nutrient accumulation in Chinese cabbage when planted in a typical Zn-deficient soil. This study illustrates that AP application benefits both compost nitrogen conservation and fertilizer quality.
Similar content being viewed by others
Introduction
China is the biggest pork producing country in the world and approximately 715 million tons of pig manure (PM) were produced in 20131. Overproduction of PM has led to serious environmental problems. Reducing the amount of PM and control the associated pollution has been regarded as one of the limiting factors for further development of the pig enterprise2. Composting is a traditional, effective method, which can not only reduce the amount of organic waste, but also transform animal manure and organic waste into an environmentally friendly organic fertilizer3, 4.
In the process of composting, feed stock materials suffer degradation during the thermophilic stage, which leads to the loss of N (mainly in form of NH3 volatilization), which lowers compost quality and increases atmospheric pollution5. In order to reduce the N loss during composting, various methods have been tried in recent years such as altering process conditions or the addition of different bulking agents and additives. For example, Shi et al. reported that increasing moisture and turning the pile can reduce N loss under a long maturity period with cow manure composting6. Ventilation control was an effective way to reduce \({{\rm{NO}}}_{3}^{-}\)-N loss at the later stage of compost making7. Other methods include using mineral or biological amendments to absorb ammonia8. For example, the microbial inoculant additive, urease inhibitors can help NH3 emission reduction9. Some adsorbents including zeolite, bentonite, peat, fly ash, biochar, etc.10,11,12 also could significantly reduce compost NH3 emission due to their extensive porosity and large surface area which can provide large NH3 adsorption capacity. Besides these, some chemical additives such as Ca3(PO4)2, MgCl2, FeCl3, Al2(SO4)3, HNO3, and CaCl2, etc.13 could be employed in composting for NH3 emission reduction through their chemical reactions with NH3. Although these commercial additives (mineral and biological) are effective in improving compost quality by inhibiting N loss, their usage is still restricted due to high cost and risk of excessive accompanying salt ions4. In view of economical waste disposal, more research is needed to enhance capacity of N conservation in compost, reduce composting cost and improve environmental friendliness14.
Recent studies showed that reducing the initial pH of the compost mixture by adding olive pomace can conserve N15. In addition, this method could lead to maximizing utilization of local resources. China is the largest apple producer in the world and total production reached 31.7 million tons in 201316, and Shaanxi province accounted for more than 1/3 of the country’s total apple output1. As a by-product of the cider-processing industry, more than 70,000 tons of apple pomace (AP) are generated each year in Shaanxi province, which need to be dealt with17. Recently, some reports pointed out that AP may be a suitable material to aid composting. Kopčić et al., for example, studied the temperature variation of laboratory-scale in-vessel co-composting of tobacco and apple waste18. Hanc and Chadimova vermicomposted AP waste with wheat straw and found that the addition of straw to AP did not enhance earthworm biomass19, but did increase the available content of nutrients (N, P, K, Mg etc.) during composting. Jiang et al. proved that AP can effectively reduce the NH3 release during the pig manure composting17. However, emission of N2O during composting reduced N conservation and contributed to greenhouse gas (GHG) emission20. There has been little research on reducing N2O during composting AP with PM which has limited the application of AP in composting.
Hence, the aims of this study were to investigate the effect of AP addition with different amounts on the gaseous emissions of NH3 and N2O during the compost N conservation process. In the composting, treatments with different amounts of AP addition were evaluated by aerobic composting of the mixture of PM and wheat straw (WS) in compost reactors. Concurrent treatment with citric acid (CA) was included in order to compare the effects of AP with an organic acid in composting.
Results and Discussion
Changes in compost temperature, pH, EC and germination index (GI)
The changes of temperature observed in the five treatments are shown in Fig. 1a. The ambient temperature was maintained from 20 °C to 30 °C during the process. The highest temperatures were obtained after 2 days with 62.6, 62.9, 61.4, 58.8, and 60.6 °C in control, and treatments of AP1, AP2, AP3, and CA, respectively. The thermophilic phase was maintained about one week for the feedstock composition, which was necessary for obtaining a successful product21. There were no differences with time for all treatments to reach the thermophilic phase. The temperature decreased rapidly after 10 days, indicating the end of the thermophilic phase, and then gradually decreased to about 30 °C. The additive amended treatments did not show any stimulatory or inhibitory effects on the temperature profile, which was similar to that reported by Li et al.4.
The pH of the compost mixture appeared to increase sharply in the first 5 days and then decrease slightly to gradually reach pH 7.7 to 8.3 in the later process (Fig. 1b). In the first phase, the mineralization of organic matter generally leads to the release of ammonium and volatile ammonia, which increases pH level22. With the composting prolonged, pH decreased due to the formation of low molecular weight fatty acids and CO2 during organic matter (OM) degradation, and the accumulation of organic acid with the addition of AP. At the end of the process, pH increased with the decomposition of organic acid. For AP additive treatments, AP3 was significantly lower than other treatments at the later composting process, due to more organic acid produced with more AP.
The change of electrical conductivity (EC) during the composting process revealed the amount of soluble salt contents in the compost (Fig. 1c). EC value increased in all treatments with composting time. After composting, the additive treatments had significantly higher EC than control, which reached 2.20, 2.52, 2.58, and 2.22 mS cm−1 for AP1, AP2, AP3, and CA treatments, respectively, compared with the relative lower EC of 1.81 mS cm−1 for the control. In the end, EC increased by 40% for control, while 46%, 67%, 72%, and 48% for AP1, AP2, AP3, and CA treatments, respectively. Increases in EC were caused by organic matter mineralization that can increase concentration of soluble salts23. Compared to the different additive treatments, AP3 obtained the highest increasing rate, which can be explained by more organic acid being produced with more AP addition, which can lead to an increase of soluble salts concentration in the compost17.
Figure 1d clearly shows a tendency of gradual decrease in compost phytotoxicity. Compared with the control, addition of AP and CA improved germination index (GI) variation, and at the end of composting the GI value reached 0.97, 1.14, 1.27, 1.38, and 1.20 for control, AP1, AP2, AP3, and CA, respectively. Generally, acceptable GI value of mature compost was above 0.524. In the study, GI of the final compost were higher than 0.9, indicating the compost in all the treatments was mature. The GI values of the compost with AP and CA additive were higher than that of compost in control, which illustrated that AP and CA amendment can help compost material detoxification.
Variation of NH3 and N2O emission during composting
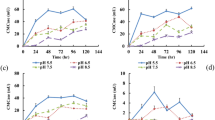
The NH3 and N2O emissions were important in this study both for the effect of N transformation and global warming influence. As shown in Fig. 2a, ammonia concentrations peaked after 5 d during the composting. Compared with control, NH3 concentrations were significantly reduced in the additives treatments, especially in the AP3. The emissions of NH3 from animal manure were influenced by pH and the \({{\rm{NH}}}_{4}^{+}\)-NH3 transformation equilibrium12. The amendments, especially AP and CA used in this study, had a pH under 7, which tended to prevent NH3 losses21. However, the losses of NH3 in the AP3 treatment was lower, which might be caused by the NH3 adsorption potentials at lower pH since the amount of AP was higher than other treatments and the compost pH at the start was lower. This phenomenon was in accordance with the findings in previous reports during composting of olive pomace with animal waste15. Beside this, the AP additive with its smaller particle size compared with wheat straw and pig manure, can lead to high bulk density of mixed initial compost material which can reduce NH3 emission, as reported, by 30–70%10. The accumulated emission of NH3 was calculated to clarify the effect of treatments (Fig. 2b). There existed similar curve with all treatments that increased rapidly in the first 7 days and then reached a platform. The total emission of NH3 of all the additive treatments of AP and CA showed significant inhibition with control. The inhibited rates with control were 26%, 46%, 57%, and 47%, for AP1, AP2, AP3, and CA treatments, respectively. The results confirmed that AP and CA additive can help to reduce NH3 emission during composting, which probably indicated there was more organic acid in the initial materials or more was produced during the composting process.
The N2O emission profile in the composting process was studied by many researchers for its important effects both in N conservation during the process and GHGs emission for its global warming influence25. As reported by former studies, N2O can be produced under both aerobic and anaerobic conditions26. During denitrification, N2O can be synthesized where there is a lack of O2 and/or a nitrate (or nitrite) accumulation27, while during nitrification, N2O is produced in the presence of O2 and/or low availability of degradable carbohydrates. In this study, N2O emission increased rapidly in the first phase with all treatments and peaked in the fifth day, reached with 4.45, 3.68, 2.98, 3.05, and 3.52 g kg−1 d−1 for the treatments of control, AP1, AP2, AP3, and CA, respectively (Fig. 2c), and decreased gradually after the peak time. All the N2O emission profile approached zero after 15th days in composting. The accumulated emission of N2O was shown clearly with all treatments (Fig. 2d). The additive treatments inhibited the N2O accumulation compared with control, by 20%, 24%, 24%, and 18% for AP1, AP2, AP3, and CA, respectively. It should be pointed out that the mechanism and tendency of N2O emission during composting is still disputed, according to earlier reports. El Kader et al. reported that a high concentration of N2O was found at the beginning of composting with farm manure28. Our study also showed the N2O emission increased rapidly in the first phase with all treatments, and the N2O emission could be decreased with AP addition. These results were in accordance with the finding of Awasthi20, who reported the high concentration of N2O was found at the beginning of composting with sewage sludge, while some additives such as 30% zeolite and 1% lime could reduce greatly the N2O emission during the first composting stage. However, N2O was mostly reported previously to be emitted during the thermophilic phase, due to the activity of nitrifiers was inhibited by high temperature29, 30. These results suggest that the N2O emission in composting is a complicated system which could be influenced by many factors.
Variation of TKN, \({{\bf{NH}}}_{{\bf{4}}}^{{\boldsymbol{+}}}\)-N, \({{\bf{NO}}}_{{\bf{2}}}^{{\boldsymbol{-}}}\)-N, and \({{\bf{NO}}}_{{\bf{3}}}^{{\boldsymbol{-}}}\)-N during composting
The total Kjeldahl nitrogen (TKN) contents in the composting mixtures with all treatments decreased during the first 5 days and increased afterwards (Fig. 3a). The loss of TKN at the beginning of the composting stage might be due to the loss of ammonia by volatilization with increasing compost temperatures. Compared with the control, the TKN losses of AP and CA adding treatments in the first five days were lower than that in the control, which were 19%, 15%, 8%, and 16% in AP1, AP2, AP3, and CA, respectively, while 22% in control. Similar results were reported by Chen et al.11, that TKN loss from treatments of 3%, 6%, and 9% bamboo charcoal addition in pig manure composting were reduced by 28%, 61%, and 65%, respectively, compared to the control after composting. The increase of TKN at the later stage in all treatments was possibly because of the continuous degradation of organic compounds31, while in this study, with the AP addition, the TKN content in the composting mixtures remained higher than other treatments throughout the entire composting. In the end of composting, the TKN contents were 24.9, 26.3, 28.6, 29.6, and 26.2 g kg−1 with the treatments of control, AP1, AP2, AP3, and CA, respectively. Similar results were obtained by other researchers with the compost additives of biochar12, bentonite2, bamboo charcoal32. This might be caused by the fact that the AP could act as an ammonia sorbent which inhibited ammonia emission in the early phases17, or the acidic AP could inhibit the ammonia release through the reaction H+ + NH3 = \({{\rm{NH}}}_{4}^{+}\) 21. And this point correlates with \({{\rm{NH}}}_{4}^{+}\)-N evolution to some extend during composting, as shown in Fig. 3b.
The \({{\rm{NH}}}_{4}^{+}\)-N contents increased during the first 5 d and the peak \({{\rm{NH}}}_{4}^{+}\)-N contents were 510.6, 550.4, 569.9, 649.6, and 567.0 mg kg−1 in the control, AP1, AP2, AP3, and CA treatments, respectively (Fig. 3b). The higher \({{\rm{NH}}}_{4}^{+}\)-N content of the compost occurred during the first 5 d, which is in accord with the traditional composting process. In general, the evolution of N forms shows the mineralization of the organic compounds during the active phase of composting with the formation of \({{\rm{NH}}}_{4}^{+}\)-N. This was because the higher pH and temperature during the thermophilic phase resulted in more NH3 volatilization release, then NH3 was subsequently adsorbed by the wet mixture, and thus formed \({{\rm{NH}}}_{4}^{+}\) 33. However, in the AP and CA added treatments, the peak concentration of \({{\rm{NH}}}_{4}^{+}\)-N was higher than that in control, which suggests that the acid addition helps the NH3 immobilization through reaction NH3 + H+ = \({{\rm{NH}}}_{4}^{+}\). After 5 d, the \({{\rm{NH}}}_{4}^{+}\)-N contents rapidly decreased as composting progressed, and only small amounts of \({{\rm{NH}}}_{4}^{+}\)-N existed in the final composts (lower than 200 mg kg−1), which proved the maturity and stability of the final composts21. The later \({{\rm{NH}}}_{4}^{+}\)-N content decrease was relevant with the \({{\rm{NH}}}_{4}^{+}\)-N microorganism immobilization and \({{\rm{NO}}}_{3}^{-}\)-N formation during the aerobic composting process34. Generally, nitrate nitrogen is the final sink of inorganic N in the composting system. The \({{\rm{NO}}}_{3}^{-}\)-N contents steady increase (Fig. 3c) was accompanied by the reduction of the \({{\rm{NH}}}_{4}^{+}\)-N during the aerobic composting process12, 21. The result showed that the addition of AP can improve the N reserve with the form of \({{\rm{NO}}}_{3}^{-}\)-N in compost according with the decrease of ammonia volatilization. In composting process, the transformation of ammonia to nitrates was caused by the action of nitrifying bacteria with the optimum nitrification pH around 7–835. Gujer & Jenkins36 pointed out that a suitable amount of alkali was needed to oxidize ammonia nitrogen and hence reduction of pH would be expected in the nitrification process. In this study, the compost pH decreased as more of the acidic AP was added. The AP1 might provide a suitable pH condition for nitrification in the beginning 10 days than others, which may lead to a higher \({{\rm{NO}}}_{3}^{-}\)-N content (~248 mg kg−1 at 10 days) in AP1 than that of other treatments. The result partly agreed with the findings reported by Wong et al.37, who found in treatment of 15% coal fly ash + lime 0.75% the \({{\rm{NO}}}_{3}^{-}\)-N content was higher than that in treatment of control, 1.5% lime, 3% lime, 5% coal fly ash + 2.25% lime, and 10% coal fly ash +1.5% lime, respectively, at the beginning of 10 days composting.
\({{\rm{NO}}}_{2}^{-}\)-N is an intermediate product of N transformation during the composting process38. The \({{\rm{NO}}}_{2}^{-}\)-N concentration in all treatments increased steadily during the early stage and then declined (Fig. 3d). The peak value of \({{\rm{NO}}}_{2}^{-}\)-N in all treatments was observed on day 16 and decreased with the AP adding amount increase, which implicating AP amendment inhibit the \({{\rm{NO}}}_{2}^{-}\)-N formation. Meanwhile, during the composting process, the former increase in \({{\rm{NO}}}_{2}^{-}\)-N content in all treatments was attributed to the \({{\rm{NH}}}_{4}^{+}\)-N converting to \({{\rm{NO}}}_{2}^{-}\)-N during nitrification39, while the later decrease was caused by the \({{\rm{NO}}}_{2}^{-}\)-N transforming to \({{\rm{NO}}}_{3}^{-}\)-N or N2O through nitrification/denitrification20, 40. The \({{\rm{NO}}}_{3}^{-}\)-N concentration significant increase (Fig. 3c) indicated the increase in nitrate formation during the continuous aerobic process21, 38. At the end of the composting, the AP amendment treatments exhibited higher contents of \({{\rm{NO}}}_{3}^{-}\)-N than the control, and the final \({{\rm{NO}}}_{3}^{-}\)-N were 236.86, 327.71, 342.53, 348.38, and 315.90 g kg−1, for the control, AP1, AP2, AP3, and CA treatments, respectively. The similar trend of nitrate was observed by other researchers during composting organic waste with bentonite2, lime20 and biochar11.
Effect of the mature compost on Chinese cabbage growth
The effect of AP amended composts on Chinese cabbage growth in pot experiments is given in Table 1. The highest SPAD content in leaves was found with AP3-treated compost, which was 25% and 62% higher than that obtained with control-treated compost and blank treatment, respectively. Compared with the blank treatment, yields of Chinese cabbage treated with composts increased significantly, and the peak weights of biomass were obtained with the AP3-treated compost, and the average weight of AP-treated compost was 26% and 248% higher than that obtained with control-treated compost and blank treatment, respectively. The differences in yields of Chinese cabbage biomass indicated that AP-amended compost effectively promoted Chinese cabbage growth. Li et al. also found that ryegrass (Lolium perenne L.) yields were significantly improved with 17.1–52.5% higher by the addition of charred biomass (CB) treated compost12. Similar results reported by Hua et al. that the biomass of fescue grown in substrate with 40% sludge compost (SC) increased 27% in a red soil and 44% in a yellow loamy soil compared to the control, while the biomass of ryegrass grown with 60% SC increased 120% in the red soil and 86% in the yellow loamy soil41. The Cu and Zn concentrations in Chinese cabbage seedlings increased as a result of the application of composted pig manure. The treatment with the AP3-treated compost significantly increased the Zn concentration in the Chinese cabbage by 22% and 138%, compared with the control-treated compost and blank treatment, respectively. The average Cu concentration in the Chinese cabbage seedlings with treatments of five kinds of compost was 53% higher than that of blank treatment. Li et al. reported that CB treated compost improved Cu content of upground ryegrass by average of 27.2% compared with blank treatment while reduced Zn content by average of 6.4%12. Hua et al. reported that Cu content in fescue can be increased significantly by 123% and 208% when grown in red soil and yellow loamy soil, respectively, while ryegrass can be increased significantly by 88% and 86% with the addition of 80% SC. For Zn content, that in fescue can be increased significantly by 378% and 313% that grown with red soil and yellow loamy soil, respectively, while ryegrass can be increased significantly by 338% and 340% with the addition of 80% SC41. The reports indicated that the content of Zn and Cu in plant tissues might be influenced by the plant species, soil types, and compost addition in such pot trials. In our study, the AP amended PM compost can provide an efficient way for the available Zn enhancement in the typical Zn-deficient soil and for improvement of the Zn content in food crops since many Asian populations experience Zn deficiency42.
Conclusions
This study indicated that during composting, compared with the control and CA treatment, AP addition in composting matrix can improve TKN concentration and conserve N content by increasing \({{\rm{NH}}}_{4}^{+}\)-N and \({{\rm{NO}}}_{3}^{-}\)-N contents. The gaseous emissions of NH3 and N2O and the accumulation emission were inhibited with AP addition caused by the acid material input at the beginning of composting. As an important agricultural by-product, AP can be regarded as a suitable additive for pig manure aerobic composting with the aim of agricultural waste utilization and compost quality improvement, while the addition ratio should be monitored with the quality evaluation of pH, EC, N conservation and the economic benefit.
Materials and Methods
Raw materials
The fresh PM was collected from the Northwest A&F University campus’s ecological testing farm, Yangling, Shaanxi, China. WS was collected from the village around the campus (within 5 km) and crushed to 3–5 cm fragments before being mixed with the pig manure. Fresh apple pomace (AP) was provided by Tongda fruit plant (Xianyang City, Shaanxi Province) which is 50 km from the campus and citric acid (CA, Chemical pure) was purchased from Sinopharm Chemical Reagent Co., Ltd. The main characteristics of the raw materials are shown in Table 2.
Composting procedure and sampling
About 63 kg of PM was mixed with 6 kg WS on wet weight ratio of 10.5: 117 for each treatment, and then AP was supplemented 0%, 5%, 10% and 20% in dry weight basis, while the treatment of CA was conducted with citric acid adding rate of 1% in dry weight basis of the mixture and denoted as control, AP1, AP2, AP3, and CA, respectively. All treatments were performed in triplicate. The moisture of the mixtures in all treatments was kept around 65% after fully mixing. After that, about 60 kg mixture was put into a laboratory automatic-mixing composter (volume 120 L). Air was pumped from the bottom into the composter with a constant air flow of about 60 L min−1 during the thermophilic phase, and the frequency of ventilation was twice per day for 30 mins, once at about 9 a.m. and the other at about 3 p.m. Temperature was monitored every 8 h by a probe fixed in the middle of the composting mass and averaged. The room temperature was also recorded. Samples around 1 kg were collected at scheduled times (0, 6, 11, 16, 23, and 30 days) according to the change of temperature after the start of the experiment. Before sampling, all the piles were thoroughly turned. Sampling was performed in triplicate and each sample was divided into two portions. One portion was stored at 4 °C before analyses while the other was freeze-dried, crushed to pass through a 0.15 mm sieve and stored in desiccator. Another fresh sample portion was used for parameters including moisture, pH, EC, \({{\rm{NO}}}_{3}^{-}\)-N, \({{\rm{NO}}}_{2}^{-}\)-N, \({{\rm{NH}}}_{4}^{+}\)-N, GI etc. determination. Gas samples were collected every day with a 1-LTedlar® PLV gas sampling bag as described by Awasthi et al.20.
Pot experiment
The pot experiment was conducted in the greenhouse of Northwest A & F University. The soil used in the pot experiment was a calcareous loam that collected from Yangmazhuang village, Yongshou County, Shaanxi Province, with the 0–20 cm depth sampling. The main characteristics of the soil are shown in Table 3. Air-dried soil and composts were ground to pass 2 mm nylon sieve before mixing. All the compost was added to soil with 5 wt.% application dosage as in our previous study12 except the blank treatment (only soil was added to the pot, without any fertilizer input), and the main characteristics of the matured compost are shown in Table 3. A plastic basin (the bottom diameter = 19 cm and the mouth diameter = 30 cm, height = 23 cm) filled with 2.0 kg soil/compost mixtures was incubated for 10 days with water content of around 80% field capacity, fifteen Chinese cabbage (Brassica chinensis L.) seeds were planted while thinned to 5 per pot at the growth stage of four leaves. Four replications were set for each treatment. During growth period, chlorophyll contents in the leaves were recorded by using a SPAD meter (Minolta, Japan-SPAD-502). After 35 days, the aboveground biomass of each pot was harvested. Plant samples were dried at 60 °C in an oven to constant weight for the dry biomass determination and stored in a dehydrator before Cu and Zn accumulation assessment.
Analytical methods
\({{\rm{NH}}}_{4}^{+}\)-N, \({{\rm{NO}}}_{3}^{-}\)-N, \({{\rm{NO}}}_{2}^{-}\)-N, moisture, EC, and pH were determined with fresh samples as methods used by Li et al.4 \({{\rm{NO}}}_{2}^{-}\)-N content was measured by using a colorimeter at wavelength 538 nm43. Total Kjeldahl nitrogen (TKN) was monitored in air-dried samples followed by the Kjeldahl digestion method4. A mass balance was carried out before and after composting to calculate all results for each treatment. The N2O content was analyzed with gas chromatography (Agilent Technologies 6890 N Network GC system, China) in 12 h as described by Colon et al.44 Ammonia emission was measured by adsorbing the exhaust gas with H3BO3 before titrated with HCl3. Phytotoxicity of the compost was evaluated by calculating germination index (GI) as described by Li et al.4 with potherb mustard (Ardisia squamulosa Presl) as an indicator plant.
The total contents of Cu and Zn in compost and in the plant samples were determined by an atomic absorption spectrophotometer (Hitachi Z-2000) after being digested by concentrated HNO3-H2O2. Reference samples (GBW-08501) were used for quality control meanwhile.
All analyses were repeated four times. Statistical analysis was performed by SPSS 18.0 software using one-way ANOVA at significance P < 0.05 level to separate treatment means for the mass data.
References
NBSC. China Statistical Yearbook. National Bureau of Statistics of China (2014).
Wang, Q. et al. Improving pig manure composting efficiency employing Ca-bentonite. Ecol. Eng. 87, 157–161 (2016).
Awasthi, M. K. et al. Role of biochar amendment in mitigation of nitrogen loss and greenhouse gas emission during sewage sludge composting. Bioresour. Technol. 219, 270–280 (2016).
Li, R. et al. Nutrient transformations during composting of pig manure with bentonite. Bioresour. Technol. 121, 362–368 (2012).
Lelieveld, J., Evans, J., Fnais, M., Giannadaki, D. & Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 525, 367–371 (2015).
Shi, W., Norton, J. M., Miller, B. E. & Pace, M. G. Effects of aeration and moisture during windrow composting on the nitrogen fertilizer values of dairy waste composts. Appl. Soil Ecol. 11, 17–28 (1999).
Wang, K., Wei, B., Huang, D., Li, H. & Ye, Z. A Case Study of Effects of Sulfuric Acid Sprayon Ammonia and Odor Emissions fromSwine Manure Composting. Appl. Eng. Agric. 30, 267–276 (2014).
Hong, J., Park, K. & Choi, W. Using biofilters to reduce ammonia emissions. Biocycle 43, 43–46 (2002).
Parker, D. B. et al. Rate and frequency of urease inhibitor application for minimizing ammonia emissions from beef cattle feedyards. T. ASAE 48, 787–793 (2005).
Chan, M. T., Selvam, A. & Wong, J. W. Reducing nitrogen loss and salinity during ‘struvite’ food waste composting by zeolite amendment. Bioresour. Technol. 200, 838–844 (2016).
Chen, Y. X. et al. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 78, 1177–1181 (2010).
Li, R. et al. Nutrient transformation during aerobic composting of pig manure with biochar prepared at different temperatures. Environ. Technol. 36, 815–826 (2015).
Jeong, Y. K. & Hwang, S. J. Optimum doses of Mg and P salts for precipitating ammonia into struvite crystals in aerobic composting. Bioresour. Technol. 96, 1–6 (2005).
Jiang, T. et al. Combined use of nitrification inhibitor and struvite crystallization to reduce the NH3 and N2O emissions during composting. Bioresour. Technol. 217, 210–218 (2016).
Canet, R. et al. Composting olive mill pomace and other residues from rural southeastern Spain. Waste Manage 28, 2585–2592 (2008).
World Apple and Pear Association (WAPA). World data reports, http://www.wapa-association.org/docs/fact_sheets/World_production_apple_pears_2003-2013.xlsx (2013).
Jiang, J., Huang, Y., Liu, X. & Huang, H. The effects of apple pomace, bentonite and calcium superphosphate on swine manure aerobic composting. Waste Manage. 34, 1595–1602 (2014).
Kopčić, N., Vuković Domanovac, M., Kučić, D. & Briški, F. Evaluation of laboratory-scale in-vessel co-composting of tobacco and apple waste. Waste Manage. 34, 323–328 (2014).
Hanc, A. & Chadimova, Z. Nutrient recovery from apple pomace waste by vermicomposting technology. Bioresour. Technol 168, 240–244 (2014).
Awasthi, M. K. et al. Influence of zeolite and lime as additives on greenhouse gas emissions and maturity evolution during sewage sludge composting. Bioresour. Technol. 216, 172–181 (2016).
Bernal, M. P., Alburquerque, J. & Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 100, 5444–5453 (2009).
Gigliotti, G. et al. Co-composting of olive husks with high moisture contents: organic matter dynamics and compost quality. Int. Biodeter. Biodegr. 67, 8–14 (2012).
Huang, G., Wu, Q., Wong, J. & Nagar, B. Transformation of organic matter during co-composting of pig manure with sawdust. Bioresour. Technol. 97, 1834–1842 (2006).
Zucconi, F., Pera, A., Forte, M. & De Bertoldi, M. Evaluating toxicity of immature compost. Biocycle 22, 54–57 (1981).
Maulini-Duran, C., Artola, A., Font, X. & Sánchez, A. Gaseous emissions in municipal wastes composting: Effect of the bulking agent. Bioresour. Technol. 172, 260–268 (2014).
Yang, F., Li, G. X., Yang, Q. Y. & Luo, W. H. Effect of bulking agents on maturity and gaseous emissions during kitchen waste composting. Chemosphere 93, 1393–1399 (2013).
Philippe, F. X., Laitat, M., Nicks, B. & Cabaraux, J. F. Ammonia and greenhouse gas emissions during the fattening of pigs kept on two types of straw floor. Agr. Ecosyst. Environ. 150, 45–53 (2012).
El Kader, N. A., Robin, P., Paillat, J. M. & Leterme, P. Turning, compacting and the addition of water as factors affecting gaseous emissions in farm manure composting. Bioresour. Technol. 98, 2619–2628 (2007).
Yamulki, S. Effect of straw addition on nitrous oxide and methane emissions from stored farmyard manures. Agr. Ecosyst. Environ. 112, 140–145 (2006).
Fukumoto, Y., Osada, T., Hanajima, D. & Haga, K. Patterns and quantities of NH3, N2O and CH4 emissions during swine manure composting without forced aeration-effect of compost pile scale. Bioresour. Technol. 89, 109–114 (2003).
Steiner, C., Das, K., Melear, N. & Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 39, 1236–1242 (2010).
Hua, L., Wu, W., Liu, Y., McBride, M. B. & Chen, Y. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ. Sci. Pollut. Res. 16, 1–9 (2009).
Fang, M., Wong, J., Ma, K. & Wong, M. Co-composting of sewage sludge and coal fly ash: nutrient transformations. Bioresour. Technol 67, 19–24 (1999).
Lu, Y., Wu, X. & Guo, J. Characteristics of municipal solid waste and sewage sludge co-composting. Waste manage. 29, 1152–1157 (2009).
USEPA, Nitrification. http://www.epa.gov/safewater/tcr/pdf/nitrification.pdf (2002).
Gujer, W. & Jenkins, D. A nitrification model for contact stabilization activated sludge process. Water Res. 9, 561–566 (1975).
Wong, J. W. C., Fung, S. O. & Selvam, A. Coal fly ash and lime addition enhances the rate and efficiency of decomposition of food waste during composting. Bioresour. Technol. 100, 3324–3331 (2009).
Chen, R. et al. N2O emissions and nitrogen transformation during windrow composting of dairy manure. J. Environ. Manage. 160, 121–127 (2015).
Szanto, G., Hamelers, H., Rulkens, W. & Veeken, A. NH3, N2O and CH4 emissions during passively aerated composting of straw-rich pig manure. Bioresour. Technol. 98, 2659–2670 (2007).
Wang, C. et al. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 47, 7341–7349 (2013).
Hua, L., Wang, Y., Wu, W., McBride, M. B. & Chen, Y. Biomass and Cu and Zn uptake of two turfgrass species grown in sludge compost–soil mixtures. Water Air Soil Pollut. 188, 225–234 (2008).
Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Bio. 23, 281–289 (2009).
Biey, E. M., Mortier, H. & Verstraete, W. Nitrogen transfer from grey municipal solid waste to high quality compost. Bioresour. Technol. 73, 47–52 (2000).
Colon, J. et al. Determination of the energy and environmental burdens associated with the biological treatment of source-separated municipal solid wastes. Energy Environ. Sci. 5, 5731–5741 (2012).
Acknowledgements
We gratefully acknowledge the National Natural Science Foundation of China (41571282, 41201280), the Fundamental Research Funds for the Central Universities (2452015047), the Natural Key Technologies R&D Program (2015BAD23B04), and the Special Fund for Ago-scientific Research in the Public Interest (201503124).
Author information
Authors and Affiliations
Contributions
H.M. wrote the main manuscript and conducted the experiments, R.L. planned the experiments and prepared figures, T.Z., B.Z. and Q.W. charged for the analysis of samples, Z.W. and Z.Z. gave the explanations of the figures and tables. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mao, H., Zhang, T., Li, R. et al. Apple pomace improves the quality of pig manure aerobic compost by reducing emissions of NH3 and N2O. Sci Rep 7, 870 (2017). https://doi.org/10.1038/s41598-017-00987-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00987-y
This article is cited by
-
The Use of Apple Pomace as a Soil Amendment Enhances the Activity of Soil Microorganisms and Nitrogen Transformations and Affects Crop Growth
Journal of Soil Science and Plant Nutrition (2021)
-
Challenges and Control Strategies of Odor Emission from Composting Operation
Applied Biochemistry and Biotechnology (2021)
-
Effects of additives on nitrogen transformation and greenhouse gases emission of co-composting for deer manure and corn straw
Environmental Science and Pollution Research (2021)
-
Greenhouse gas and ammonia emissions from stored manure from beef cattle supplemented 3-nitrooxypropanol and monensin to reduce enteric methane emissions
Scientific Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.