Abstract
Infantile haemangiomas (IHs) are the most common congenital vascular tumours of infancy. Propranolol has been demonstrated to be effective for IHs; however, the factors affecting its therapeutic effect remain unknown. We enrolled 169 infants with IHs of the head and neck region treated with oral propranolol at a dose of 2.0 mg/kg/day. We evaluated the therapeutic responses 6 months after treatment and the end of treatment, which were categorized into four grades. The type and location of the lesions and the infant age at treatment initiation were analysed. The clinical response rate (III + IV) was 91.72% at 6 months after treatment and 97.63% at the end of treatment. The average treatment duration was 9.99 (2–24) months. The group aged 4–6 months exhibited a greater therapeutic response rate (98.48%). The treatment duration was shorter (9.52 months) for mixed-type IHs. Better therapeutic responses were observed for IHs located around the parotid, periorbital, cheek, and neck regions and for multiple IH lesions. Our study indicated that propranolol is effective for IHs affecting the head and neck. The age at treatment initiation and the location of the lesions had a significant effect on the therapeutic response, whereas the lesion type might affect the treatment duration.
Similar content being viewed by others
Introduction
Infantile haemangiomas (IHs) are the most common vascular tumours in infants with a prevalence as high as 10%1, approximately 60% of which are located on the head and neck2. IHs may cause many complications, particularly during their proliferative phase, including ulceration, visual impairment, and airway obstruction3. Following the report by Leaute-Labreze C et al. in 2008 indicating that propranolol may treat IHs effectively4, propranolol has increasingly become the first-line agent for the treatment of IHs.
Propranolol is generally effective for IHs on the head and neck5, 6. However, reports have indicated that propranolol is not effective for all IHs, with a failure rate as high as 10%7. In our clinical experience of treating IHs with oral propranolol, the therapeutic response and treatment duration have varied widely according to the different locations of the lesions involving the head and neck. Expectations regarding the therapeutic response and treatment duration are extremely important for clinicians and the parental guardians of the infants.
In this study, we report our clinical observations of head and neck IHs treated with oral propranolol and an analysis of the factors affecting the therapeutic effect.
Infants and Methods
Inclusion and exclusion criteria
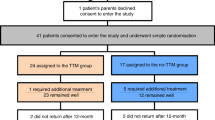
This study was conducted in the Department of Oral and Maxillofacial Surgery of the Qilu Hospital, Shandong University, China from June 2009 to November 2016. Approval for this study was obtained by the institutional ethics committee of Qilu Hospital, Shandong University. The inclusion criteria were as follows: IHs diagnosed by a medical history and physical examination according to the International Society for the Study of Vascular Anomalies (ISSVA) Classification of Vascular Anomalies8 and ultrasonographic or MRI examination. None of the infants were previously treated with other medications. The exclusion criteria were a history or risk of asthma, reactive airway disease, impaired renal or liver function, heart defects or arrhythmias, hypotension, hypersensitivity to propranolol, and lesions involving other regions in addition to the head and neck. Infants who did not adhere to the treatments or follow-up visits were also excluded from the study. The study followed the tenets of the Declaration of Helsinki for research involving human subjects, and informed consent was obtained from all parents whose infants participated in the study. The methods were performed in accordance with the approved guidelines (http://jama.jamanetwork.com/article.aspx?articleid=1760318).
Propranolol treatment protocol
All infants were administered oral propranolol in two divided doses (at 9 am and 2 pm). The initial dosage was determined by the infant age (1–3 months, 1 mg/kg/day; 4–12 months, 1.5 mg/kg/day). All infants underwent electrocardiographic monitoring 30 min prior to the medication administration and 30 min and 60 min after medication delivery during the first three days. If well tolerated over 2 to 3 days of observation, the dose was increased up to 2.0 mg/kg/day prior to hospital discharge, after which the patient was followed-up at an outpatient clinic every 2 months. The propranolol therapy was stopped when the lesions had completely regressed or when the lesions remained stable for longer than 2 months after at least 1 year of treatment. No other treatment was adopted during the oral propranolol course in our study.
Clinical evaluation
The following infant data were collected: gender, type and location of lesions, and age at therapy initiation and end dates. The infants were divided into four groups (0–3 months, 4–6 months, 7–9 months and 10–12 months) according to their age at the initiation of treatment. The treatment efficacy was evaluated 6 months after treatment initiation to determine the factors affecting the therapeutic effect of propranolol on IHs of the head and neck. We also evaluated the treatment efficacy at the end of treatment, which was based on the following three tumour-associated parameters: (a) volume reduction; (b) colour improvement; and (c) texture improvement. The evaluations were based on the photographic, ultrasonographic or MR images obtained before and after treatment. The results were categorized into four grades based on the Achauer system9: grade I, poor response (0–25% regression); grade II, fair response (26–50% regression); grade III, good response (51–75% regression); and grade IV, excellent response (76–100% regression). We considered grades III and IV indicative of clinical response.
Statistical analysis
Statistical analyses were performed using standard statistical software packages (SPSS, version20.0, Chicago). Data were expressed as numbers, percentages, or the means ± standard deviations. One-way analysis of variance (ANOVA) was used for the comparisons of treatment duration among different groups. The Kruskal-Wallis test was used for the comparison of clinical response rates among the different groups, and a p-value of 0.05 was considered statistically significant.
Results
Infants and IH characteristics
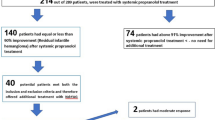
A total of 169 infants with head and neck IHs were enrolled in this study. Among the infants, 66 (39%) were male and 103 (61%) were female. Regarding type, mixed haemangioma was predominant, observed in 130 infants, whereas 21 tumours were deep and the remaining 18 were superficial. The most frequently involved head and neck sites were the parotid and perioral regions. A total of 16 patients had multifocal head and neck haemangiomas involving more than one anatomic site. The infants were begun on treatment at an average age of 3.85 months (0.5–11.4 months). The main clinical data are presented in Table 1.
Therapeutic effect of oral propranolol for IH
All infants exhibited immediate IH colour lightening, reduced tumour growth rates and obvious softening in texture during hospitalization. Clinical response (grades III + IV) regression rates were observed in 97.63% of the infants at the end of treatment at a dose of 2.0 mg/kg/day. The average treatment duration was 9.99 months (2–24 months).
The results of the therapeutic response evaluations 6 months after treatment initiation were as follows: grade I in 4 infants (2.37%), grade II in 10 infants (5.92%), grade III in 37 infants (21.89%), and grade IV in 118 infants (69.82%). The clinical response rate was 91.72%.
Effects of age at treatment initiation and lesion type and location on therapeutic effect
Table 2 presents the IH therapeutic responses and treatment durations.
An analysis by age at treatment initiation revealed that the infants in whom treatment was initiated earlier than 3 months of age had the poorest therapeutic responses and longest treatment durations. This group demonstrated an 84.93% clinical response rate and average treatment duration of 10.58 months. In contrast, the group aged 4–6 months had a 98.48% clinical response rate while the treatment duration for the group aged 7–9 months was 8.59 months. Although the clinical response rate differences among the four groups were significant (p = 0.030) for the initiation of treatment, the treatment duration differences were not significant (p = 0.375).
Regarding the haemangioma type, deep lesions had a superior therapeutic response (95.45%) than mixed (90.77%) and superficial lesions (94.44%), which, although interesting, were not significant findings (p = 0.716). The lesion type had a significant impact on the treatment duration (p = 0.033). The treatment duration for mixed lesions (9.52 months) was reduced compared with deep lesions (12.43 months), and the difference was significant (p = 0.010).
The location of the IHs had a significant impact on the therapeutic response (p = 0.020). Although the therapeutic response of parotid, periorbital, cheek, neck and multiple lesions reached 100%, perinasal (72.22%) and forehead (71.43%) lesions exhibited a poor response to propranolol. The treatment duration of IHs in different locations varied widely (cranium: 6.77 months; neck: 11.60 months); however, these differences were not significant (p = 0.284).
Discussion
Propranolol is a non-selective beta-adrenergic receptor (β-AR) blocker that has increasingly become an important treatment agent for IHs10. To date, no universally accepted protocol exists for the initiation and dosing of propranolol. A prospective study11 revealed that propranolol (2.0 mg/kg/day) was effective in Chinese infants with IH. In this study, 2.0 mg/kg/day was used as the regular dose. The clinical evaluation was performed 6 months after oral propranolol administration to assess the factors affecting the therapeutic effect. Grade IV response rates were achieved in 69.82% of infants 6 months after treatment initiation and in 88.76% of infants at the end of treatment, which were higher rates than those reported in other studies12, 13. Considering that lesions of the head and neck reportedly have better therapeutic responses to propranolol after an average treatment of 10.5 months6, a possible reason for our increased response rates may be that the lesions of the infants enrolled in our study were all located on the head and neck, whereas the lesions in other studies occurred all over the body.
Lesion location was a significant factor affecting the therapeutic response of IHs to propranolol in this study. Previous studies demonstrated that trunk haemangiomas responded poorly and required longer treatment durations than head and neck haemangiomas6. In a recent study on pharmacological therapies for IHs14, drug treatments for parotid region lesions were reportedly the most efficacious, whereas the least efficacious responses were observed with treatments of the lip region. In our cohort, better responses were observed for haemangiomas of the parotid, periorbital, cheek and neck regions. Lesions in locations where the therapeutic responses have been poor may be treated with other drugs14 while those that are not located in cosmetically sensitive areas may be treated with surgery15. Among the β-ARs, the β2-AR has a critical role in the action of propranolol16. Although no differences were observed in the β2-AR expression levels between four non-responding infants and four matched controls7, the differences in β2-AR expression among lesions at different locations and IH stages were not evaluated. We theorize that the effect of location on the therapeutic effect and treatment duration may be due to possible differences in β2-AR expression.
Investigators have observed that children who start treatment earlier have better responses17, 18 than older children. In our study, age had a significant impact on the IH treatment duration. The group aged 4–6 months had a higher clinical response rate than the other age groups. The youngest age group (1–3 months) had a poor clinical response, in contrast to previously published results. Additionally, we observed that the younger age groups (1–9 months) required lengthier treatments. We assessed the efficacy of propranolol after 6 months of treatment. The lesions in children who are begun on treatment before 3 months of age may still be in the proliferative phase19. Conversely, the lesions in older children may be in the involutional phase. These observations may explain why the younger infants in our study had longer treatment durations and relatively poorer responses. Previous results18, 20 have also demonstrated that younger infants require longer treatment than older infants.
IHs are classified into superficial, deep and mixed types according to the depth of the lesions21. In this study, deep lesions required significantly lengthier treatments. Because deeper lesions are consistently larger and and have a longer proliferative period19, they always require a longer treatment duration. The lesion type had no significant effect on the therapeutic response rates.
The limitations of this study are its retrospective nature, small sample size and high patient variability. Larger prospective studies should be undertaken to investigate the prognostic factors affecting the therapeutic effects of propranolol on infantile haemangiomas of the head and neck.
References
Haggstrom, A. N. et al. Prospective study of infantile hemangiomas: demographic, prenatal, and perinatal characteristics. The Journal of pediatrics 150, 291–294, doi:10.1016/j.jpeds.2006.12.003 (2007).
Haggstrom, A. N., Lammer, E. J., Schneider, R. A., Marcucio, R. & Frieden, I. J. Patterns of infantile hemangiomas: new clues to hemangioma pathogenesis and embryonic facial development. Pediatrics 117, 698–703, doi:10.1542/peds.2005-1092 (2006).
Gontijo, B. Complications of infantile hemangiomas. Clinics in dermatology 32, 471–476, doi:10.1016/j.clindermatol.2014.02.002 (2014).
Leaute-Labreze, C. et al. Propranolol for severe hemangiomas of infancy. The New England journal of medicine 358, 2649–2651, doi:10.1056/NEJMc0708819 (2008).
Fuchsmann, C. et al. Propranolol as first-line treatment of head and neck hemangiomas. Archives of otolaryngology–head & neck surgery 137, 471–478, doi:10.1001/archoto.2011.55 (2011).
Castaneda, S. et al. Therapeutic Effect of Propranolol in Mexican Patients with Infantile Hemangioma. Drugs - real world outcomes 3, 25–31, doi:10.1007/s40801-015-0052-3 (2016).
Phillips, R. J., Lokmic, Z., Crock, C. M. & Penington, A. Infantile haemangiomas that failed treatment with propranolol: clinical and histopathological features. Journal of paediatrics and child health 50, 619–625, doi:10.1111/jpc.12600 (2014).
Enjolras, O. & Mulliken, J. B. Vascular tumors and vascular malformations (new issues). Adv. Dermatol. 13, 375–423 (1997).
Achauer, B. M., Chang, C. J. & Vander Kam, V. M. Management of hemangioma of infancy: review of 245 patients. Plastic and reconstructive surgery 99, 1301–1308 (1997).
Hermans, D. J., Bauland, C. G., Zweegers, J., van Beynum, I. M. & van der Vleuten, C. J. Propranolol in a case series of 174 patients with complicated infantile haemangioma: indications, safety and future directions. The British journal of dermatology 168, 837–843, doi:10.1111/bjd.12189 (2013).
Chang, L. et al. Is Propranolol Safe and Effective for Outpatient Use for Infantile Hemangioma? A Prospective Study of 679 Cases From One Center in China. Ann. Plast. Surg. 76, 559–563, doi:10.1097/sap.0000000000000506 (2016).
Buckmiller, L. M., Munson, P. D., Dyamenahalli, U., Dai, Y. & Richter, G. T. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. The Laryngoscope 120, 676–681, doi:10.1002/lary.20807 (2010).
Pandey, V. et al. Propranolol for infantile haemangiomas: experience from a tertiary center. Journal of cutaneous and aesthetic surgery 7, 37–41, doi:10.4103/0974-2077.129975 (2014).
Zhang, L., Yuan, W. E. & Zheng, J. W. Pharmacological therapies for infantile hemangiomas: A clinical study in 853 consecutive patients using a standard treatment algorithm. Scientific reports 6, 21670, doi:10.1038/srep21670 (2016).
O, T. M., Scheuermann-Poley, C., Tan, M. & Waner, M. Distribution, clinical characteristics, and surgical treatment of lip infantile hemangiomas. JAMA facial plastic surgery 15, 292–304, doi:10.1001/jamafacial.2013.883 (2013).
Ji, Y., Chen, S., Xu, C., Li, L. & Xiang, B. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. The British journal of dermatology 172, 24–32, doi:10.1111/bjd.13388 (2015).
Phillips, R. J., Penington, A. J., Bekhor, P. S. & Crock, C. M. Use of propranolol for treatment of infantile haemangiomas in an outpatient setting. Journal of paediatrics and child health 48, 902–906, doi:10.1111/j.1440-1754.2012.02521.x (2012).
Andersen, I. G., Rechnitzer, C. & Charabi, B. Effectiveness of propanolol for treatment of infantile haemangioma. Danish medical journal 61, A4776 (2014).
Chang, L. C. et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 122, 360–367, doi:10.1542/peds.2007-2767 (2008).
Schupp, C. J., Kleber, J. B., Gunther, P. & Holland-Cunz, S. Propranolol therapy in 55 infants with infantile hemangioma: dosage, duration, adverse effects, and outcome. Pediatric dermatology 28, 640–644, doi:10.1111/j.1525-1470.2011.01569.x (2011).
Chiller, K. G., Passaro, D. & Frieden, I. J. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch. Dermatol. 138, 1567–1576 (2002).
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (81172572 and 81641036), the Key Research & Development Project of Shandong Province (2015GGH318012), and the Basic Scientific Research of Shandong University (2014QLKY33).
Author information
Authors and Affiliations
Contributions
Jianyong Dong, Jiexin Ning and Shaohua Liu were involved in the study design; Jianyong Dong, Jiexin Ning, Kai Li, Ronghui Li, Linlin Yue, and Yingying Huang conducted the study; Jianyong Dong and Jiexin Ning collected and analysed the data; Jianyong Dong wrote the first draft of the manuscript; Jianyong Dong, Jiexin Ning, Chao Liu and Xuxia Wang, and Shaohua Liu reviewed the manuscript; and Shaohua Liu gave the final manuscript approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Dong, JY., Ning, JX., Li, K. et al. Analysis of factors affecting the therapeutic effect of propranolol for infantile haemangioma of the head and neck. Sci Rep 7, 342 (2017). https://doi.org/10.1038/s41598-017-00495-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00495-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.